Medical Laboratories in Healthcare Delivery: A Systematic Review of Their Roles and Impact
Abstract
:1. Introduction
- Identify and categorize roles: identify and categorize the various roles that MLs play in healthcare delivery.
- Assess impact on patient outcomes: assess the impact of medical laboratory services on patient outcomes and healthcare quality.
- Explore integration challenges: explore challenges and barriers to effectively integrating MLs with other healthcare services.
- Evaluate resource utilization: evaluate the efficiency and effectiveness of resource utilization within MLs.
- Examine technological adoption: examine the adoption and impact of new technologies in MLS.
- Knowledge gap in laboratory contributions: There is a significant lack of comprehensive understanding regarding the full scope of MLs’ roles in healthcare delivery. This gap hinders the ability to optimize lab-based diagnostics and treatments critical to patient outcomes.
- Impact measurement challenges: The impact of ML on healthcare delivery is not consistently or adequately measured. Without clear metrics and evaluation frameworks, assessing how effectively labs contribute to patient care and health system efficiency is difficult.
- Integration issues: MLs often face challenges integrating their services with other healthcare delivery components. This disjointed integration can lead to inefficiencies, delays in diagnostic processes, and potential negative impacts on patient care.
- Resource allocation and utilization: MLs need to investigate how resources are allocated and utilized. Inefficiencies in resource management can affect the quality and timeliness of laboratory services, ultimately impacting healthcare delivery.
- Technological advancements and adoption: The rapid pace of technological advancements in laboratory sciences creates both opportunities and challenges. There is a need to evaluate how new technologies are adopted, their impact on laboratory performance, and their contribution to improved healthcare outcomes.
- Workforce challenges: The role of the laboratory workforce in healthcare delivery is often underappreciated and understudied. Understanding laboratory personnel’s training, workload, and professional development needs is crucial for enhancing their contribution to healthcare.
- What are MLs’ primary roles and functions in the healthcare delivery system?
- How do MLs impact patient outcomes and overall healthcare quality?
- What challenges do MLs face regarding integration with other healthcare services?
- What are the key factors influencing the efficiency and effectiveness of MLs?
- How are technological advancements being adopted in MLs, and what is their impact?
- Provide a comprehensive overview of the current state of MLs in healthcare delivery.
- Highlight the contributions of MLs to patient care and healthcare outcomes.
- Identify gaps in knowledge and areas for further research in ML science.
- Propose recommendations for improving medical laboratory services’ integration, efficiency, and effectiveness.
- Foster greater awareness and appreciation of the critical role that MLs play in the healthcare system.
2. Materials and Methods
2.1. Methodology
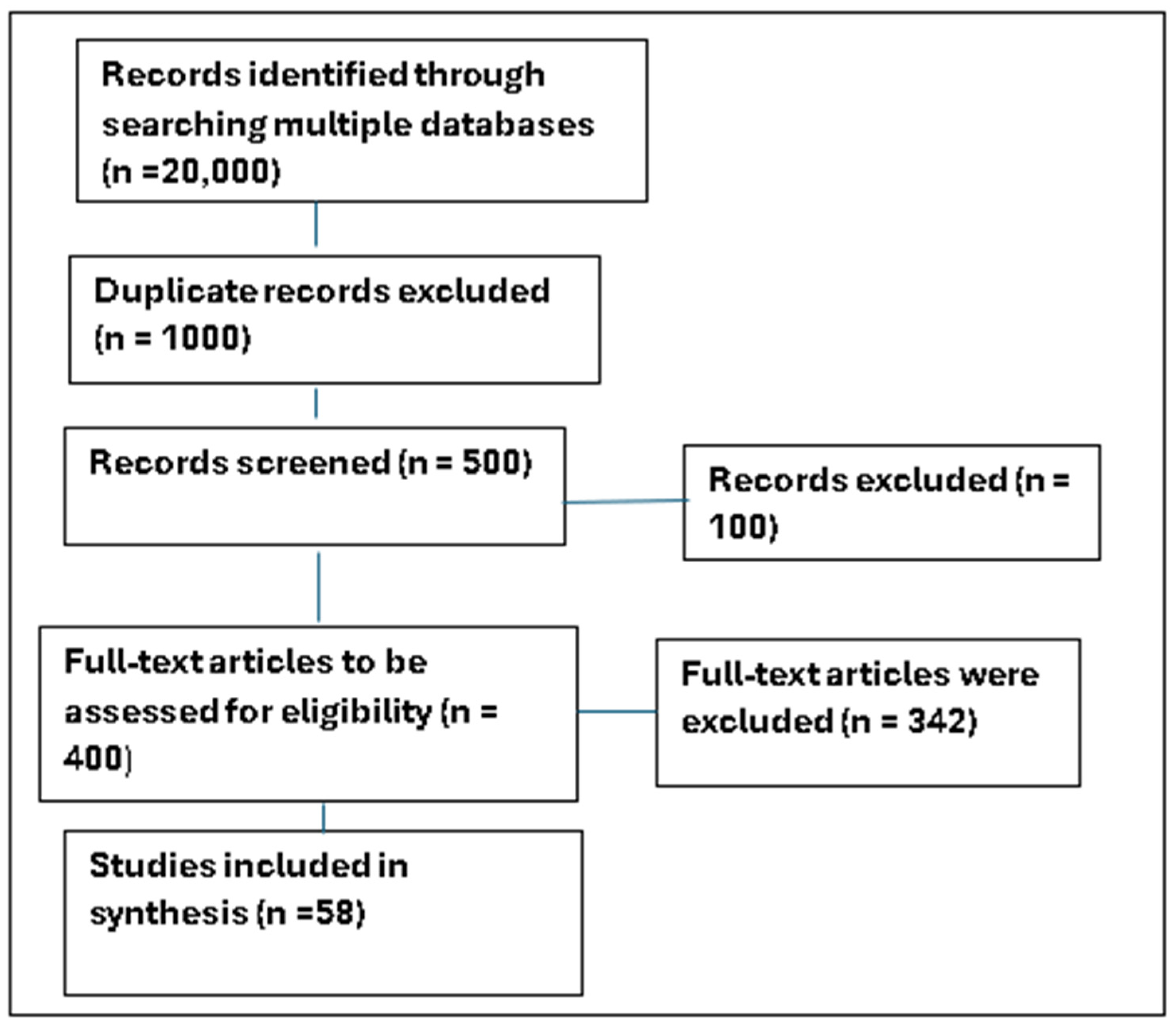
2.2. Methodology and Strategy for Searching and Filtering the Literature
2.3. Justification for the Time Frame
2.3.1. Relevance of Recent Advances
2.3.2. Focus on Contemporary Issues
2.3.3. Quality and Credibility
2.3.4. Potential Selection Bias
- Exclusion of older foundational papers: Older foundational papers may contain valuable insights and historical context that could inform current practices and developments. Excluding these papers might result in a loss of important background information and a less comprehensive understanding of the evolution of medical laboratory science.
- Language bias: The focus on English-language publications may exclude significant research published in local languages. In LMIC regions, local-language publications can be pivotal in understanding region-specific healthcare challenges and solutions. This exclusion could lead to an incomplete representation of the research landscape and potentially overlook valuable contributions from non-English-speaking researchers.
- Geographic and cultural bias: Research conducted in LMIC regions may be under-represented if it is not published in high-impact international journals. Local studies, which might be published in regional journals or in languages other than English, provide critical insights into the unique healthcare contexts of these regions. Excluding such studies could result in a biased understanding of the healthcare challenges and innovations in LMICs.
2.4. Methodology for Analyzing the Reported Information
3. Results
3.1. Medical Laboratories’ Roles in Pandemics
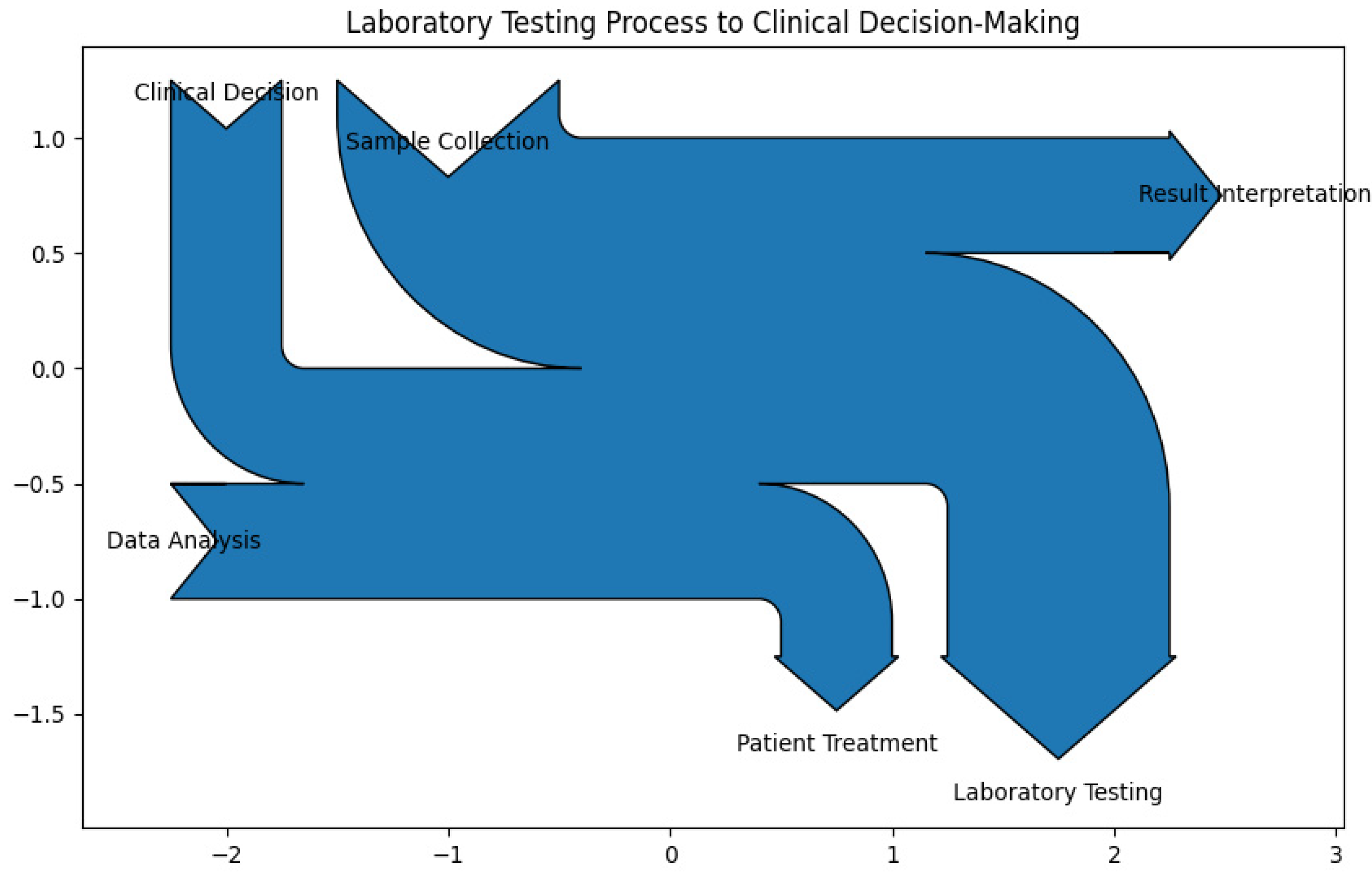
3.2. Laboratory Testing Process to Clinical Decision-Making
- Sample collection (15%): This is the initial step where biological samples (e.g., blood, urine, tissue) are collected from patients. Proper sample collection is crucial as it directly impacts the accuracy and reliability of subsequent tests. Accurate sample collection is essential to avoid contamination and ensure the integrity of the samples. Proper techniques and protocols must be followed to obtain reliable results.
- Laboratory testing (25%): Once samples are collected, they undergo various diagnostic tests. This process involves analyzing the samples using specialized equipment and techniques to detect diseases, infections, or other medical conditions. This is the core function of medical laboratories. The quality and precision of the tests directly affect the diagnosis and treatment of patients.
- Data analysis (20%): After testing, the data generated from the tests are analyzed. This includes statistical calculations, identifying patterns, trends, and anomalies and ensuring data accuracy and validity. This produces raw analytical results, such as numerical values, graphs, and charts, which need further interpretation to be meaningful. This step is critical for ensuring the accuracy and validity of the test results. A thorough analysis of test data is necessary to identify any abnormalities or patterns that may indicate a medical condition. This step requires expertise and attention to detail.
- Result interpretation (15%): The analyzed data are then interpreted to understand the patient’s condition clearly. This involves comparing the results with reference values and considering the patient’s medical history and symptoms. This guides clinicians in diagnosing conditions, determining treatment plans, and making informed medical decisions. Interpreting the results accurately is crucial for providing actionable information to clinicians. Misinterpretation can lead to incorrect diagnoses and inappropriate treatments.
- Clinical decision (15%): Based on the interpreted results, clinicians make informed decisions regarding patient care. This may include diagnosing a condition, determining the severity of a disease, or deciding on the appropriate treatment plan. The decisions made by clinicians based on laboratory results are vital for effective patient care. Accurate and timely information from the laboratory supports better clinical outcomes.
- Patient treatment (10%): The final step involves implementing the treatment plan based on the clinical decisions. This may include prescribing medications, recommending lifestyle changes, or scheduling follow-up tests and appointments. Implementing the right treatment plan based on laboratory findings ensures that patients receive appropriate care, leading to improved health outcomes.
3.3. Accessing Healthcare Through Laboratory Services
3.4. Quality Management System in Medical Laboratories—A Cost-Effective Model
- Standard operating procedures (SOPs): A set of step-by-step instructions compiled by an organization to help workers to carry out routine operations. SOPs aim to achieve efficiency, quality output, and uniformity of performance while reducing miscommunication and failure to comply with industry regulations. Laboratories develop and adhere to detailed SOPs for all testing processes to ensure consistency and accuracy in test procedures.
- Calibration and maintenance of equipment: Process of determining the relationship between the output or response of a measuring instrument and the value of the input. Calibration typically involves the use of a measuring standard. Maintenance refers to functions or actions required to ensure the proper working order of a piece of equipment. Laboratory equipment is regularly calibrated and maintained to ensure correct function and accurate results.
- Internal quality control (IQC): Process of determining the relationship between the output or response of a measuring instrument and the value of the input. Laboratories analyze control samples alongside patient samples to monitor test performance. This rigor detects any deviations or errors in the testing process in real time. Control charts are used to monitor the performance of laboratory tests over time. They help to detect trends, shifts, or unusual variations in test results.
- External quality assessment (EQA): Used to periodically assess the quality of a lab’s performance and achieve added confidence in patient test results. Results are objectively compared to other laboratories using the same methodologies, instruments, and reagents. Participation in external proficiency testing programs where laboratories analyze unknown samples from an external agency to provide an independent assessment of laboratory performance and identify areas for improvement.
- Staff training and competency assessment: laboratory personnel undergo continuous training and competency assessments, ensuring that they are skilled and knowledgeable about the latest techniques and standards.
- Documentation and record keeping: having detailed documentation of all procedures, test results, and quality control measures facilitates traceability and accountability and helps to identify and correct errors.
- Regular audits and reviews: conduct regular internal and external audits that review compliance with quality standards to identify gaps and areas for improvement in the laboratory’s quality control processes.
- Corrective and preventive actions (CAPAs): implementing CAPA processes that address any identified issues and prevent recurrence enhances laboratory services’ overall quality and reliability.
- Risk management: involves identifying and managing potential risks that could impact the quality of laboratory results, as well as proactively addressing issues before they affect test outcomes.
3.5. The Roles of Artificial Intelligence Technologies in Modern Laboratory Medicine
Transformative Capabilities of Artificial Intelligence in Medical Laboratories
- Enhanced diagnostic accuracy: AI technologies, particularly machine learning algorithms, demonstrate exceptional accuracy in analyzing medical images and interpreting complex datasets, leading to earlier and more accurate diagnoses of conditions like cancers and neurological disorders [1].
- Predictive analytics: AI’s predictive analytic capabilities enable healthcare providers to anticipate medical events and patient outcomes with unprecedented accuracy, allowing for proactive interventions and improved patient management [1].
- Personalized medicine: AI can analyze extensive patient data to develop personalized treatment plans tailored to individual needs, improving treatment efficacy and reducing adverse reactions [2].
- Operational efficiency: AI can automate routine tasks in medical laboratories, such as sample sorting and data entry, freeing up professionals to focus on more complex analyses and decision-making processes, thus improving operational efficiency [2].
- Real-time monitoring and intervention: AI extends to real-time patient monitoring, particularly in intensive care and chronic disease management, predicting critical events before they occur and allowing for timely interventions [1].
- Addressing challenges in LMICs: AI-driven solutions, such as portable diagnostic devices and telemedicine platforms, can extend healthcare services to remote and underserved areas, providing cost-effective and scalable diagnostic solutions [2].
- Ethical and regulatory considerations: AI raises ethical and regulatory concerns, such as data privacy, security, and potential biases in algorithms. Establishing robust regulatory frameworks is essential to address these issues and ensure responsible AI integration into healthcare systems [2].
3.6. Collaboration Among Hospital Clinicians and Medical Laboratory Scientists
4. Discussion
4.1. Roles in Healthcare
4.1.1. Diagnostic Services
4.1.2. Disease Surveillance
4.1.3. Clinical Decision Support
4.1.4. Emergency Response
4.2. Challenges in LMICs
4.2.1. Resource Constraints
4.2.2. Access to Diagnostics
4.2.3. Infrastructure and Policy Issues
4.3. Technological Advancements
4.3.1. Artificial Intelligence (AI)
4.3.2. Point-of-Care Technologies
4.4. Quality Management Systems (QMSs)
4.4.1. Quality Management Systems (QMSs)
- Adopt scalable frameworks: Implement scalable QMS frameworks like ISO 9001, which can be tailored to the specific needs and capacities of LMICs [31]. This allows for gradual implementation, starting with critical areas and expanding as resources permit.
- Leverage international support: Seek technical and financial assistance from international organizations such as the World Health Organization (WHO) and the World Bank. These organizations can provide funding, training, and resources to support QMS implementation [32].
- Public–private partnerships: Encourage partnerships between governments and private sector entities to share the costs and benefits of implementing a QMS. This can include joint ventures, shared infrastructure, and co-funded training programs.
- Capacity building: Invest in training and capacity-building programs to develop local expertise in QMS. This can be achieved through online courses, workshops, and collaboration with international experts.
4.4.2. Strengthening the Positioning and Rationale for the Cost-Effective QMS Model in MLS
4.5. Collaborative Efforts
4.6. AI Strategies
- Focus on high-impact areas: Prioritize AI applications that address the most pressing health challenges, such as disease surveillance, diagnostics, and telemedicine. This ensures that limited resources are used effectively.
- Build local expertise: Establish centers of excellence for AI in healthcare within LMICs. These centers can provide training, conduct research, and develop AI solutions tailored to local needs.
- Collaborate with tech companies: Form partnerships with technology companies to leverage their expertise and resources. These collaborations can help to develop cost-effective AI solutions and provide access to cutting-edge technology.
- Ethical and inclusive implementation: Ensure that AI strategies are implemented ethically and inclusively. This involves addressing potential biases in AI systems, ensuring data privacy, and involving local communities in the development and deployment of AI solutions.
- Innovative financing: explore innovative financing mechanisms such as blended finance, which combines public and private investment, and results-based financing, where funding is tied to achieving specific outcomes.
4.7. Future Directions
4.7.1. Investments in Laboratory Infrastructure
4.7.2. Adoption of Innovative Technologies
4.7.3. Establishment of Regulatory Frameworks
4.7.4. Ongoing Research and Development
5. Future Directions in Laboratory Medicine
5.1. Enhanced Diagnostic Accuracy
- Image analysis: AI excels in analyzing medical images, such as radiographs and MRIs, achieving higher accuracy than traditional methods. This allows for earlier detection of conditions like cancer.
5.2. Operational Efficiency
- Automation of routine tasks: AI automates repetitive tasks in laboratories, such as sample sorting and data entry, freeing up professionals for more complex analyses.
5.3. Improved Decision Support
- Clinical decision support systems (CDSSs): AI-driven CDSSs assist healthcare providers in selecting appropriate tests and interpreting results, ensuring clinical decisions are based on accurate data.
5.4. Challenges and Considerations
- Data quality and availability: The effectiveness of AI relies on the quality and quantity of data. In many LMICs, data scarcity and poor digital infrastructure pose significant challenges.
5.5. Latest Advancements in Laboratory Technology
- Automation and robotics: Streamlining processes, enhancing efficiency, and reducing human error. Automated systems handle repetitive tasks, allowing scientists to focus on complex analyses.
- Artificial intelligence (AI) and data analytics: AI is used for data analysis, helping to interpret complex datasets and improve diagnostic accuracy. Machine learning algorithms identify patterns in lab results that are not immediately apparent to human analysts.
- Digital pathology: allows for digitizing pathology slides, enabling remote access and collaboration among pathologists, enhancing diagnostic capabilities, and facilitating quicker decision-making.
- Green laboratory practices: emphasizing sustainability in lab operations, including using eco-friendly materials and waste reduction strategies to minimize environmental impact.
- Telemedicine and remote collaboration tools: enabling remote consultations and collaboration among healthcare professionals, improving access to laboratory services.
- CRISPR and gene editing technologies: advancements in CRISPR technology enable precise genetic modifications, which have significant implications for research and therapeutic applications.
5.6. Summary of Review Findings
- Role in healthcare: MLs are crucial for accurate diagnostics, disease surveillance, and emergency response. They significantly improve patient outcomes by guiding treatment decisions through precise laboratory results.
- Integration of AI: AI in laboratory medicine enhances diagnostic accuracy, streamlines workflows, and supports predictive analytics, leading to tailored treatment plans and improved patient management.
- Challenges in LMICs: Laboratories in LMICs face challenges such as inadequate infrastructure, limited access to technology, and insufficient data, hindering effective healthcare delivery and disease management.
- Collaborative efforts: The article advocates collaboration among governments, healthcare organizations, and laboratory professionals to strengthen laboratory systems and develop robust infrastructures to meet modern healthcare demands.
- Future directions: This paper calls for investments in laboratory infrastructure, the adoption of innovative technologies, and the establishment of regulatory frameworks to enhance laboratory services. It emphasizes ongoing research and development to address evolving healthcare challenges.
6. Conclusions and Recommendations
- Knowledge gap in laboratory contributions: This review elucidates the primary roles and functions of medical laboratories (MLs) in the healthcare delivery system, addressing the first research question. This comprehensive understanding helps optimize lab-based diagnostics and treatments critical to patient outcomes.
- Impact measurement challenges: By examining how MLs impact patient outcomes and overall healthcare quality, this review provides clear metrics and evaluation frameworks, addressing the second research question. This enables a more consistent and adequate measurement of MLs’ contributions to healthcare.
- Integration issues: This review identifies the challenges MLs face regarding integration with other healthcare services, addressing the third research question. Understanding these challenges helps mitigate inefficiencies and delays in diagnostic processes, ultimately improving patient care.
- Resource allocation and utilization: This review explores the key factors influencing the efficiency and effectiveness of MLs, addressing the fourth research question. Insights into resource management can enhance the quality and timeliness of laboratory services, positively impacting healthcare delivery.
- Technological advancements and adoption: By evaluating how technological advancements are being adopted in MLs and their impact, this review addresses the fifth research question. This assessment helps understand the opportunities and challenges posed by new technologies, contributing to improved healthcare outcomes.
- Workforce challenges: Although not explicitly listed as a separate research question, this review’s findings on the roles, training, workload, and professional development needs of laboratory personnel provide valuable insights into workforce challenges. This understanding is crucial for enhancing the contribution of laboratory staff to healthcare delivery.
- Short-Term Priorities
- 1.
- Infrastructure investments:
- 2.
- Workforce training:
- AI skill development: Training programs should focus on both technical AI skills and human-centric skills like critical thinking and problem-solving. Organizations like Jobs for the Future (JFF) provide toolkits to support this transition.
- Upskilling and reskilling: Continuous learning opportunities for employees to adapt to AI technologies are essential. This includes partnerships with educational institutions and online learning platforms.
- Long-Term Priorities
- 1.
- AI adoption:
- Strategic AI roadmap: Develop a clear AI strategy that aligns with business objectives. This includes investing in data management, building AI talent, and piloting AI projects before scaling them [55].
- AI maturity levels: progress through AI maturity levels, from awareness to transformational stages, to fully integrate AI into business processes.
- 2.
- Inter-lab networks:
- Collaborative research networks: Establishing inter-lab networks can foster collaboration and innovation. These networks can leverage AI to optimize research environments and improve scientific output [9].
- Smart lab connectivity: integrating AI and IoT in labs to create smart, connected environments that streamline workflows and enhance reproducibility.
- For Policymakers:
- Infrastructure funding: allocate resources for advanced data centers and AI-ready systems.
- Education and training: support AI skill development programs and continuous learning initiatives.
- For Funders:
- Strategic investments: invest in AI projects with clear roadmaps and potential for scalability.
- Collaborative networks: fund initiatives that promote inter-lab collaboration and smart lab connectivity.
- For Laboratory Managers:
- AI integration: develop and implement AI strategies aligned with lab objectives.
- Workforce development: prioritize upskilling and reskilling of staff to adapt to AI technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munagandla, V.B.; Dandyala, S.S.V.; Vadde, B.C. AI-Powered Cloud-Based Epidemic Surveillance System: A Framework for Early Detection. Rev. Intel. Artif. Med. 2024, 15, 673–690. [Google Scholar]
- Chintala, S.K. AI in public health: Modeling disease spread and management strategies. NeuroQuantology 2022, 20, 10830. [Google Scholar]
- Shiwlani, A.; Khan, M.; Sherani AM, K.; Qayyum, M.U.; Hussain, H.K. Revolutionizing healthcare: The impact of artificial intelligence on patient care, diagnosis, and treatment. Jurihum J. Inov. Dan Hum. 2024, 1, 779–790. [Google Scholar]
- Moher, D.; Schulz, K.F.; Simera, I.; Altman, D.G. Guidance for developers of health research reporting guidelines. PLoS Med. 2010, 7, e1000217. [Google Scholar] [CrossRef]
- Thanneru, S.; Sikka, K.; Bhalla, A.S.; Tripathi, M.; Thakar, A.; Singh, A.; Singh, C.A.; Verma, H. Deciding treatment end point in necrotizing otitis externa: Validation of a standardized clinical response assessment strategy with positron emission tomography findings. Eur. Arch. Otorhinolaryngol. 2025, 282, 1171–1177. [Google Scholar] [CrossRef]
- Pambuccian, S.E. The COVID-19 pandemic: Implications for the cytology laboratory. J. Am. Soc. Cytopathol. 2020, 9, 202–211. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin. Chem. Lab. Med. 2020, 58, 1063–1069. [Google Scholar] [CrossRef]
- Binnicker, M.J. Emergence of a novel coronavirus disease (COVID-19) and the importance of diagnostic testing: Why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clin. Chem. 2020, 66, 664–666. [Google Scholar] [CrossRef]
- Charlton, C.L.; Hull, N.; Sloma, C.R.; Bonifas, M.; Johnson, M.; Strain, A.K.; Wolford, T.; Staley, S.; Perkins, C.; Razzaque, R.; et al. How to prepare for the unexpected: A public health laboratory response. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef]
- Reller, L.B.; Weinstein, M.P.; Peterson, L.R.; Hamilton, J.D.; Baron, E.J.; Tompkins, L.S.; Miller, J.M.; Wilfert, C.M.; Tenover, F.C.; Thomson, R.B. Role of clinical microbiology laboratories in the management and control of infectious diseases and the delivery of health care. Clin. Infect. Dis. 2001, 32, 605–610. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Obeagu, G.B.U. Strengthening Laboratory Systems for Ensuring Accurate Diagnoses in Mother-to-Child Transmission (MTCT) Prevention Programs in Uganda: A Narrative Review. Ann. Med. Surg. 2024, 86, 10–1097. [Google Scholar]
- Watson, I.D.; Wilkie, P.; Hannan, A.; Beastall, G.H. Role of laboratory medicine in collaborative healthcare. Clin. Chem. Lab. Med. 2019, 57, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; Braga, F.; Panteghini, M. Laboratory medicine in the new healthcare environment. Clin. Chem. Lab. Med. 2016, 54, 523–533. [Google Scholar] [CrossRef]
- Oduoye, M.O.; Fatima, E.; Muzammil, M.A.; Dave, T.; Irfan, H.; Fariha, F.N.U.; Marbell, A.; Ubechu, S.C.; Scott, G.Y.; Elebesunu, E.E. Impacts of the advancement in artificial intelligence on laboratory medicine in low-and middle-income countries: Challenges and recommendations—A literature review. Health Sci. Rep. 2024, 7, e1794. [Google Scholar] [CrossRef]
- da Silva, S.J.R.; Silva, C.T.A.D.; Guarines, K.M.; Mendes, R.P.G.; Pardee, K.; Kohl, A.; Pena, L. Clinical and laboratory diagnosis of SARS-CoV-2, the virus causing COVID-19. ACS Infect. Dis. 2020, 6, 2319–2336. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R. Role of Medical Laboratory Technology in Health Care. In Clinical Laboratory Management; Springer: Berlin/Heidelberg, Germany, 2024; pp. 3–6. [Google Scholar]
- Turgeon, M.L. Clinical Laboratory Science-E-Book: Clinical Laboratory Science-E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2022. [Google Scholar]
- Opeyemi, A.A.; Obeagu, E.I.; Hassan, A.O. Enhancing quality healthcare in Nigeria through medical laboratory services: A review. Medicine 2024, 103, e36869. [Google Scholar] [CrossRef]
- Babyar, J. Laboratory science and a glimpse into the future. Int. J. Healthc. Manag. 2020, 13, 456–463. [Google Scholar] [CrossRef]
- Swanson, K.; Dodd, M.R.; VanNess, R.; Crossey, M. Improving the Delivery of Healthcare through Clinical Diagnostic Insights: A Valuation of Laboratory Medicine through “Clinical Lab 2.0”. J. Appl. Lab. Med. 2018, 3, 487–497. [Google Scholar] [CrossRef]
- Parsons, L.M.; Somoskovi, A.; Lee, E.; Paramasivan, C.N.; Schneidman, M.; Birx, D.; Roscigno, G.; Nkengasong, J.N. Global health: Integrating national laboratory health systems and services in resource-limited settings. Afr. J. Lab. Med. 2012, 1, 5. [Google Scholar] [CrossRef]
- Ahmadi, A. Digital health transformation: Leveraging ai for monitoring and disease management. Int. J. BioLife Sci. (IJBLS) 2024, 3, 10–24. [Google Scholar]
- Greaves, R.F.; Bernardini, S.; Ferrari, M.; Fortina, P.; Gouget, B.; Gruson, D.; Lang, T.; Loh, T.P.; Morris, H.A.; Park, J.Y.; et al. Key questions about the future of laboratory medicine in the next decade of the 21st century: A report from the IFCC-Emerging Technologies Division. Clin. Chim. Acta 2019, 495, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Santarsiero, F.; Schiuma, G.; Carlucci, D.; Helander, N. Digital transformation in healthcare organisations: The role of innovation labs. Technovation 2023, 122, 102640. [Google Scholar] [CrossRef]
- Funnye-Doby, C. Awareness of Clinical Laboratory Sciences and Shortage of Clinical Laboratory Scientists in the 21st Century. Ph.D. Thisis, Walden University, Washington, MN, USA, 2016. [Google Scholar]
- Ondoa, P.; Ndlovu, N.; Keita, M.-S.; Massinga-Loembe, M.; Kebede, Y.; Odhiambo, C.; Mekonen, T.; Ashenafi, A.; Kebede, A.; Nkengasong, J. Preparing national tiered laboratory systems and networks to advance diagnostics in Africa and meet the continent’s health agenda: Insights into priority areas for improvement. Afr. J. Lab. Med. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Vian, T. Review of corruption in the health sector: Theory, methods and interventions. Health Policy Plan. 2008, 23, 83–94. [Google Scholar] [CrossRef]
- Pradhan, S.; Gautam, K.; Pant, V. ISO 15189: 2022; what’s new in new? J. Pathol. Nepal 2023, 13, 2027–2028. [Google Scholar] [CrossRef]
- Nega, D.; Abebe, A.; Abera, A.; Gidey, B.; G/Tsadik, A.; Tasew, G. Comprehensive competency assessment of malaria microscopists and laboratory diagnostic service capacity in districts stratified for malaria elimination in Ethiopia. PLoS ONE 2020, 15, e0235151. [Google Scholar] [CrossRef]
- Westgard, J.O. Managing quality vs. measuring uncertainty in the medical laboratory. Clin. Chem. Lab. Med. 2010, 48, 31–40. [Google Scholar] [CrossRef]
- Sayed, S.; Cherniak, W.; Lawler, M.; Tan, S.Y.; El Sadr, W.; Wolf, N.; Silkensen, S.; Brand, N.; Looi, P.L.M.; Pai, S.A.; et al. Improving pathology and laboratory medicine in low-income and middle-income countries: Roadmap to solutions. Lancet 2018, 391, 1939–1952. [Google Scholar] [CrossRef]
- Chowdhury, A.T.; Newaz, M.; Saha, P.; Majid, M.E.; Mushtak, A.; Kabir, M.A. Application of Big Data in Infectious Disease Surveillance: Contemporary Challenges and Solutions. In Surveillance, Prevention, and Control of Infectious Diseases: An AI Perspective; Springer: Berlin/Heidelberg, Germany, 2024; pp. 51–71. [Google Scholar]
- Olatunji, A.O.; Olaboye, J.A.; Maha, C.C.; Kolawole, T.O.; Abdul, S. Revolutionizing infectious disease management in low-resource settings: The impact of rapid diagnostic technologies and portable devices. Int. J. Appl. Res. Soc. Sci. 2024, 6, 1417–1432. [Google Scholar] [CrossRef]
- Hanson, K.E.; Altayar, O.; Caliendo, A.M.; Arias, C.A.; Englund, J.A.; Hayden, M.K.; Lee, M.K.; Loeb, M.; Patel, R.; Alayli, A.E.; et al. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: Antigen testing (June 2021). Clin. Infect. Dis. 2024, 78, e208–e229. [Google Scholar] [CrossRef]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial intelligence in disease diagnosis: A systematic literature review, synthesizing framework and future research agenda. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 8459–8486. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, N.; Wahl, B. Artificial intelligence and the future of global health. Lancet 2020, 395, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- McClure, K. Student perceptions of the clinical laboratory science profession. Am. Soc. Clin. Lab. Sci. 2009, 22, 16–21. [Google Scholar]
- Sautter, R.; Halstead, D. The importance of medical laboratory scientists and the number of doctoral scientists that began their career by working on the front lines of laboratory medicine. Lab. Med. 2023, 54, e121–e123. [Google Scholar] [CrossRef] [PubMed]
- Church, D.L.; Naugler, C. Using a systematic approach to strategic innovation in laboratory medicine to bring about change. Crit. Rev. Clin. Lab. Sci. 2022, 59, 178–202. [Google Scholar] [CrossRef]
- Panteghini, M. Redesigning the surveillance of in vitro diagnostic medical devices and medical laboratory performance by quality control in the traceability era. Clin. Chem. Lab. Med. 2023, 61, 759–768. [Google Scholar] [CrossRef]
- Pabbaraju, K.; Wong, A.A.; Douesnard, M.; Ma, R.; Gill, K.; Dieu, P.; Fonseca, K.; Zelyas, N.; Tipples, G.A. A public health laboratory response to the pandemic. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef]
- Alemnji, G.A.; Zeh, C.; Yao, K.; Fonjungo, P.N. Strengthening national health laboratories in sub-S aharan A frica: A decade of remarkable progress. Trop. Med. Int. Health 2014, 19, 450–458. [Google Scholar] [CrossRef]
- Durant, T.J.S.; Peaper, D.R.; Ferguson, D.; Schulz, W.L. Impact of COVID-19 pandemic on laboratory utilization. J. Appl. Lab. Med. 2020, 5, 1194–1205. [Google Scholar] [CrossRef]
- Stern, D.B. Philip M. Bromberg (1931–2020). In Trauma, Dissociation, and the Multiple Self; Taylor & Francis: New York, NY, USA, 2021. [Google Scholar]
- Ahn, C.; Amer, H.; Anglicheau, D.; Ascher, N.; Baan, C.; Battsetset, G.; Bat-Ireedui, B.; Berney, T.; Betjes, M.; Bichu, S.; et al. Global Transplantation COVID Report March 2020. Transplantation 2020, 104, 1974–1983. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061. [Google Scholar] [CrossRef] [PubMed]
- Perkins, T.A.; Siraj, A.S.; Ruktanonchai, C.W.; Kraemer, M.U.G.; Tatem, A.J. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat. Microbiol. 2016, 1, 16126. [Google Scholar] [CrossRef] [PubMed]
- Nyenswah, T.G.; Kateh, F.; Bawo, L.; Massaquoi, M.; Gbanyan, M.; Fallah, M.; Nagbe, T.K.; Karsor, K.K.; Wesseh, C.S.; Sieh, S.; et al. Ebola and its control in liberia, 2014–2015. Emerg. Infect. Dis. 2016, 22, 169–177. [Google Scholar] [CrossRef]
- Berkley, S. Health Security’s Blind Spot; American Association for the Advancement of Science: Washington, DC, USA, 2018; p. 1075. [Google Scholar]
- Shaw, J.; Rudzicz, F.; Jamieson, T.; Goldfarb, A. Artificial intelligence and the implementation challenge. J. Med. Internet Res. 2019, 21, e13659. [Google Scholar] [CrossRef]
- Adeniyi, A.O.; Arowoogun, J.O.; Chidi, R.; Okolo, C.A.; Babawarun, O. The impact of electronic health records on patient care and outcomes: A comprehensive review. World J. Adv. Res. Rev. 2024, 21, 1446–1455. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, N.; Del Ser, J.; Coeckelbergh, M.; de Prado, M.L.; Herrera-Viedma, E.; Herrera, F. Connecting the dots in trustworthy Artificial Intelligence: From AI principles, ethics, and key requirements to responsible AI systems and regulation. Inf. Fusion 2023, 99, 101896. [Google Scholar] [CrossRef]
- Reed, D.; Gannon, D.; Dongarra, J. Reinventing high performance computing: Challenges and opportunities. arXiv 2022, arXiv:2203.02544. [Google Scholar]
- Babashahi, L.; Barbosa, C.E.; Lima, Y.; Lyra, A.; Salazar, H.; Argôlo, M.; Almeida, M.A.d.; Souza, J.M.d. AI in the Workplace: A Systematic Review of Skill Transformation in the Industry. Adm. Sci. 2024, 14, 127. [Google Scholar] [CrossRef]
- Proksch, M.; Paliwal, N.; Bielert, W. The Secrets of AI Value Creation: A Practical Guide to Business Value Creation with Artificial Intelligence from Strategy to Execution; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Challoumis, C. Building a sustainable economy-how ai can optimize resource allocation. In Proceedings of the XVI International Scientific Conference, Philadelphia, PA, USA, 3–4 October 2024. [Google Scholar]
- Kong, J.D.; Akpudo, U.E.; Effoduh, J.O.; Bragazzi, N.L. Leveraging Responsible, Explainable, and Local Artificial Intelligence Solutions for Clinical Public Health in the Global South. Healthcare 2023, 11, 457. [Google Scholar] [CrossRef]

| Role of Medical Laboratories | Description | Impact on Healthcare | Sources |
|---|---|---|---|
| Diagnostic services | Provide essential tests for disease detection and monitoring. | Early diagnosis leads to timely treatment and better patient outcomes. | [5,8,9,10,11,12,13,14] |
| Disease surveillance | Monitor disease prevalence and outbreaks. | Supports public health initiatives and informs policy decisions. | [6,10,15,16,17] |
| Clinical decision support | Assist clinicians in selecting appropriate tests and interpreting results. | Enhances the accuracy of diagnoses and treatment plans. | [9,17,18,19,20] |
| Research and development | Contribute to clinical trials and the development of new diagnostic technologies. | Drives innovation in healthcare and improves diagnostic capabilities. | [10,12,15,16,18] |
| Emergency response | Play a critical role during pandemics (e.g., COVID-19 testing). | Facilitates rapid response to health crises, minimizing disease spread. | [9,14,17,19,20,21] |
| Quality assurance | Ensure the reliability and accuracy of laboratory tests. | Maintains high standards of care and patient safety. | [13,15,16,19,22] |
| Education and training | Train healthcare professionals in laboratory practices and test interpretation. | Builds a knowledgeable workforce that improves healthcare delivery. | [8,13,15,18,21] |
| Substance abuse testing | Conduct tests to detect drug use and monitor rehabilitation. | Supports treatment programs and public safety initiatives. | [9,10,12,16,18] |
| Genetic testing | Analyze genetic material to identify hereditary conditions. | Aids in personalized medicine and risk assessment for diseases. | [12,13,14,15,17] |
| Transfusion services | Ensure safe blood transfusions through compatibility testing. | Reduces the risk of transfusion reactions and improves patient outcomes. | [10,12,16,21,23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekoya, A.; Okezue, M.A.; Menon, K. Medical Laboratories in Healthcare Delivery: A Systematic Review of Their Roles and Impact. Laboratories 2025, 2, 8. https://doi.org/10.3390/laboratories2010008
Adekoya A, Okezue MA, Menon K. Medical Laboratories in Healthcare Delivery: A Systematic Review of Their Roles and Impact. Laboratories. 2025; 2(1):8. https://doi.org/10.3390/laboratories2010008
Chicago/Turabian StyleAdekoya, Adebola, Mercy A. Okezue, and Kavitha Menon. 2025. "Medical Laboratories in Healthcare Delivery: A Systematic Review of Their Roles and Impact" Laboratories 2, no. 1: 8. https://doi.org/10.3390/laboratories2010008
APA StyleAdekoya, A., Okezue, M. A., & Menon, K. (2025). Medical Laboratories in Healthcare Delivery: A Systematic Review of Their Roles and Impact. Laboratories, 2(1), 8. https://doi.org/10.3390/laboratories2010008








