Multi-Level Drug Delivery System Integrated with Injectable Hydrogels and ZIF-8 for Sustained Release of Lidocaine
Abstract
1. Introduction
2. Materials and Methods
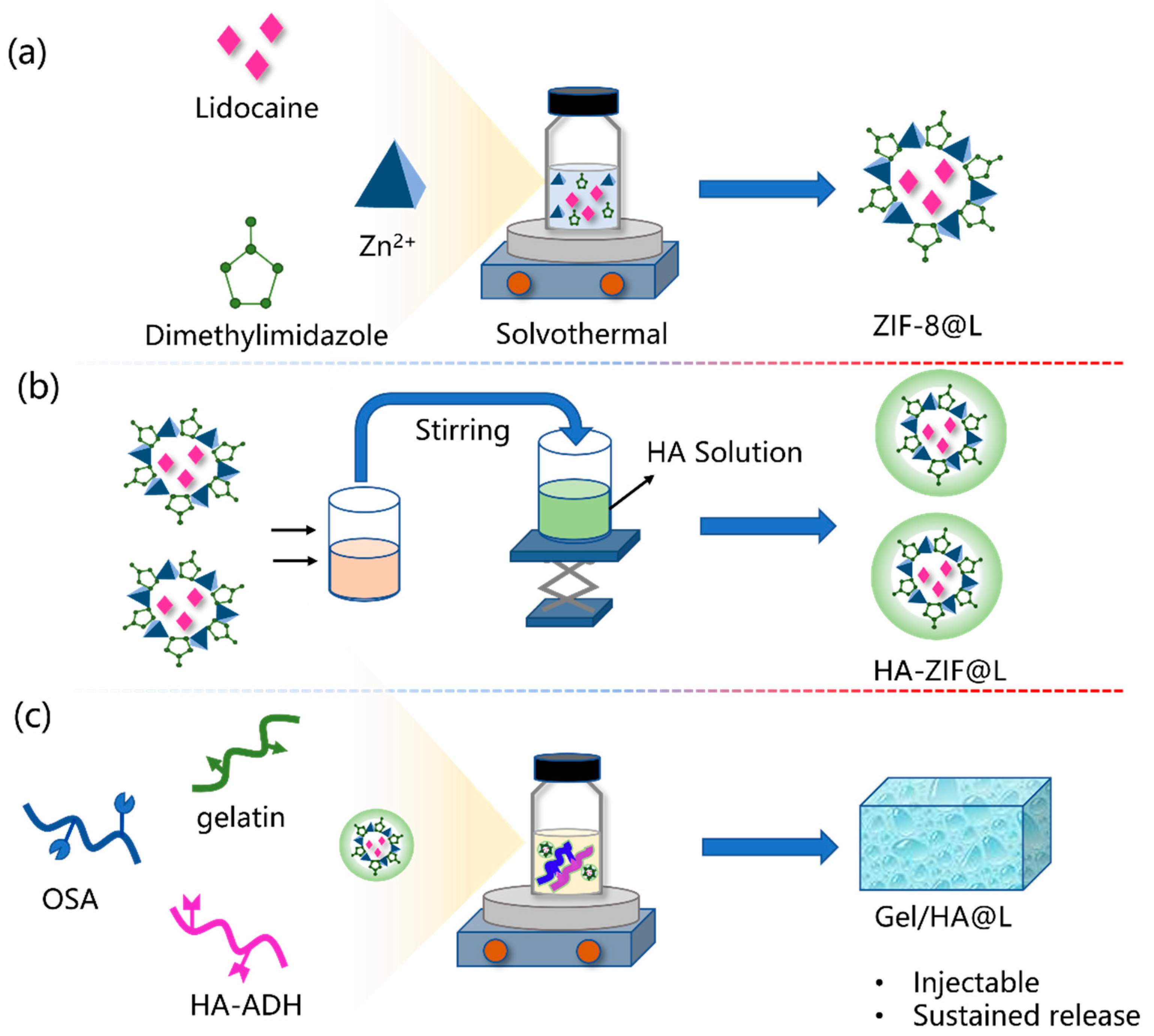
2.1. Synthesis of ZIF-8@L
2.2. Synthesis of HA/ZIF@L
2.3. Preparation of Gel/HZ@L
2.3.1. Synthesis of OSA
2.3.2. Synthesis of HA-ADH
2.3.3. Synthesis of Gel/HZ@L
2.4. Rheological Analysis
2.5. In Vitro Release Study
2.6. Biocompatibility of the Hydrogel
3. Results
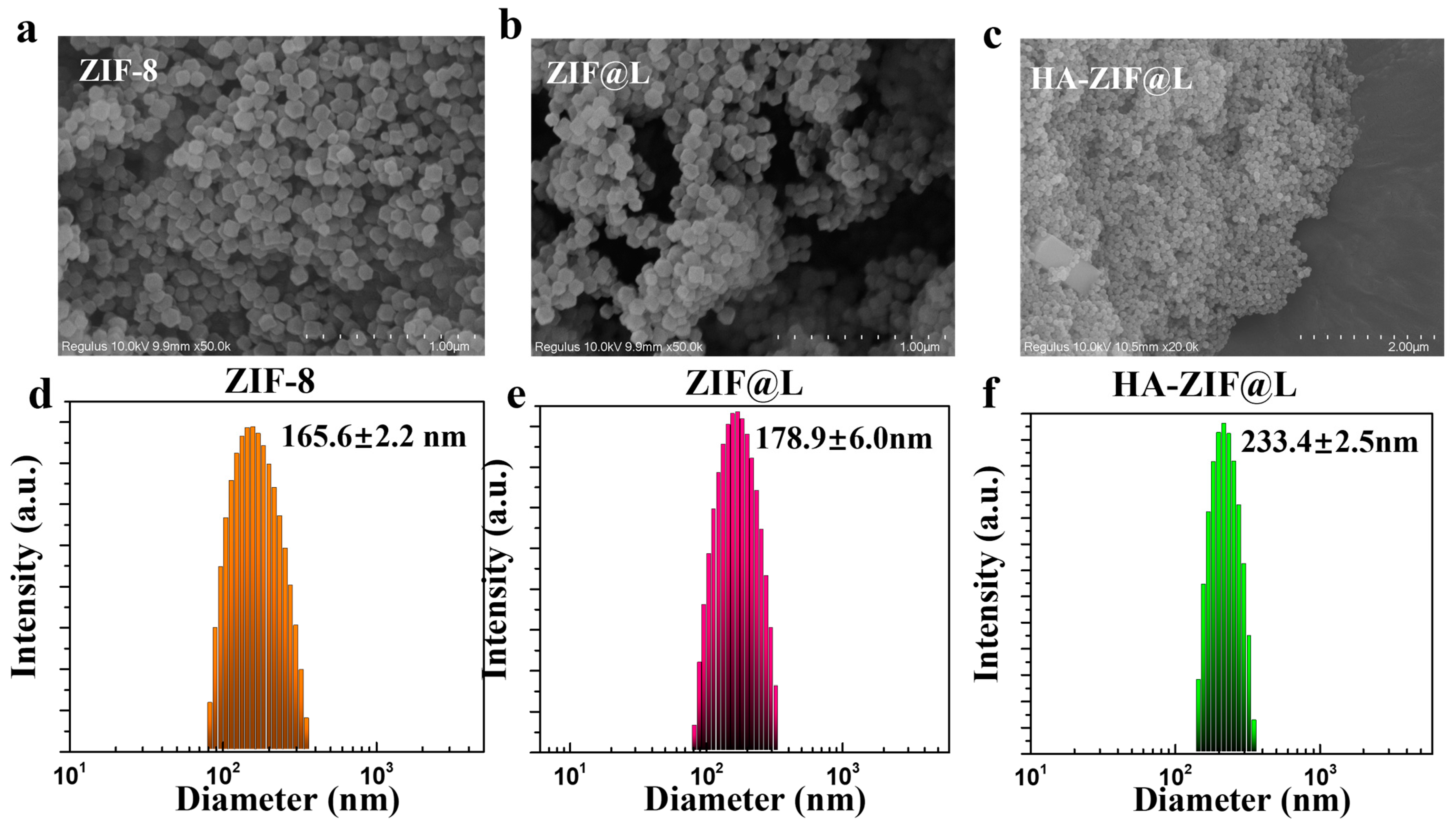
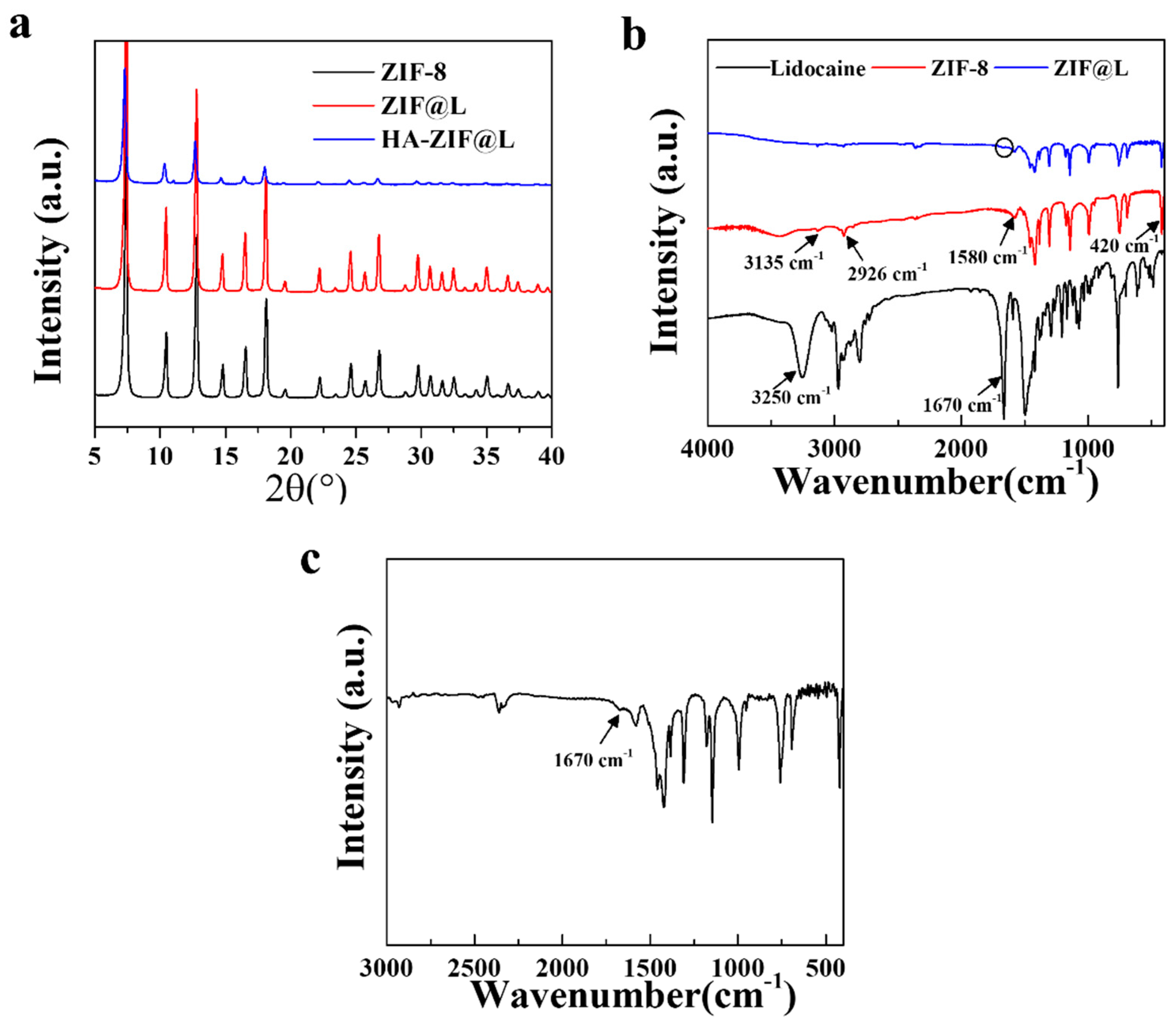
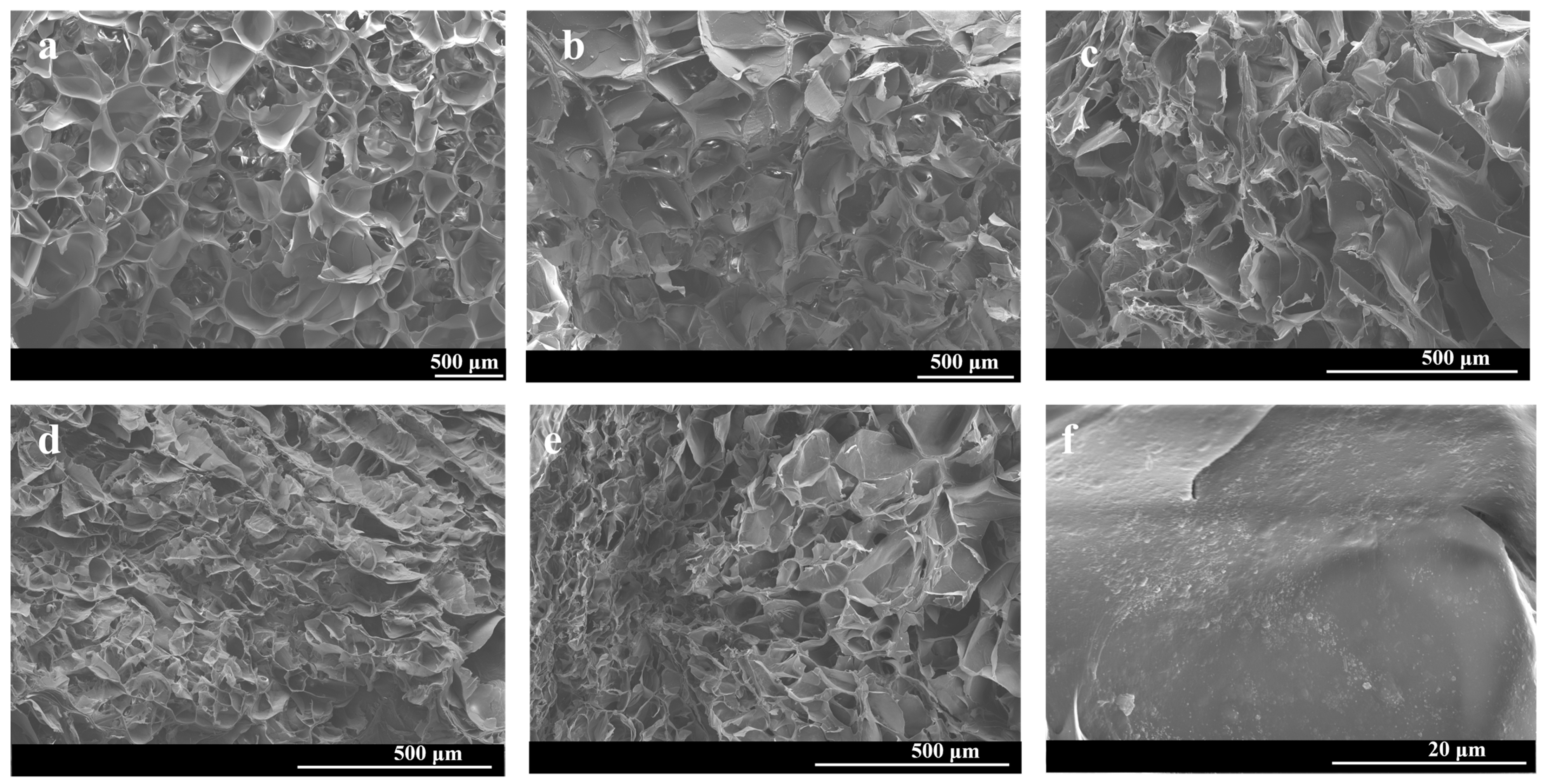
3.1. Fabrication and Characterization of Gel/HZ@L
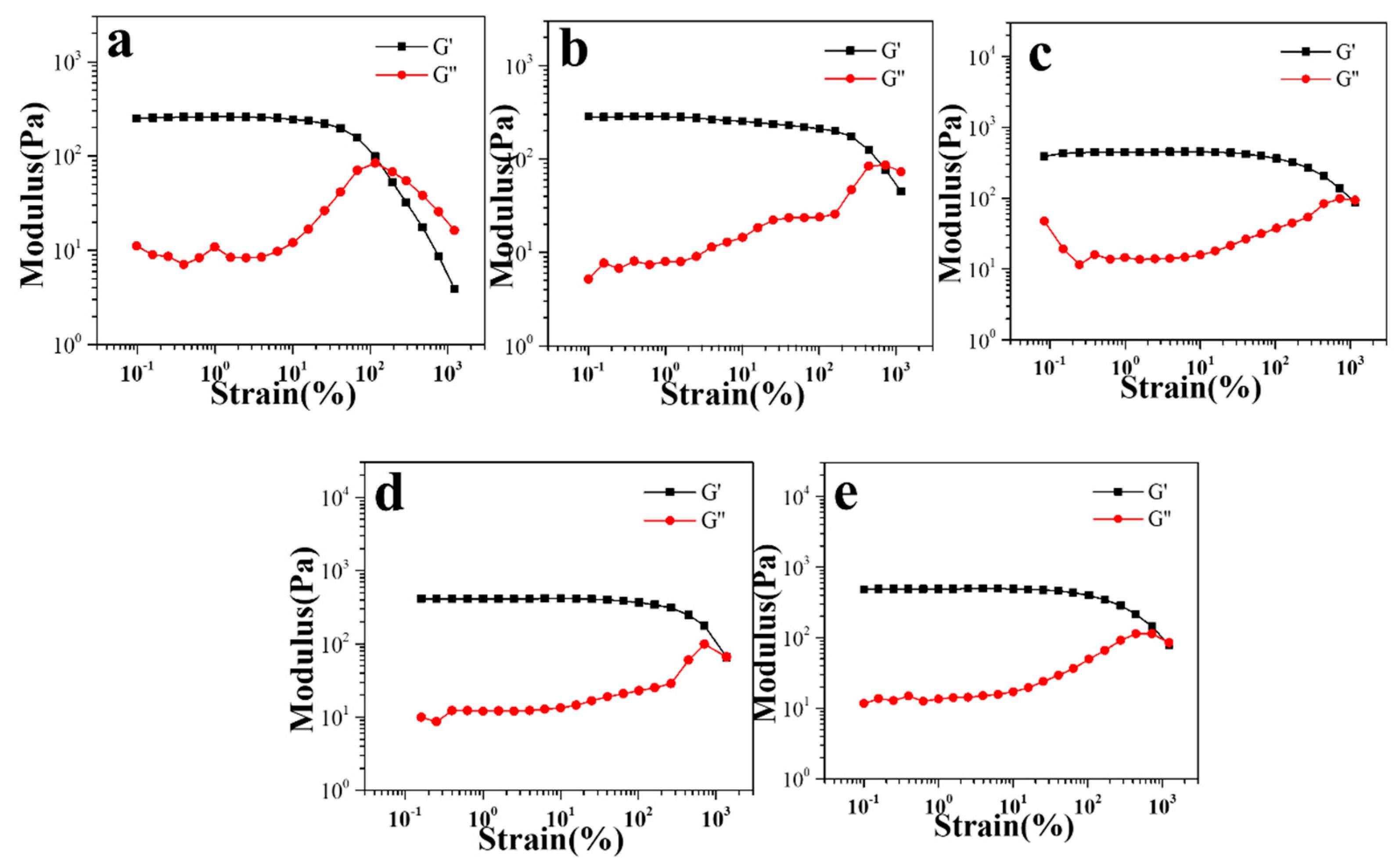
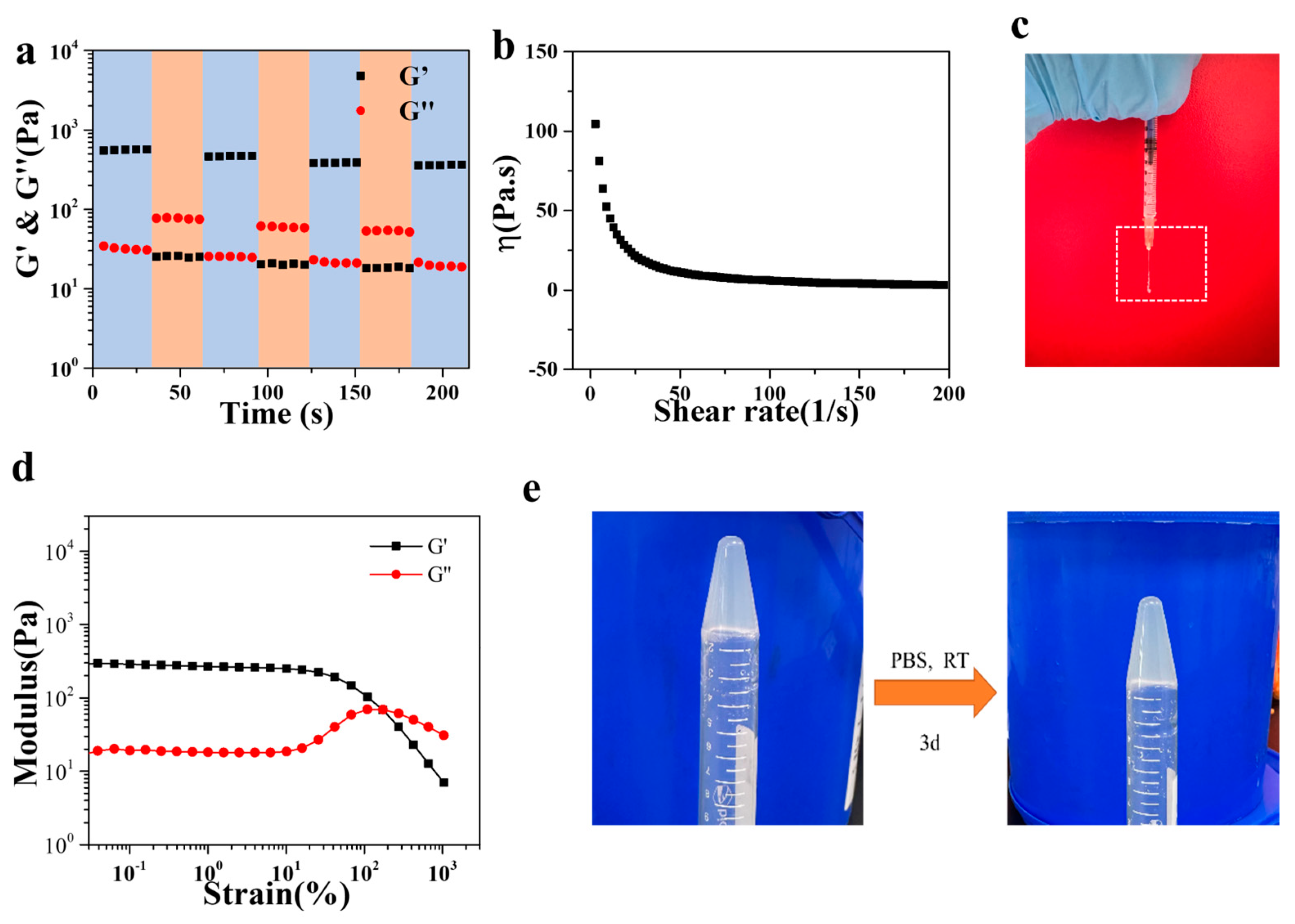
3.2. Rheological Properties of Gel/HZ@L
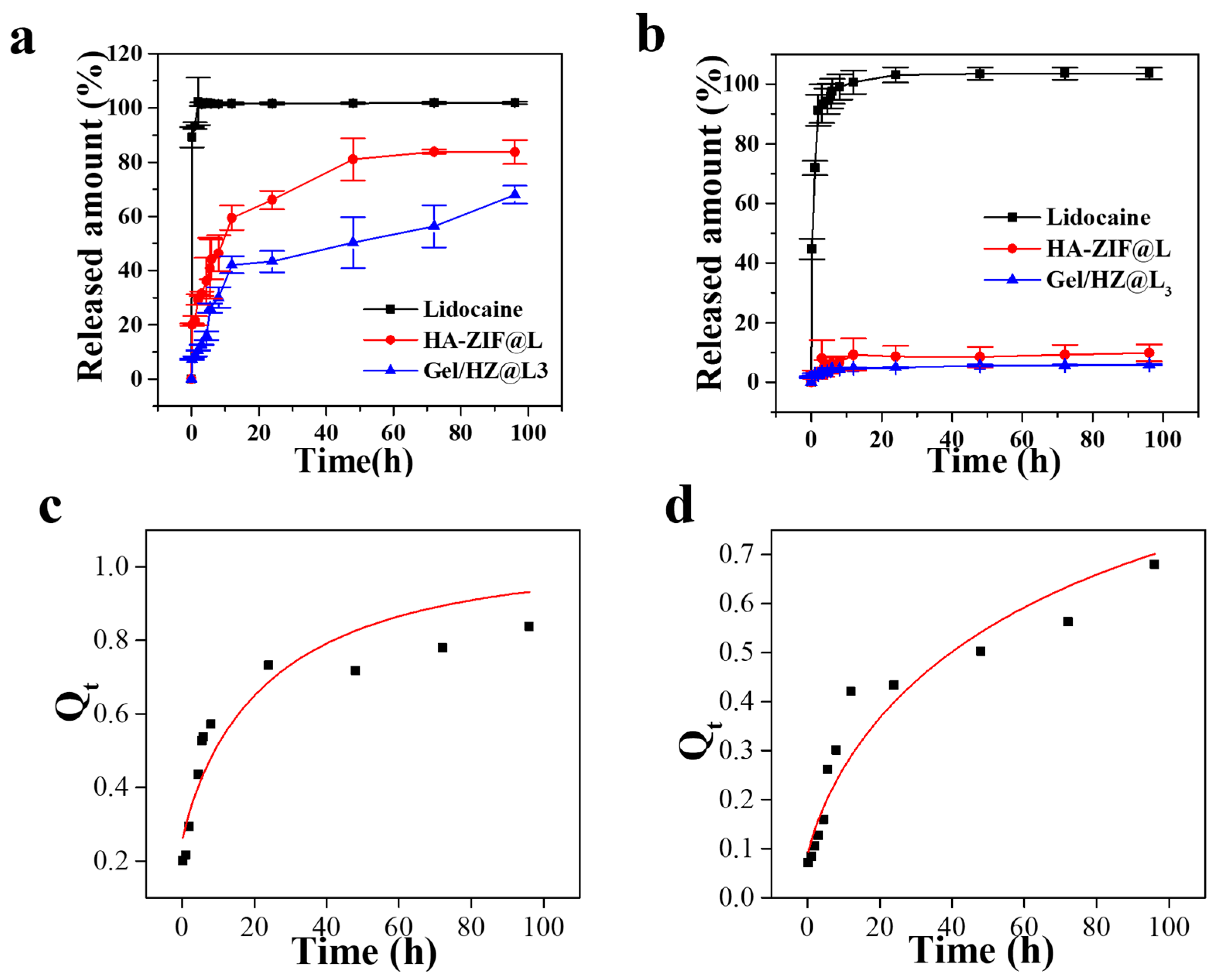
3.3. Release Behavior of HZ@L and Gel/HZ@L
3.4. Lidocaine Release Mechanism
3.5. Cytotoxicity and Biocompatibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, F.; Liu, J.; Chen, J.; Wu, W.; Zhang, Y.; Zhao, G.; Kang, Y.; Gong, D.; He, L.; Wang, J.; et al. Nanocrystals Slow-Releasing Ropivacaine and Doxorubicin to Synergistically Suppress Tumor Recurrence and Relieve Postoperative Pain. ACS Nano 2023, 17, 20135–20152. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qin, L.; Fang, Y.; Dan, Z.; Shen, Y.; Tan, G.; Huang, Y.; Ma, C. Electrospun PLGA nanomembrane: A novel formulation of extended-release bupivacaine delivery reducing postoperative pain. Mater. Des. 2020, 193, 108768. [Google Scholar] [CrossRef]
- Brigham, N.C.; Ji, R.-R.; Becker, M.L. Degradable polymeric vehicles for postoperative pain management. Nat. Commun. 2021, 12, 1367. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Xu, X.; Wang, A. An injectable mesoporous silica-based analgesic delivery system prolongs the duration of sciatic nerve block in mice with minimal toxicity. Acta Biomater. 2021, 135, 638–649. [Google Scholar] [CrossRef]
- Callahan, G.; Veith, A.; Madariaga, A.; Rausch, M.K.; Stromberg, D.; Baker, A.B. Drug eluting chest tube with sustained release of local anesthetic agents for pain reduction. Appl. Mater. Today 2023, 32, 101817. [Google Scholar] [CrossRef]
- Peng, F.; Liu, J.; Zhang, Y.; Fan, J.; Gong, D.; He, L.; Zhang, W.; Qiu, F. Designer self-assembling peptide nanofibers induce biomineralization of lidocaine for slow-release and prolonged analgesia. Acta Biomater. 2022, 146, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Steverink, J.G.; van Tol, F.R.; Oosterman, B.J.; Vermonden, T.; Verlaan, J.-J.; Malda, J.; Piluso, S. Robust gelatin hydrogels for local sustained release of bupivacaine following spinal surgery. Acta Biomater. 2022, 146, 145–158. [Google Scholar] [CrossRef]
- Wang, P.; Wang, G.; Tang, H.; Feng, S.; Tan, L.; Zhang, P.; Wei, G.; Wang, C. Preparation of Ropivacaine Encapsulated by Zeolite Imidazole Framework Microspheres as Sustained-Release System and Efficacy Evaluation. Chem. Eur. J. 2023, 29, e202203458. [Google Scholar] [CrossRef]
- Blair, H.A. Bupivacaine/Meloxicam Prolonged Release: A Review in Postoperative Pain. Drugs 2021, 81, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, X.; Li, H.; Peng, S.; Huang, H.; Yu, Z.; Chen, L.; Liu, X.; Bai, J. pH-Triggered Transformable Peptide Nanocarriers Extend Drug Retention for Breast Cancer Combination Therapy. Adv. Healthc. Mater. 2024, 2400031. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, S.; Bian, H.; Xing, X.; Yu, J.; Wu, X.; Zhang, L.; Liang, X.; Lu, A.; Huang, C. Extracellular matrix-mimicking nanofibrous chitosan microspheres as cell micro-ark for tissue engineering. Carbohyd. Polym. 2022, 292, 119693. [Google Scholar] [CrossRef]
- Khalili, Z.; Kazemi, N.M.; Azar, Z.J.; Mosavi, Z.; Hasanzadeh, M. Fabrication and characterization of a Bi2O3-modified chitosan@ZIF-8 nanocomposite for enhanced drug loading-releasing efficacy. Int. J. Biol. Macromol. 2024, 263, 130295. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Pei, X.; Yang, L.; Wang, L.; Chen, L.; Yang, G.; Pei, X.; Wan, Q.; Wang, J. Drug-loading ZIF-8 for modification of microporous bone scaffold to promote vascularized bone regeneration. Chin. Chem. Lett. 2024, 35, 108889. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, S.; Shen, Z.-T.; Hou, T.; Zhao, Y.-H.; Huang, M.-S.; Li, J.; Chen, H.; Hu, P.-H.; Luo, Z.-J.; et al. Core-Shell Reactor Partitioning Enzyme and Prodrug by ZIF-8 for NADPH-Sensitive In Situ Prodrug Activation. Angew. Chem. Int. Edit. 2023, 62, e202314025. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Lu, Z.-W.; Wu, Y.-C.; Liao, Y.-J.; Kuo, C.-Y. An injectable, chitosan-based hydrogel prepared by Schiff base reaction for anti-bacterial and sustained release applications. Int. J. Biol. Macromol. 2024, 269, 131808. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, Y.; Bai, S.; Zheng, Z.; Liu, Y.; Gui, S.; Shan, S.; Wu, J.; He, N. In situ formation of injectable organogels for punctal occlusion and sustained release of therapeutics: Design, preparation, in vitro and in vivo evaluation. Int. J. Pharmaceut. 2023, 638, 122933. [Google Scholar] [CrossRef]
- Fahmy-Garcia, S.; Mumcuoglu, D.; de Miguel, L.; Dieleman, V.; Witte-Bouma, J.; van der Eerden, B.C.J.; van Driel, M.; Eglin, D.; Verhaar, J.A.N.; Kluijtmans, S.G.J.M.; et al. Novel In Situ Gelling Hydrogels Loaded with Recombinant Collagen Peptide Microspheres as a Slow-Release System Induce Ectopic Bone Formation. Adv. Healthc. Mater. 2018, 7, 1800507. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Resnick, J.L.; Chaudhry, A.B.; Gertsman, I.; Nischal, K.K.; DiLeo, M.V. Ocular biodistribution of cysteamine delivered by a sustained release microsphere/thermoresponsive gel eyedrop. Int. J. Pharmaceut. 2022, 624, 121992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ning, C.; Xu, W.; Hu, H.; Li, M.; Zhao, G.; Ding, J.; Chen, X. Precision-guided long-acting analgesia by Gel-immobilized bupivacaine-loaded microsphere. Theranostics 2018, 8, 3331–3347. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Ning, C.; Li, M.; Zhao, G.; Jiang, W.; Ding, J.; Chen, X. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials 2018, 181, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Kuo, C.-H.; Chou, L.-Y.; Liu, D.-Y.; Weerapana, E.; Tsung, C.-K. Optimized Metal–Organic-Framework Nanospheres for Drug Delivery: Evaluation of Small-Molecule Encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, Z.; Lyu, Y.; Yang, J.; Lin, L.; Bai, H.; Li, Y.; Feng, Y.; Chen, Y. Electroactive injectable hydrogel based on oxidized sodium alginate and carboxymethyl chitosan for wound healing. Int. J. Biol. Macromol. 2023, 230, 123231. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Ariga, K.; Shrestha, L.K.; Hsu, S.-H. Development of MOF Reinforcement for Structural Stability and Toughness Enhancement of Biodegradable Bioinks. Biomacromolecules 2021, 22, 1053–1064. [Google Scholar] [CrossRef]
- Sun, Q.; Bi, H.; Wang, Z.; Li, C.; Wang, X.; Xu, J.; Zhu, H.; Zhao, R.; He, F.; Gai, S.; et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials 2019, 223, 119473. [Google Scholar] [CrossRef]
- Bakhtiari, S.E.; Zhu, Z.; Magdysyuk, O.V.; Brocchini, S.; Williams, G.R. Amorphous solid dispersions of lidocaine and lidocaine HCl produced by ball milling with well-defined RAFT-synthesised methacrylic acid polymers. Int. J. Pharmaceut. 2023, 644, 123291. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Ma, H. Selenium release kinetics and mechanism from Cordyceps sinensis exopolysaccharide-selenium composite nanoparticles in simulated gastrointestinal conditions. Food Chem. 2021, 350, 129223. [Google Scholar] [CrossRef] [PubMed]
- Dao, L.; Chen, S.; Sun, X.; Pang, W.; Zhang, H.; Liao, J.; Yan, J.; Pang, J. Construction and sustained release of konjac glucomannan/naringin composite gel spheres. Front. Nutr. 2023, 9, 1123494. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, W.; Chen, Z. Magnetic Fe3O4@ZIF-8 nanoparticles as a drug release vehicle: pH-sensitive release of norfloxacin and its antibacterial activity. Colloid Surf. B 2023, 223, 113170. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bi, H.-Y.; Li, H.; Mao, X.-M.; Liang, Y.-Q. Synthesis; characterization, and sustained release property of Fe3O4@(enrofloxacin-layered double hydroxides) nanocomposite. Mat. Sci. Eng C-Mater. 2017, 78, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Zhao, X.; Su, Z.; Wang, C.; Lin, Y. Layer-by-Layer Decorated Nanoscale ZIF-8 with High Curcumin Loading Effectively Inactivates Gram-Negative and Gram-Positive Bacteria. ACS Appl. Bio Mater. 2020, 3, 3673–3680. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Fan, F.; Wang, X.; Ali, S.; Zhou, F.; Zhang, J. Multi-Level Drug Delivery System Integrated with Injectable Hydrogels and ZIF-8 for Sustained Release of Lidocaine. J. Pharm. BioTech Ind. 2025, 2, 3. https://doi.org/10.3390/jpbi2010003
Jiang L, Fan F, Wang X, Ali S, Zhou F, Zhang J. Multi-Level Drug Delivery System Integrated with Injectable Hydrogels and ZIF-8 for Sustained Release of Lidocaine. Journal of Pharmaceutical and BioTech Industry. 2025; 2(1):3. https://doi.org/10.3390/jpbi2010003
Chicago/Turabian StyleJiang, Lei, Fan Fan, Xuemei Wang, Shaukat Ali, Feng Zhou, and Jiantao Zhang. 2025. "Multi-Level Drug Delivery System Integrated with Injectable Hydrogels and ZIF-8 for Sustained Release of Lidocaine" Journal of Pharmaceutical and BioTech Industry 2, no. 1: 3. https://doi.org/10.3390/jpbi2010003
APA StyleJiang, L., Fan, F., Wang, X., Ali, S., Zhou, F., & Zhang, J. (2025). Multi-Level Drug Delivery System Integrated with Injectable Hydrogels and ZIF-8 for Sustained Release of Lidocaine. Journal of Pharmaceutical and BioTech Industry, 2(1), 3. https://doi.org/10.3390/jpbi2010003






