A Short Review of Second-Generation Isobutanol Production by SHF and SSF
Abstract
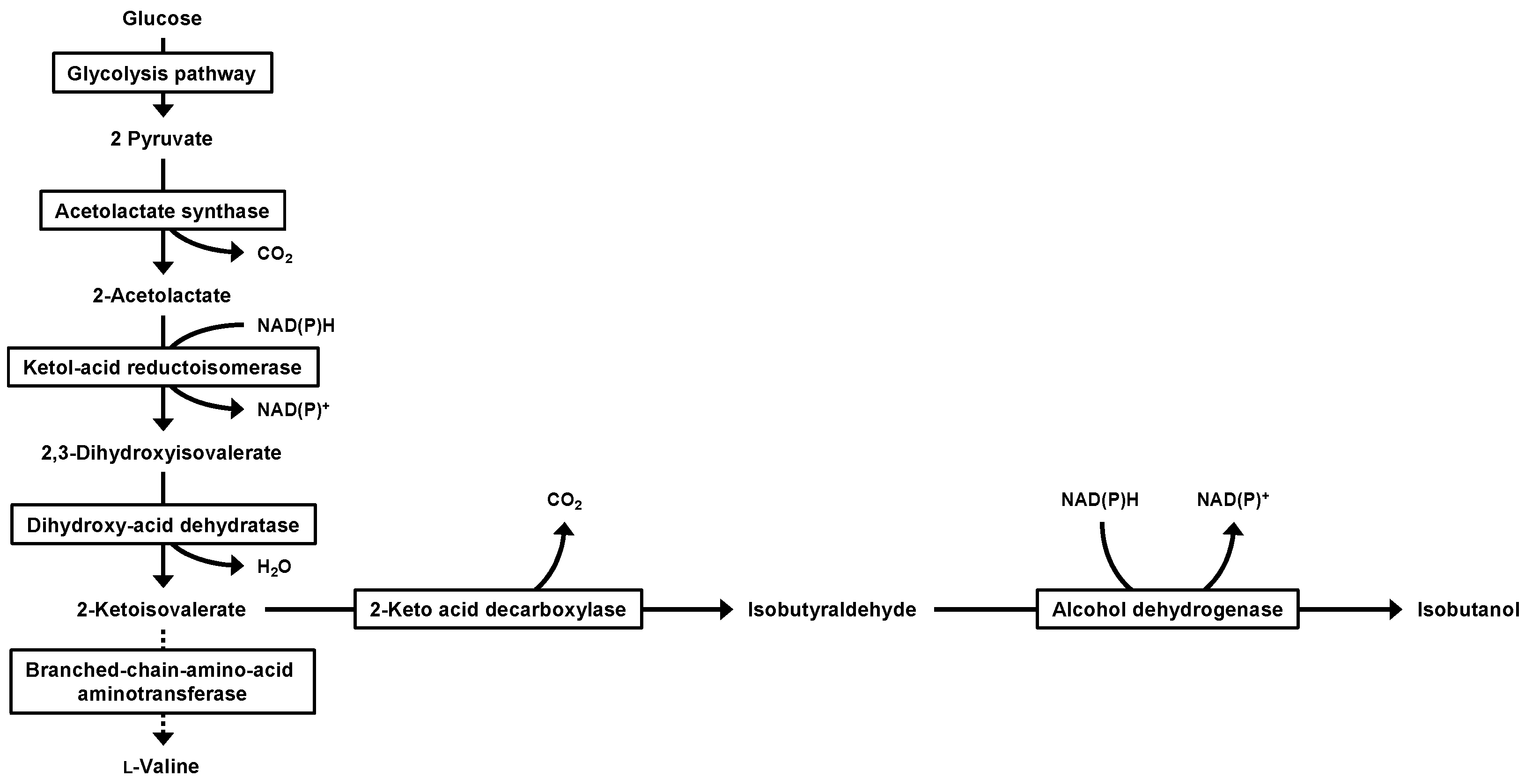
1. Introduction
2. Development of Isobutanol Production Capacity
3. Second-Generation Isobutanol Production by SHF
4. Second-Generation Isobutanol Production by SSF
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rial, R.C. Biofuels versus climate change: Exploring potentials and challenges in the energy transition. Renew. Sustain. Energy Rev. 2024, 196, 114369. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. Int. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Michael, K.; Steffi, N.; Peter, D. The past, present, and future of biofuels—Biobutanol as promising alternative. In Biofuel Production—Recent Developments and Prospects; dos Santos, M.A., Ed.; IntechOpen: London, UK, 2011; pp. 451–486. [Google Scholar]
- Food and Agriculture Organization (FAO). Crops for biofuels: Economic and technical assessment for sustainable production and utilization. In Biofuels and the Sustainability Challenge: A Global Assessment of Sustainability Issues, Trends and Policies for Biofuels and Related Feedstocks; Elbehri, A., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; pp. 15–48. [Google Scholar]
- Soccol, C.R.; Vandenberghe, L.P.S.; Costa, B.; Woiciechowski, A.L.; de Carvalho, J.C.; Medeiros, A.B.P.; Francisco, A.M.; Bonomi, L.J. Brazilian biofuel program: An overview. J. Sci. Ind. Res. 2005, 64, 897–904. [Google Scholar]
- EPA. Renewable Fuel Standard Program (RFS2) Regulatory Impact Analysis. 2010. Available online: https://19january2017snapshot.epa.gov/sites/production/files/2015-08/documents/420r10006.pdf (accessed on 14 May 2024).
- European Commission. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the Use of Energy from Renewable Sources. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L2001 (accessed on 14 May 2024).
- International Renewable Energy Agency (IRENA). Innovation Outlook: Advanced Liquid Biofuels. 2016. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2016/IRENA_Innovation_Outlook_Advanced_Liquid_Biofuels_2016.pdf (accessed on 14 May 2024).
- IEA. Renewables 2019. 2019. Available online: https://www.iea.org/reports/renewables-2019 (accessed on 14 May 2024).
- The New York Times. Air New Zealand Flies on Engine with Jatropha Biofuel Blend. Available online: https://archive.nytimes.com/green.blogs.nytimes.com/2008/12/30/air-new-zealand-flies-on-engine-with-jatropha-biofuel-blend/ (accessed on 14 May 2024).
- The New York Times. Virgin Atlantic Flies Jumbo Jet Powered by Biofuel. Available online: https://www.nytimes.com/2008/02/24/business/worldbusiness/24iht-biofuel.4.10340286.html (accessed on 14 May 2024).
- CFM International. Continental Airlines Flight Demonstrates Use of Sustainable Biofuels as Energy Source for Jet Travel. Available online: https://www.cfmaeroengines.com/press-articles/continental-airlines-flight-demonstrates-use-of-sustainable-biofuels-as-energy-source-for-jet-travel/ (accessed on 14 May 2024).
- Japan Airlines. JAL Flight Brings Aviation One Step Closer to Using Biofuel. Available online: https://press.jal.co.jp/en/uploads/01.%20Jan%2030%20Biofuel%20Press%20Release%20(English).pdf (accessed on 14 May 2024).
- The New York Times. KLM Carries Passengers in Biofuel Test Flight. Available online: https://archive.nytimes.com/www.nytimes.com/gwire/2009/11/23/23greenwire-klm-carries-passengers-in-biofuel-test-flight-92575.html?scp=2&sq=klm&st=cse (accessed on 14 May 2024).
- GreenAir News. United Airlines Makes Historic First US Commercial Biofuel Flight Using Solazyme’s Algae-Derived Solajet. Available online: https://archives.greenairnews.com/www.greenaironline.com/news0d35.html?viewStory=1369 (accessed on 14 May 2024).
- Aeromexico Business. Flying to a Greener Future. Available online: https://www.aeromexicobusiness.com/en/article/flying_to_a_greener_future (accessed on 14 May 2024).
- LATAM Airlines Group. Annual Report 2012. Available online: https://www.latamairlinesgroup.net/static-files/87aca1fc-f919-4f39-abcb-5161a2e2f091 (accessed on 14 May 2024).
- China Daily. Air China Conducts First Biofuel Test Flight. Available online: https://www.chinadaily.com.cn/china/2011-10/28/content_13998217.htm (accessed on 14 May 2024).
- AirFrance Corporate. Sustainable Aviation Fuel. Available online: https://corporate.airfrance.com/en/sustainable-aviation-fuel (accessed on 14 May 2024).
- Biofuels International. Interjet and Airbus Test First Biofuel Flight in Mexico. Available online: https://biofuels-news.com/news/interjet-and-airbus-test-first-biofuel-flight-in-mexico/ (accessed on 14 May 2024).
- KLM Royal Dutch Airlines. Sustainable Aviation Fuel (SAF) for Reduced CO2 Emissions. Available online: https://www.klm.co.jp/en/information/sustainability/sustainable-aviation-fuel?showredirectnotice=1&localmarketredirectedfrom=US (accessed on 14 May 2024).
- All Nippon Airways. The Benefits of ANA’s Commitment to Cleaner Fuel. Available online: https://www.anahd.co.jp/ana_news/en/2021/04/22/20210422-1.html (accessed on 14 May 2024).
- Etihad Airways. Etihad Sustainability Report. Available online: https://www.etihadaviationgroup.com/content/dam/eag/corporate/etihadaviation/en-ae/pdfs/Etihad%20Sustainability%20Report%20-%202020-2021.pdf (accessed on 14 May 2024).
- Neste. Nippon Cargo Airlines to Use Neste MY Sustainable Aviation Fuel for Their Cargo Flights Reducing the Emissions of Cargo Transport. Available online: https://www.neste.com/news/nippon-cargo-airlines-to-use-neste-my-sustainable-aviation-fuel-for-their-cargo-flights-reducing-the-emissions-of-cargo-transport (accessed on 14 May 2024).
- Reuters. Solazyme Shoots High with Algae Makeover. Available online: https://jp.reuters.com/article/idUSTRE81L203/ (accessed on 14 May 2024).
- Finnair. Annual Report 2014. Available online: https://investors.finnair.com/~/media/Files/F/Finnair-IR/documents/en/reports-and-presentation/2015/annual-report-2014.pdf (accessed on 14 May 2024).
- The New York Times. Airlines Fly the Skies on a Sugar High. Available online: https://www.nytimes.com/2014/10/08/business/energy-environment/airlines-fly-the-skies-on-a-sugar-high.html (accessed on 14 May 2024).
- Alaska Airlines. Alaska Airlines Flies First Commercial Flight with New Biofuel Made from Forest Residuals. Available online: https://news.alaskaair.com/alaska-airlines/company-news/nara-flight/ (accessed on 14 May 2024).
- United Airlines. Roadmap to Net Zero by 2050. Available online: https://crreport.united.com/environmental-sustainability/roadmap-to-net-zero (accessed on 14 May 2024).
- Singapore Airlines. Sustainability Report FY2020-21. Available online: https://www.singaporeair.com/saar5/pdf/Investor-Relations/Annual-Report/sustainabilityreport2021.pdf (accessed on 14 May 2024).
- The Indian Express. SpiceJet Flies India’s First Biofuel Flight, from Dehradun to Delhi. Available online: https://indianexpress.com/article/business/aviation/spicejet-operates-indias-first-biofuel-powered-flight-5326913/ (accessed on 14 May 2024).
- American Airlines. 2021 ESG Report. Available online: https://www.aa.com/content/images/customer-service/about-us/corporate-governance/esg/aag-esg-report-2021.pdf (accessed on 14 May 2024).
- All Nippon Airways. ANA Conducted a Flight Using Sustainable Aviation Fuel Produced from Microalgae. Available online: https://www.anahd.co.jp/group/en/pr/202106/20210618.html (accessed on 14 May 2024).
- Ministry of Economy, Trade and Industry. Japanese Airlines Fly with Domestically Produced Sustainable Aviation Fuel (SAF). Available online: https://www.meti.go.jp/english/press/2021/0618_003.html (accessed on 14 May 2024).
- United Airlines. Our Sustainable Aviation Fuel (SAF) Program. Available online: https://www.united.com/en/us/fly/company/responsibility/sustainable-aviation-fuel.html (accessed on 14 May 2024).
- Algae Planet. First Sustainable Aviation Fuel-Powered Fuji Dream Air Flight. Available online: https://algaeplanet.com/first-sustainable-aviation-fuel-powered-fuji-dream-air-flight/ (accessed on 14 May 2024).
- Lufthansa Group. Sustainable Aviation Fuel. Available online: https://www.lufthansagroup.com/en/responsibility/climate-environment/sustainable-aviation-fuel.html (accessed on 14 May 2024).
- Malaysia Airlines. SMalaysia Airlines Flies First Passenger Flight with Neste MY Sustainable Aviation Fuel Supplied by PETRONAS. Available online: https://www.malaysiaairlines.com/my/en/mh-media-centre/news-releases/2022/malaysia-airlines-flies-first-passenger-flight-with-neste-my-sustainable-aviation-fuel-supplied-by-petronas.html (accessed on 14 May 2024).
- Qantas Group. Sustainable Aviation Fuel. Available online: https://www.qantas.com/au/en/qantas-group/sustainability/our-planet/sustainable-aviation-fuel.html (accessed on 14 May 2024).
- Air India Express. Making History, AirAsia India, Praj and IOCL Join Hands to Fly First Commercial Flight in India Powered by a Blend of ‘Indigenous’ Sustainable Aviation Fuel. Available online: https://www.praj.net/wp-content/uploads/2023/05/230519-Press-Release-Praj-IOCL-AirAsia.pdf (accessed on 14 May 2024).
- Emirates. Emirates Operates Milestone Demonstration Flight Powered with 100% Sustainable Aviation Fuel. Available online: https://www.emirates.com/media-centre/emirates-operates-milestone-demonstration-flight-powered-with-100-sustainable-aviation-fuel/ (accessed on 14 May 2024).
- Finnair. Sustainable Aviation Fuel Has a Key Role in Decreasing Air Travel Emissions. Available online: https://www.finnair.com/jp-ja/bluewings/sustainability/sustainable-aviation-fuel-has-a-key-role-in-decreasing-air-travel-emissions--2890100 (accessed on 14 May 2024).
- Cathay Pacific Airlines. Sustainability Report 2023. Available online: https://www.cathaypacific.com/content/dam/cx/about-us/sustainability/sustainability-reports/en/cathay-pacific-sustainable-development-report-2023-en.pdf (accessed on 14 May 2024).
- Virgin Atlantic Airways. Virgin Atlantic Flies World’s First 100% Sustainable Aviation Fuel Flight from London Heathrow to New York JFK. Available online: https://corporate.virginatlantic.com/gb/en/media/press-releases/worlds-first-sustainable-aviation-fuel-flight.html (accessed on 14 May 2024).
- Times of India. Vistara Operates First Commercial India Flight with Sustainable Fuel. Available online: https://timesofindia.indiatimes.com/city/mumbai/vistara-operates-first-commercial-india-flight-with-sustainable-fuel/articleshow/99998141.cms (accessed on 14 May 2024).
- U.S. Department of Energy. Starch- and Sugar-Based Ethanol Feedstocks. Available online: https://afdc.energy.gov/fuels/ethanol-feedstocks (accessed on 14 May 2024).
- U.S. Energy Information Administration. Biofuels Explained—Ethanol and Biomass-BASED Diesel. 2020. Available online: https://www.eia.gov/energyexplained/biofuels/ (accessed on 14 May 2024).
- Ribeiro, B.E. Beyond commonplace biofuels: Social aspects of ethanol. Energy Policy 2013, 57, 355–362. [Google Scholar] [CrossRef]
- Havlík, P.; Schneider, U.A.; Schmid, E.; Böttcher, H.; Fritz, S.; Skalský, R.; Aoki, K.; De Cara, S.; Kindermann, G.; Kraxner, F.; et al. Global land-use implications of first and second generation biofuel targets. Energy Policy 2011, 39, 5690–5702. [Google Scholar] [CrossRef]
- Renewable Fuels Association (RFA). 2021 Ethanol Industry Outlook. Available online: https://d35t1syewk4d42.cloudfront.net/file/314/RFA_Outlook_2021_fin_low.pdf (accessed on 14 May 2024).
- Dedov, A.G.; Karavaev, A.A.; Loktev, A.S.; Osipov, A.K. Bioisobutanol as a promising feedstock for production of “Green” hydrocarbons and petrochemicals (A review). Pet. Chem. 2021, 61, 1139–1157. [Google Scholar] [CrossRef]
- SkyQuest Technology, Isobutanol market size, share, growth analysis, by type (synthetic and bio-based), by application (pharmaceuticals, chemicals and textiles, paint and coating, oil and gas), by region-Industry forecast 2024–2031. Available online: https://www.skyquestt.com/report/isobutanol-market (accessed on 14 May 2024).
- Tao, L.; Tan, E.C.; McCormick, R.; Zhang, M.; Aden, A.; He, X.; Zigler, B.T. Techno-economic analysis and life-cycle assessment of cellulosic isobutanol and comparison with cellulosic ethanol and n-butanol. Biofuels Bioprod. Biorefining 2014, 8, 30–48. [Google Scholar] [CrossRef]
- Lin, Z.; Cong, W.; Zhang, J. Biobutanol production from acetone–butanol–ethanol fermentation: Developments and prospects. Fermentation 2023, 9, 847. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef]
- Sauer, U.; Canonaco, F.; Heri, S.; Perrenoud, A.; Fischer, E. The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J. Biol. Chem. 2004, 279, 6613–6619. [Google Scholar] [CrossRef]
- Atsumi, S.; Wu, T.Y.; Eckl, E.M.; Hawkins, S.D.; Buelter, T.; Liao, J.C. Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes. Appl. Microbiol. Biotechnol. 2010, 85, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Bastian, S.; Liu, X.; Meyerowitz, J.T.; Snow, C.D.; Chen, M.M.Y.; Arnold, F.H. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab. Eng. 2011, 13, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Baez, A.; Cho, K.M.; Liao, J.C. High-flux isobutanol production using engineered Escherichia coli: A bioreactor study with in situ product removal. Appl. Microbiol. Biotechnol. 2011, 90, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Inoue, S.; Yoshida, M. High solid concentrations during the hydrothermal pretreatment of eucalyptus accelerate hemicellulose decomposition and subsequent enzymatic glucose production. Bioresour. Technol. Rep. 2018, 4, 16–20. [Google Scholar] [CrossRef]
- Akita, H.; Yusoff, M.Z.M.; Fujimoto, S. Preparation of oil palm empty fruit bunch hydrolysate. Fermentation 2021, 7, 81. [Google Scholar] [CrossRef]
- Jung, H.M.; Lee, J.Y.; Lee, J.H.; Oh, M.K. Improved production of isobutanol in pervaporation-coupled bioreactor using sugarcane bagasse hydrolysate in engineered Enterobacter aerogenes. Bioresour. Technol. 2018, 259, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Nakashima, N.; Hoshino, T. Bacterial production of isobutanol without expensive reagents. Appl. Microbiol. Biotechnol. 2015, 99, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, J.K.; Ahn, J.O.; Song, Y.H.; Shin, C.S.; Park, Y.C.; Kim, K.H. Isobutanol production from empty fruit bunches. Renew. Energy 2020, 157, 1124–1130. [Google Scholar] [CrossRef]
- Akita, H.; Shibata, S.; Komoriya, T.; Kamei, S.; Asamoto, H.; Matsumoto, M. Simultaneous saccharification and fermentation for isobutanol production from banana peel. Fermentation 2024, 10, 161. [Google Scholar] [CrossRef]
- Jarboe, L.R.; Chi, Z. Inhibition of microbial biocatalysts by biomass-derived aldehydes and methods for engineering tolerance. In New Developments in Aldehydes Research; Torrioni, L., Pescasseroli, E., Eds.; Nova Science Publishers: New York, NY, USA, 2013; pp. 101–120. [Google Scholar]
- Mills, T.Y.; Sandoval, N.R.; Gill, R.T. Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol. Biofuels 2009, 2, 26. [Google Scholar] [CrossRef]
- Akita, H.; Watanabe, M.; Suzuki, T.; Nakashima, N.; Hoshino, T. Characterization of the Kluyveromyces marxianus strain DMB1 YGL157w gene product as a broad specificity NADPH-dependent aldehyde reductase. AMB Express 2015, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhuge, B.; Qin, Y.; Zong, H.; Lu, X. Candida glycerinogenes strains overexpressing transcription factors have improved furfural tolerance in ethanol production from non-detoxified cellulose hydrolysate. Curr. Microbiol. 2022, 79, 196. [Google Scholar] [CrossRef] [PubMed]
- de Lima, A.E.P.; Wrobel, R.L.; Paul, B.; Anthony, L.C.; Sato, T.K.; Zhang, Y.; Hittinger, C.T.; Maravelias, C.T. High yield co-production of isobutanol and ethanol from switchgrass: Experiments, and process synthesis and analysis. Sustain. Energy Fuels 2023, 7, 3266–3275. [Google Scholar] [CrossRef]
- Desai, S.H.; Rabinovitch-Deere, C.A.; Fan, Z.; Atsumi, S. Isobutanol production from cellobionic acid in Escherichia coli. Microb. Cell Fact. 2015, 14, 52. [Google Scholar] [CrossRef]
- Bumrungtham, P.; Promdonkoy, P.; Prabmark, K.; Bunterngsook, B.; Boonyapakron, K.; Tanapongpipat, S.; Champreda, V.; Runguphan, W. Engineered production of isobutanol from sugarcane trash hydrolysates in Pichia pastoris. J. Fungi 2022, 8, 767. [Google Scholar] [CrossRef]
- Su, H.; Lin, J.; Wang, G. Metabolic engineering of Corynebacterium crenatium for enhancing production of higher alcohols. Sci. Rep. 2016, 6, 39543. [Google Scholar] [CrossRef]
- Hoffman, S.M.; Alvarez, M.; Alfassi, G.; Rein, D.M.; Garcia-Echauri, S.; Cohen, Y.; Avalos, J.L. Cellulosic biofuel production using emulsified simultaneous saccharification and fermentation (eSSF) with conventional and thermotolerant yeasts. Biotechnol. Biofuels 2021, 14, 157. [Google Scholar] [CrossRef]
- Gauss, W.F.; Suzuki, S.; Takagi, M. Manufacture of Alcohol from Cellulosic Materials Using Plural Ferments. U.S. Patent 3,990,944, 9 November 1976. [Google Scholar]
- Olofsson, K.; Bertilsson, M.; Liden, G. A short review on SSF-an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef]
- Wingren, A.; Galbe, M.; Zacchi, G. Techno-economic evaluation of producing ethanol from softwood: Comparison of SSF and SHF and identification of bottleneck. Biotechnol. Prog. 2003, 19, 1109–1117. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, Y.; Qin, T.; Wang, L.; Tang, Y.; Sun, Y.; Wan, P. Lignin depolymerization via an integrated approach of anode oxidation and electro-generated H2O2 oxidation. RSC Adv. 2014, 4, 6232–6238. [Google Scholar] [CrossRef]
- Akita, H.; Goshima, T.; Suzuki, T.; Itoiri, Y.; Kimura, Z.-i.; Matsushika, A. Application of Pichia kudriavzevii NBRC1279 and NBRC1664 to simultaneous saccharification and fermentation for bioethanol production. Fermentation 2021, 7, 83. [Google Scholar] [CrossRef]
- Akita, H.; Matsushika, A. Transcription analysis of the acid tolerance mechanism of Pichia kudriavzevii NBRC1279 and NBRC1664. Fermentation 2023, 9, 559. [Google Scholar] [CrossRef]
- Gevo. An Overview of Gevo’s Biobased Isobutanol Production Process. 2023. Available online: https://gevo.com/wp-content/uploads/2023/03/Gevo-WP_Isobutanol.5.26.23.pdf (accessed on 14 May 2024).
- Bertacchi, S.; Jayaprakash, P.; Morrissey, J.P.; Branduardi, P. Interdependence between lignocellulosic biomasses, enzymatic hydrolysis and yeast cell factories in biorefineries. Microb. Biotechnol. 2022, 15, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Bhandiwad, A.; Shaw, A.J.; Guss, A.; Guseva, A.; Bahl, H.; Lynd, L.R. Metabolic engineering of Thermoanaerobacterium saccharolyticum for n-butanol production. Metab. Eng. 2014, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Qu, C.; Feng, J.; Lan, Y.; Fu, H.; Wang, J. Metabolic engineering of Thermoanaerobacterium aotearoense strain SCUT27 for biofuels production from sucrose and molasses. Biotechnol. Biofuels 2023, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Conway, P.M.; Cervenka, N.D.; Cui, J.; Maloney, M.; Olson, D.G.; Lynd, L.R. Metabolic engineering of Clostridium thermocellum for n-butanol production from cellulose. Biotechnol. Biofuels 2019, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Chen, M.; Liu, L.; Rui, B.; Deng, Z.; Zhang, Z.; Shen, T. 13C metabolic flux analysis: Classification and characterization from the perspective of mathematical modeling and application in physiological research of neural cell. Front. Mol. Neurosci. 2022, 15, 883466. [Google Scholar] [CrossRef]
- Noda, S.; Mori, Y.; Oyama, S.; Kondo, A.; Araki, M.; Shirai, T. Reconstruction of metabolic pathway for isobutanol production in Escherichia coli. Microb. Cell Fact. 2019, 18, 124. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels. 2019, 12, 240. [Google Scholar] [CrossRef]

| Year | Airline * | Primary Feedstock | Mixture Ratio (%) | Reference |
|---|---|---|---|---|
| 2007 | Air New Zealand | Jatropha | 50 | [10] |
| 2008 | Virgin Atlantic | Coconut, babassu | 20 | [11] |
| 2009 | Continental Airlines | Algae, jatropha | 50 | [12] |
| Japan Airlines | Algae, jatropha, camelina | 50 | [13] | |
| KLM Royal Dutch Airlines | Camelina | 50 | [14] | |
| United Airlines | Algae | N/A | [15] | |
| 2010 | Aeromexico | N/A | N/A | [16] |
| LATAM Airlines Brasil | Jatropha | 50 | [17] | |
| 2011 | Air China | Jatropha | 50 | [18] |
| Air France | Waste cooking oil | N/A | [19] | |
| InterJet | Jatropha | 27 | [20] | |
| KLM Royal Dutch Airlines | Waste cooking oil | 20 | [21] | |
| 2012 | All Nippon Airways | Waste cooking oil | N/A | [22] |
| Etihad Airways | Waste cooking oil | N/A | [23] | |
| Nippon Cargo Airlines | Waste cooking oil | N/A | [24] | |
| United Airlines | Algae | 40 | [25] | |
| 2014 | Finnair | Waste cooking oil | N/A | [26] |
| Lufthansa Airlines | Farnesene sugar | 100 | [27] | |
| 2016 | Alaska Airlines | Forest residual | 20 | [28] |
| United Airlines | Algae | N/A | [29] | |
| 2017 | Singapore Airlines | Palm oil | N/A | [30] |
| 2018 | SpiceJet | Cooking oil, seeds of oil-bearing plants | N/A | [31] |
| 2020 | American Airlines | Waste nonedible oil, waste wood | N/A | [32] |
| Etihad Airways | Waste cooking oil | 50 | [23] | |
| Singapore Airlines | N/A | N/A | [30] | |
| 2021 | All Nippon Airways | Algae | N/A | [33] |
| Etihad Airways | Waste cooking oil | 38 | [23] | |
| Japan Airlines | Algae, discarded clothes, wood chips | N/A | [34] | |
| United Airlines | Algae | 100 | [35] | |
| 2022 | Fuji Dream Airline | Algae, waste cooking oil | N/A | [36] |
| Lufthansa | Waste cooking oil | N/A | [37] | |
| Malaysia Airlines | Animal fat waste | N/A | [38] | |
| Qantas | Agricultural waste, waste cooking oil, energy crops | N/A | [39] | |
| 2023 | AirAsia | Agricultural residual | N/A | [40] |
| Emirates | Plant sugar, waste fats | 100 | [41] | |
| Finnair | Agricultural waste, waste cooking oil | N/A | [42] | |
| Cathay Pacific Airlines | Animal fats, waste cooking oil | N/A | [43] | |
| Virgin Atlantic Airways | Plant sugar, waste fats | 100 | [44] | |
| Vistara | Jatropha | 17 | [45] |
| Producer Organism | Feedstock | Benefits | Limitations |
|---|---|---|---|
| First-generation | Edible biomass (starch, sugar, etc.) | Simple pretreatment process High production yield | Competition with food supply |
| Yeast | |||
| Second-generation | Inedible biomass (lignocellulose, food residual, etc.) | Abundant carbon resources No competition with the food supply | Complex pretreatment process Low production yield |
| Yeast or bacteria | |||
| Third-generation | Carbon dioxide | No competition with the food supply More than 10-fold the carbon dioxide fixation capacity of higher plants Simple production process | Challenges in mass-production technology Low production yield |
| Algae |
| Characteristic | Isobutanol | Ethanol | Gasoline |
|---|---|---|---|
| Research octane number | 106 | 110 | 88–98 |
| Motor octane number | 90 | 90 | 80–88 |
| Boiling point (°C) | 108 | 78 | 27–225 |
| Flash point (°C) | 28 | 13 | 7.6 |
| Autoignition temperature (°C) | 415 | 363 | −43 |
| Energy density (MJ/L) | 26.6 | 21.4 | 30–33 |
| Reid vapor pressure (kPa) | 3.3 | 16 | 54–103 |
| Lower heating value (MJ/kg) | 33.1 | 26.8 | 41–44 |
| Heat of evaporation (MJ/kg) | 0.69 | 0.92 | 0.36 |
| Lower flammability limit concentration (vol%) | 1.7 | 3.3 | 0.37–0.44 |
| Upper flammability limit concentration (vol%) | 11.8 | 19 | 1.4 |
| Strain | Feedstock | Concentration (g/L) | Productivity [(g/(L·h)] | Yield (%) | Reference |
|---|---|---|---|---|---|
| SHF | |||||
| E. aerogenes EHM02 | Sugarcane bagasse | 23.0 | 0.319 | 14 | [63] |
| Saccharomyces cerevisiae HRW253 | Switchgrass | 5.52 | 0.115 | 24 | [71] |
| E. coli mlcXT7-LAFC-AAKCD | Japanese cedar | 3.70 | 0.0386 | 14 | [64] |
| E. coli AL17 | Cellulose | 1.40 | 0.0300 | 36 | [72] |
| E. coli JK209 | Empty fruit bunch | 1.40 | 0.0167 | 26 | [65] |
| Pichia pastoris PPY0311 | Sugarcane trash | 0.0482 | 0.000669 | N.D. | [73] |
| SSF | |||||
| Corynebacterium crenatium MA11C | Duckweed | 5.61 | 0.0688 | 25 | [74] |
| E. coli mlcXT7-LAFC-AAKCD | Banana peel | 1.27 | 0.0148 | N.D. | [66] |
| S. cerevisiae YEZ546-2 | Cellulose | 0.364 | 0.00758 | N.D. | [75] |
| Process | Advantages | Disadvantages |
|---|---|---|
| SHF | Performance of enzymatic hydrolysis and fermentation under optimal conditions High production yield | Long production time based on a multistep production process |
| SSF | Short production time and low energy input based on a simple production process Easy to perform | Mismatch of optimal condition between enzymatic hydrolysis and fermentation Low production yield |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akita, H.; Matsushika, A. A Short Review of Second-Generation Isobutanol Production by SHF and SSF. Appl. Biosci. 2024, 3, 296-309. https://doi.org/10.3390/applbiosci3030020
Akita H, Matsushika A. A Short Review of Second-Generation Isobutanol Production by SHF and SSF. Applied Biosciences. 2024; 3(3):296-309. https://doi.org/10.3390/applbiosci3030020
Chicago/Turabian StyleAkita, Hironaga, and Akinori Matsushika. 2024. "A Short Review of Second-Generation Isobutanol Production by SHF and SSF" Applied Biosciences 3, no. 3: 296-309. https://doi.org/10.3390/applbiosci3030020
APA StyleAkita, H., & Matsushika, A. (2024). A Short Review of Second-Generation Isobutanol Production by SHF and SSF. Applied Biosciences, 3(3), 296-309. https://doi.org/10.3390/applbiosci3030020








