Response of Aeroponically Cultivated Baby-Leaf Lettuce (Lactuca sativa L.) Plants with Different Zinc, Copper, Iodine, and Selenium Concentrations
Abstract
:1. Introduction
2. Materials and Methods
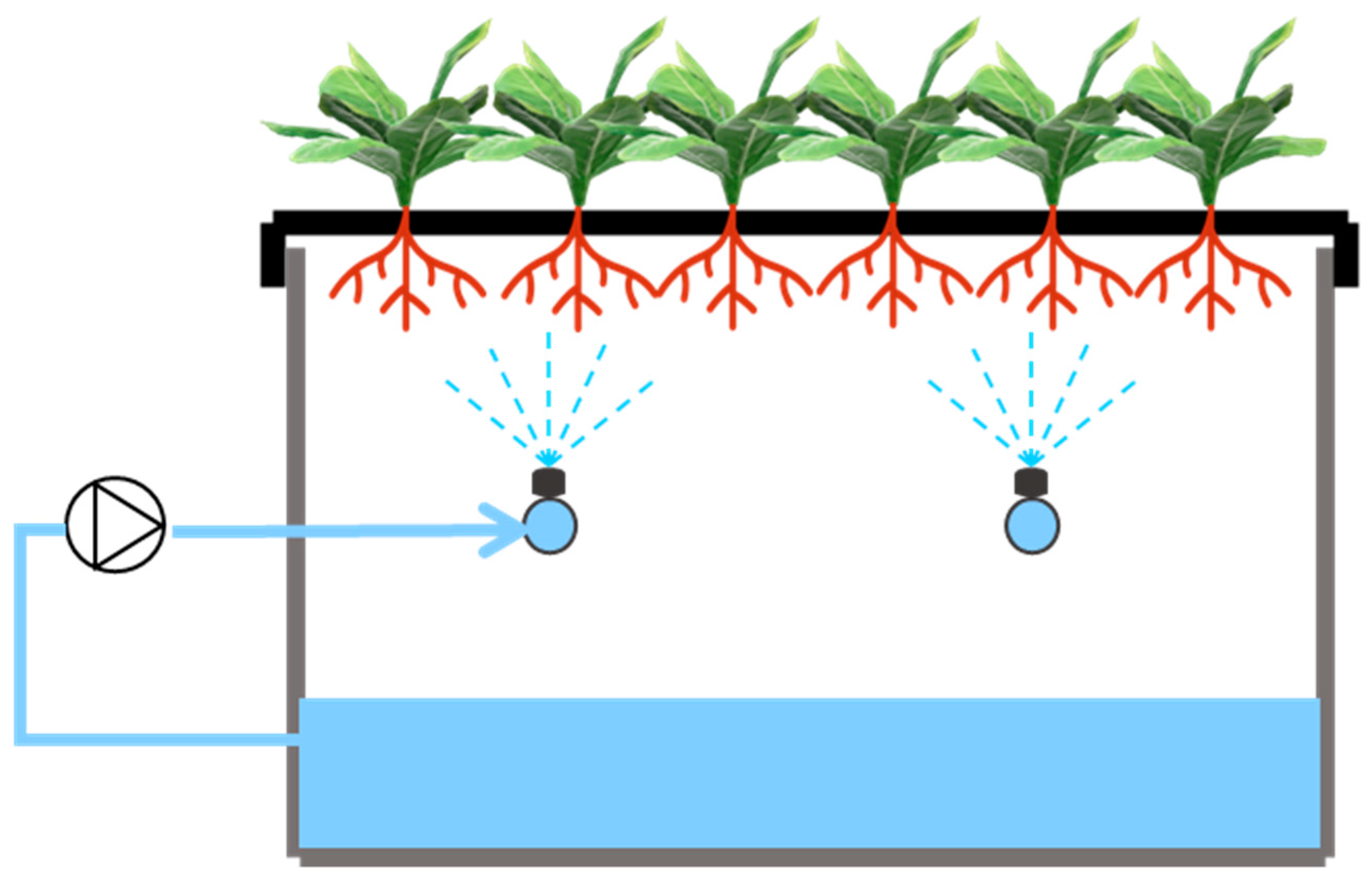
2.1. Experimental Site and Environmental Condition
2.2. Plant Material
2.3. Experimental Design and Treatments
2.4. Nutrient Solution Management
2.5. Plant Growth
2.6. Leaf Quality Attributes
2.7. Mineral Concentration
2.8. Contribution to Copper, Zinc, Selenium, or Iodine Dietary Intake and Maximum Daily Intake
2.9. Statistical Analysis
3. Results and Discussion
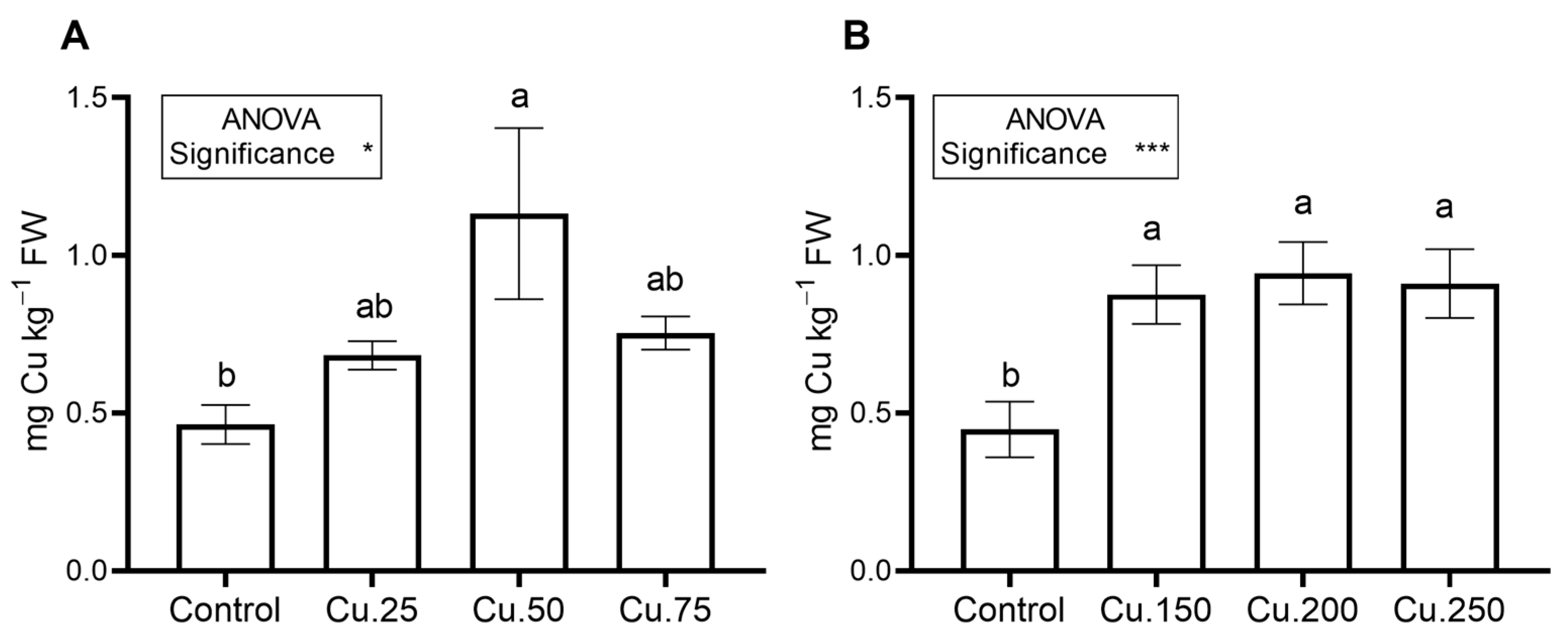
3.1. Biofortification with Copper (Experiments Cu_1 and Cu_2)
3.2. Biofortification with Zinc (Experiments Zn_1 and Zn_2)
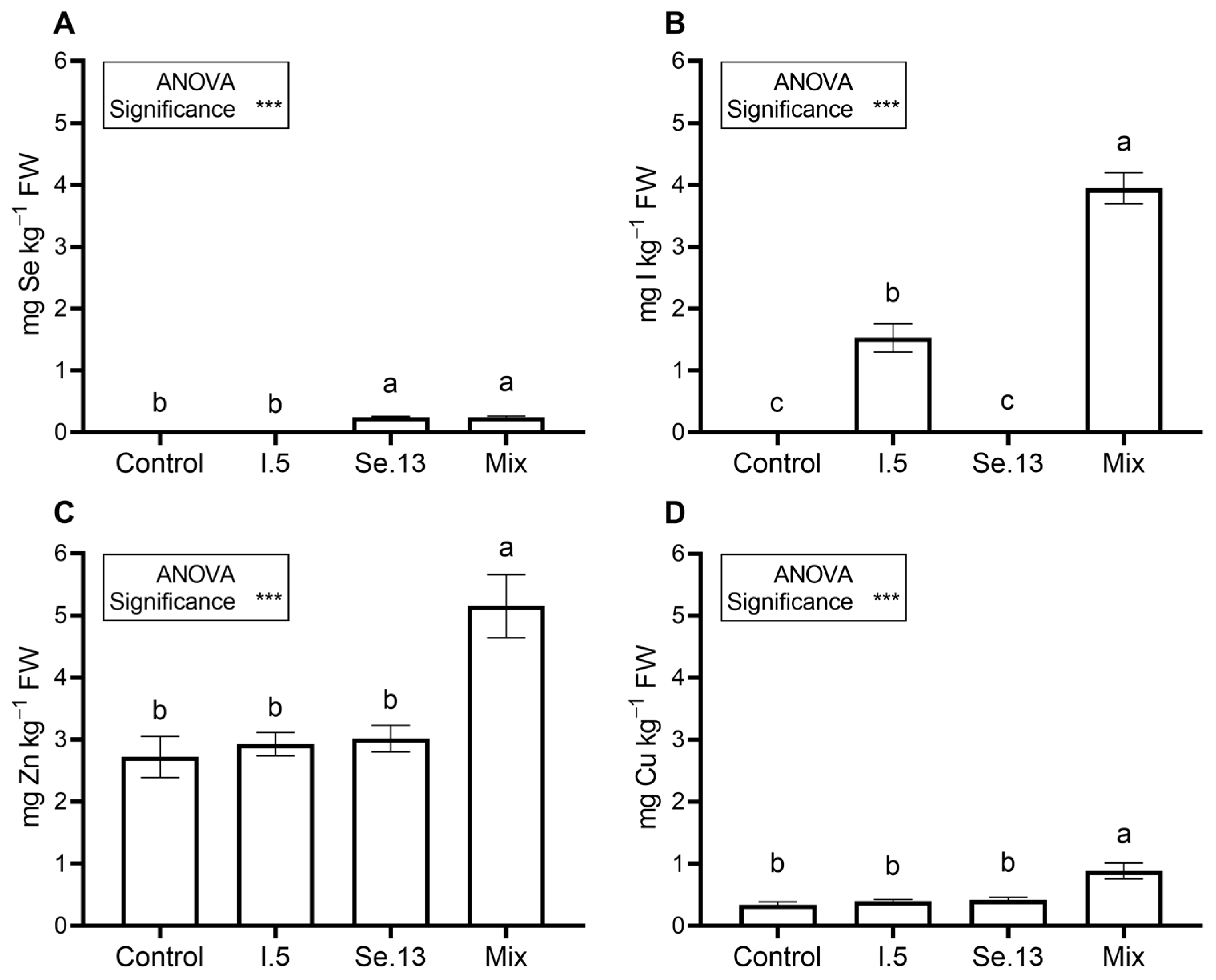
3.3. Biofortification with Se, I, or Simultaneously with Zn, Cu, Se, and I (Experiment Mix)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheoran, S.; Kumar, S.; Ramtekey, V.; Kar, P.; Meena, R.S.; Jangir, C.K. Current Status and Potential of Biofortification to Enhance Crop Nutritional Quality: An Overview. Sustainability 2022, 14, 3301. [Google Scholar] [CrossRef]
- Stein, A.J. Global Impacts of Human Mineral Malnutrition. Plant Soil 2010, 335, 133–154. [Google Scholar] [CrossRef]
- Hotz, C.; Brown, K.H. Assessment of the Risk of Zinc Deficiency in Populations and Options for Its Control. Food Nutr. Bull. 2004, 25, S94–S203. [Google Scholar]
- Gibson, R.S. Zinc Deficiency and Human Health: Etiology, Health Consequences, and Future Solutions. Plant Soil 2012, 361, 291–299. [Google Scholar] [CrossRef]
- Prohaska, J.R. Impact of Copper Deficiency in Humans. Ann. N. Y. Acad. Sci. 2014, 1314, 1–5. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Aburto, N.J.; Abudou, M.; Candeias, V.; Wu, T. Effect of Salt Iodization to Prevent Iodine Deficiency Disorders: A Systematic Review with Meta-Analyses; World Health Organization: Geneva, Switzerland, 2014; ISBN 9789241508285. [Google Scholar]
- Ríos, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and Induction of the Antioxidant Capacity in Lettuce Plants. Sci. Hortic. 2008, 116, 248–255. [Google Scholar] [CrossRef]
- Aziz, M.Z.; Yaseen, M.; Abbas, T.; Naveed, M.; Mustafa, A.; Hamid, Y.; Saeed, Q.; XU, M. gang Foliar Application of Micronutrients Enhances Crop Stand, Yield and the Biofortification Essential for Human Health of Different Wheat Cultivars. J. Integr. Agric. 2019, 18, 1369–1378. [Google Scholar] [CrossRef]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine Biofortification of Crops: Agronomic Biofortification, Metabolic Engineering and Iodine Bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Zhou, B.; Cao, H.; Wu, Q.; Mao, K.; Yang, X.; Su, J.; Zhang, H. Agronomic and Genetic Strategies to Enhance Selenium Accumulation in Crops and Their Influence on Quality. Foods 2023, 12, 4442. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.; Twyman, R.M.; Zhu, C.; Farré, G.; Serrano, J.C.E.; Portero-Otin, M.; Muñoz, P.; Sandmann, G.; Capell, T.; Christou, P. Biofortification of Crops with Nutrients: Factors Affecting Utilization and Storage. Curr. Opin. Biotechnol. 2016, 44, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.; Kumar, V.; Rizwanuddin, S.; Mishra, S.; Kumar, V.; Saris, P.E.J.; Khanduri, N.; Kumar, A.; Pandey, P.; Gupta, A.K.; et al. Biofortification as a Solution for Addressing Nutrient Deficiencies and Malnutrition. Heliyon 2024, 10, e30595. [Google Scholar] [CrossRef] [PubMed]
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific Opinion on Dietary Reference Values for Copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E. Selenium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press Taylor & Francis Group: Abingdon, UK, 2007; pp. 515–549. [Google Scholar]
- Voogt, W.; Holwerda, H.T.; Khodabaks, R. Biofortification of Lettuce (Lactuca sativa L.) with Iodine: The Effect of Iodine Form and Concentration in the Nutrient Solution on Growth, Development and Iodine Uptake of Lettuce Grown in Water Culture. J. Sci. Food Agric. 2010, 90, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Photorespiration Process and Nitrogen Metabolism in Lettuce Plants (Lactuca sativa L.): Induced Changes in Response to Iodine Biofortification. J. Plant Growth Regul. 2010, 29, 477–486. [Google Scholar] [CrossRef]
- Puccinelli, M.; Landi, M.; Maggini, R.; Pardossi, A.; Incrocci, L. Iodine Biofortification of Sweet Basil and Lettuce Grown in Two Hydroponic Systems. Sci. Hortic. 2021, 276, 109783. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Incrocci, L.; Rosellini, I.; Pezzarossa, B. Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems. Horticulturae 2021, 7, 590. [Google Scholar] [CrossRef]
- Bian, Z.-H.; Lei, B.; Cheng, R.-F.; Wang, Y.; Li, T.; Yang, Q.-C. Selenium Distribution and Nitrate Metabolism in Hydroponic Lettuce (Lactuca sativa L.): Effects of Selenium Forms and Light Spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar] [CrossRef]
- Kowalska, I.; Smoleń, S.; Czernicka, M.; Halka, M.; Kęska, K.; Pitala, J. Effect of Selenium Form and Salicylic Acid on the Accumulation of Selenium Speciation Forms in Hydroponically Grown Lettuce. Agriculture 2020, 10, 584. [Google Scholar] [CrossRef]
- Puccinelli, M.; Rosellini, I.; Malorgio, F.; Pardossi, A.; Pezzarossa, B. Hydroponic Production of Selenium-Enriched Baby Leaves of Swiss Chard (Beta vulgaris Var. cicla) and Its Wild Ancestor Sea Beet (Beta vulgaris Ssp. maritima). Horticulturae 2023, 9, 909. [Google Scholar]
- Barrameda-Medina, Y.; Lentini, M.; Esposito, S.; Ruiz, J.M.; Blasco, B. Zn-Biofortification Enhanced Nitrogen Metabolism and Photorespiration Process in Green Leafy Vegetable Lactuca sativa L. J. Sci. Food Agric. 2017, 97, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Barrameda-Medina, Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D.A.; Ruiz, J.M. Zinc Biofortification Improves Phytochemicals and Amino-Acidic Profile in Brassica Oleracea Cv. Bronco. Plant Sci. 2017, 258, 45–51. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Pongrac, P.; Sneddon, C.C.; Thompson, J.A.; Wright, G. Limits to the Biofortification of Leafy Brassicas with Zinc. Agriculture 2018, 8, 32. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Kyriacou, M.; Soteriou, G.A.; Graziani, G.; De Pascale, S.; Rouphael, Y. Zinc Biofortification of Hydroponically Grown Basil: Stress Physiological Responses and Impact on Antioxidant Secondary Metabolites of Genotypic Variants. Front. Plant Sci. 2022, 13, 1049004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Xu, Y.; Yin, Y.; Guo, H.; Du, W. Divergence in Response of Lettuce (Var. Ramosa Hort.) to Copper Oxide Nanoparticles/Microparticles as Potential Agricultural Fertilizer. Environ. Pollut. Bioavailab. 2019, 31, 80–84. [Google Scholar] [CrossRef]
- Fortis-Hernández, M.; Ortiz-Lopez, J.; Preciado-Rangel, P.; Trejo-Valencia, R.; Lagunes-Fortiz, E.; Andrade-Sifuentes, A.; Rueda-Puente, E.O. Biofortification with Copper Nanoparticles (Nps Cu) and Its Effect on the Physical and Nutraceutical Quality of Hydroponic Melon Fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12568. [Google Scholar] [CrossRef]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. A Decade of Improving Nutritional Quality of Horticultural Crops Agronomically (2012−2022): A Systematic Literature Review. Sci. Total Environ. 2024, 911, 168665. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Huang, Y.; Hu, Y.; Liu, Y.; Christie, P. Interactions between Selenium and Iodine Uptake by Spinach (Spinacia oleracea L.) in Solution Culture. Plant Soil 2004, 261, 99–105. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of Biofortification with Iodine and Selenium of Lettuce Cultivated in the NFT Hydroponic System. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; et al. Iodine and Selenium Biofortification of Lettuce (Lactuca sativa L.) by Soil Fertilization with Various Compounds of These Elements. Acta Sci. Pol. Hortorum Cultus 2016, 15, 69–91. [Google Scholar]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Halka, M.; Sady, W. Biofortification of Six Varieties of Lettuce (Lactuca sativa L.) with Iodine and Selenium in Combination with the Application of Salicylic Acid. Front. Plant Sci. 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz-Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous Biofortification of Wheat with Zinc, Iodine, Selenium, and Iron through Foliar Treatment of a Micronutrient Cocktail in Six Countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef] [PubMed]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern Plant Cultivation Technologies in Agriculture under Controlled Environment: A Review on Aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Qureshi, W.A.; Sheikh, S.A.; Chen, J.; Chandio, F.A.; Lakhiar, I.A.; Solangi, K.A. Effects of Droplet Size and Spray Interval on Root-to-Shoot Ratio, Photosynthesis Efficiency, and Nutritional Quality of Aeroponically Grown Butter Head Lettuce. Int. J. Agric. Biol. Eng. 2022, 15, 79–88. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving Vegetable Quality in Controlled Environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.-S. Minimally Processed Ready-to-Eat Baby-Leaf Vegetables: Production, Processing, Storage, Microbial Safety, and Nutritional Potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Zhu, X.; Marcelis, L. Vertical Farming for Crop Production. Mod. Agric. 2023, 1, 13–15. [Google Scholar] [CrossRef]
- Puccinelli, M.; Incrocci, L.; Malorgio, F.; Pardossi, A.; Vernieri, P. Plant Factory with Artificial Light: Pros and Cons. Agrochimica 2023, 67, 65–74. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV—VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, E.L. Phosphorus. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil. Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Baker, W.H.; Thompson, T.L. Determination of Total Nitrogen Plant Samples by Kjeldahl. In Plant Analysis Reference Procedures for the Southern Region of the United States; Southern Cooperative Series Bulletin; The University of Georgia Crop & Soil Science Dept: Athens, Greece, 1992; Volume 368, ISBN 1-58161-368-7. [Google Scholar]
- UNI EN 13657; Characterization of Waste—Digestion for Subsequent Determination of Aqua Regia Soluble Portion of Elements. Ente Nazionale Italiano di Unificazione (UNI): Rome, Italy, 2004.
- UNI EN ISO 17294-2; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. Ente Nazionale Italiano di Unificazione (UNI): Rome, Italy, 2016.
- Fortis-Hernández, M.; González-Rodríguez, T.; Espinosa-Palomeque, B.; Preciado-Rangel, P.; Gallegos-Robles, M.A.; Rueda-Puente, E.O. Foliar Biofortification with Copper Nanoparticles and Its Effect on Phytochemical Quality and Enzymatic Activity in Lettuce. Hortic. Bras. 2024, 42, e2617. [Google Scholar] [CrossRef]
- Wairich, A.; De Conti, L.; Lamb, T.I.; Keil, R.; Neves, L.O.; Brunetto, G.; Sperotto, R.A.; Ricachenevsky, F.K. Throwing Copper Around: How Plants Control Uptake, Distribution, and Accumulation of Copper. Agronomy 2022, 12, 994. [Google Scholar] [CrossRef]
- Obrador, A.; Gonzalez, D.; Alvarez, J.M. Effect of Inorganic and Organic Copper Fertilizers on Copper Nutrition in Spinacia oleracea and on Labile Copper in Soil. J. Agric. Food Chem. 2013, 61, 4692–4701. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- European Parliament and Council of the European Union Commission Regulation (EU) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs. Off. J. Eur. Union 2011, 320, 15–17.
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The Effect of Excess Copper on Growth and Physiology of Important Food Crops: A Review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- de Lima, B.M.; Noboa, C.S.; de Lima, F.M.; da Costa Mello, S.; Purquerio, L.F.V.; Sala, F.C. Agronomic Biofortification with Zinc in Hydroponically Cultivated Lettuce. Aust. J. Crop Sci. 2023, 17, 198–205. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc Absorption in Plants: Uptake, Transport, Translocation and Accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc Toxicity in Plants: A Review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Rangel, P.; Campos-Ortiz, A.; Chávez, E.S.; Reyes-Gonzalez, A.; Ruiz-Espinoza, F.; Ojeda-Barrios, D.; Hernandez-Montiel, L. Zinc Biofortification Improves Yield, Nutraceutical Quality and Antioxidant Capacity in Lettuce. Trop. Subtrop. Agroecosyst. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume 22, ISBN 0123456789. [Google Scholar]
- Hernández-Castro, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Rodríguez-Mendoza, M.N.; Sánchez-García, P.; Robledo-Paz, A. Bioaccumulation of Iron, Selenium, Nitrate, and Proteins in Chard Shoots. J. Soil Sci. Plant Nutr. 2015, 15, 694–710. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and Partitioning of Selenium in Basil (Ocimum basilicum L.) Plants Grown in Hydroponics. Sci. Hortic. 2017, 225, 271–276. [Google Scholar] [CrossRef]
- Francini, A.; Quattrini, E.; Giuffrida, F.; Ferrante, A. Biofortification of Baby Leafy Vegetables Using Nutrient Solution Containing Selenium. J. Sci. Food Agric. 2023, 103, 5472–5480. [Google Scholar] [CrossRef]
- Mezeyova, I.; Hegedusova, A.; Andrejiová, A.; Mezeyová, I.; Hegedűsová, A.; Hegedűs, O.; Golian, M. Phytomass and Content of Essential Oils in Ocimum basilicum after Foliar Treatment with Selenium. J. Int. Sci. Publ. 2016, 4, 19–27. [Google Scholar]
- Smoleń, S.; Baranski, R.; Ledwożyw-Smoleń, I.; Skoczylas, Ł.; Sady, W. Combined Biofortification of Carrot with Iodine and Selenium. Food Chem. 2019, 300, 125202. [Google Scholar] [CrossRef]
- Weng, H.X.; Yan, A.L.; Hong, C.L.; Xie, L.L.; Qin, Y.C.; Cheng, C.Q. Uptake of Different Species of Iodine by Water Spinach and Its Effect to Growth. Biol. Trace Elem. Res. 2008, 124, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P.; Pardossi, A. Iodine Accumulation and Tolerance in Sweet Basil (Ocimum basilicum L.) with Green or Purple Leaves Grown in Floating System Technique. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Iodine Application Affects Nitrogen-Use Efficiency of Lettuce Plants (Lactuca sativa L.). Acta Agric. Scand. B Soil Plant Sci. 2011, 61, 378–383. [Google Scholar] [CrossRef]
- Smoleń, S.; Wierzbińska, J.; Sady, W.; Kołton, A.; Wiszniewska, A.; Liszka-Skoczylas, M. Iodine Biofortification with Additional Application of Salicylic Acid Affects Yield and Selected Parameters of Chemical Composition of Tomato Fruits (Solanum lycopersicum L.). Sci. Hortic. 2015, 188, 89–96. [Google Scholar] [CrossRef]
- Puccinelli, M.; Rosellini, I.; Malorgio, F.; Pardossi, A.; Pezzarossa, B. Iodine Biofortification of Swiss Chard (Beta vulgaris Ssp. vulgaris Var. cicla) and Its Wild Ancestor Sea Beet (Beta vulgaris Ssp. maritima) Grown Hydroponically as Baby Leaves: Effects on Leaf Production and Quality. J. Sci. Food Agric. 2023, 103, 7888–7895. [Google Scholar] [CrossRef]
- Sahin, O. Combined Biofortification of Soilless Grown Lettuce with Iodine, Selenium and Zinc and Its Effect on Essential and Non-Essential Elemental Composition. J. Plant Nutr. 2021, 44, 673–678. [Google Scholar] [CrossRef]
- European Food Safety Authority. Overview on Tolerable Upper Intake Levels. EFSA J. 2018, 3, 4. [Google Scholar]

| Experiment | Treatment | Concentration (µM) | Concentration (mg L−1) | |
|---|---|---|---|---|
| Cu_1 | Control | Cu | 3 | 0.19 |
| Cu.25 | 25 | 1.59 | ||
| Cu.50 | 50 | 3.18 | ||
| Cu.75 | 75 | 4.76 | ||
| Cu_2 | Control | Cu | 3 | 0.19 |
| Cu.150 | 150 | 9.53 | ||
| Cu.200 | 200 | 12.70 | ||
| Cu.250 | 250 | 15.88 | ||
| Zn_1 | Control | Zn | 10 | 0.65 |
| Zn.50 | 50 | 3.27 | ||
| Zn.100 | 100 | 6.54 | ||
| Zn.150 | 150 | 9.51 | ||
| Zn_2 | Control | Zn | 10 | 0.65 |
| Zn.250 | 250 | 16.35 | ||
| Zn.350 | 350 | 22.89 | ||
| Zn.450 | 450 | 29.43 | ||
| Mix | Control | Cu | 3 | 0.19 |
| Zn | 10 | 0.65 | ||
| Se | 0 | 0 | ||
| I | 0 | 0 | ||
| Se.13 | Se | 13 | 1.03 | |
| I.5 | I | 5 | 0.63 | |
| Mix | Cu | 150 | 9.53 | |
| Zn | 250 | 16.35 | ||
| Se | 13 | 1.03 | ||
| I | 5 | 0.63 |
| Experiment | Treatment | Concentration (µM) | % AI or % PRI per 100 g FW | g FW 100% AI or PRI | |
|---|---|---|---|---|---|
| Cu_1 | Control | Cu | 3 | 3.20 | 3154.6 |
| Cu.25 | 25 | 4.70 | 2131.2 | ||
| Cu.50 | 50 | 7.81 | 1319.0 | ||
| Cu.75 | 75 | 5.20 | 1929.3 | ||
| Cu_2 | Control | Cu | 3 | 3.09 | 3333.4 |
| Cu.150 | 150 | 6.04 | 1672.0 | ||
| Cu.200 | 200 | 6.49 | 1553.6 | ||
| Cu.250 | 250 | 6.25 | 1616.6 | ||
| Zn_1 | Control | Zn | 10 | 2.73 | 3677.0 |
| Zn.50 | 50 | 3.65 | 2750.4 | ||
| Zn.100 | 100 | 4.74 | 2109.6 | ||
| Zn.150 | 150 | 4.71 | 2133.1 | ||
| Zn_2 | Control | Zn | 10 | 3.18 | 3167.6 |
| Zn.250 | 250 | 5.55 | 1805.1 | ||
| Zn.350 | 350 | 4.62 | 2172.3 | ||
| Zn.450 | 450 | 5.42 | 1863.3 | ||
| Mix | Control | Cu | 0 | 2.33 | 4368.5 |
| Zn | 0 | 2.37 | 4274.6 | ||
| Se | 0 | 0 | - | ||
| I | 0 | 0.83 | 15,655.1 | ||
| Se.13 | Se | 13 | 35.79 | 279.9 | |
| I.5 | I | 5 | 101.89 | 99.8 | |
| Mix | Cu | 150 | 6.12 | 1656.8 | |
| Zn | 250 | 4.48 | 2249.2 | ||
| Se | 13 | 35.32 | 284.8 | ||
| I | 5 | 262.9 | 38.2 |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| u.m. | Control | Zn.50 | Zn.100 | Zn.150 | ANOVA | |
| Fresh weight | g m−2 | 2062.7 ± 200.0 | 2060.1 ± 112.5 | 1982.1 ± 262.0 | 1915.1 ± 182.9 | ns |
| Dry weight | g m−2 | 82.8 ± 8.0 | 80.6 ± 2.9 | 75.5 ± 6.7 | 71.2 ± 1.5 | ns |
| Dry matter content | % FW | 4.01 ± 0.00 | 3.93 ± 0.36 | 3.83 ± 0.17 | 3.76 ± 1.44 | ns |
| N-tot | g kg−1 FW | 2.80 ± 0.22 | 3.01 ± 0.37 | 2.78 ± 0.13 | 2.80 ± 0.14 | ns |
| K | g kg−1 FW | 3.83 ± 0.40 | 3.64 ± 0.40 | 3.88 ± 0.13 | 4.05 ± 0.14 | ns |
| P | g kg−1 FW | 0.215 ± 0.038 | 0.402 ± 0.013 | 0.339 ± 0.057 | 0.364 ± 0.102 | ns |
| Ca | g kg−1 FW | 0.894 ± 0.142 | 0.861 ± 0.110 | 0.881 ± 0.100 | 0.909 ± 0.120 | ns |
| Na | g kg−1 FW | 0.025 ± 0.002 b | 0.079 ± 0.012 a | 0.061 ± 0.006 ab | 0.096 ± 0.009 a | * |
| Mg | g kg−1 FW | 0.207 ± 0.008 | 0.168 ± 0.003 | 0.217 ± 0.010 | 0.216 ± 0.015 | ns |
| Mn | mg kg−1 FW | 6.63 ± 0.01 | 5.19 ± 0.12 | 6.70 ± 0.11 | 6.23 ± 0.93 | ns |
| Fe | mg kg−1 FW | 14.55 ± 0.23 | 13.22 ± 4.27 | 16.63 ± 0.85 | 13.79 ± 6.35 | ns |
| Cu | mg kg−1 FW | 0.637 ± 0.012 a | 0.540 ± 0.030 b | 0.570 ± 0.002 b | 0.541 ± 0.014 b | ns |
| Chlorophylls | mg g−1 FW | 0.871 ± 0.140 | 1.041 ± 0.078 | 1.051 ± 0.068 | 1.081 ± 0.006 | ns |
| Carotenoids | mg g−1 FW | 0.145 ± 0.009 | 0.179 ± 0.013 | 0.166 ± 0.001 | 0.183 ± 0.004 | ns |
| Flavonoids | mg g−1 FW | 0.563 ± 0.107 | 0.772 ± 0.151 | 0.723 ± 0.094 | 0.991 ± 0.079 | ns |
| Phenols | mg g−1 FW | 1.50 ± 0.15 | 1.89 ± 0.29 | 1.40 ± 0.04 | 1.87 ± 0.07 | ns |
| Antioxidant capacity | mmol Fe (II) kg−1 FW | 7.68 ± 1.40 | 8.78 ± 1.54 | 7.83 ± 0.09 | 9.93 ± 0.08 | ns |
| NO3 | mg kg−1 PF | 2599.9 ± 263.8 | 2902.9 ± 695.7 | 2539.7 ± 164.8 | 2744.6 ± 672.5 | ns |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| u.m. | Control | Zn.250 | Zn.350 | Zn.450 | ANOVA | |
| Fresh weight | g m−2 | 1892.3 ± 71.8 | 1637.1 ± 98.1 | 1726.5 ± 224.5 | 1762.5 ± 129.6 | ns |
| Dry weight | g m−2 | 86.3 ± 3.5 | 74.6 ± 7.8 | 68.5 ± 6.4 | 78.3 ± 6.5 | ns |
| Dry matter content | % FW | 4.59 ± 0.28 | 4.53 ± 0.27 | 4.02 ± 0.16 | 4.33 ± 0.19 | ns |
| N-tot | g kg−1 FW | 3.13 ± 0.14 | 3.05 ± 0.12 | 2.64 ± 0.10 | 2.99 ± 0.14 | ns |
| K | g kg−1 FW | 3.66 ± 0.27 | 3.16 ± 0.31 | 2.74 ± 0.07 | 3.36 ± 0.46 | ns |
| P | g kg−1 FW | 0.617 ± 0.080 | 0.544 ± 0.052 | 0.435 ± 0.022 | 0.453 ± 0.018 | ns |
| Ca | g kg−1 FW | 0.594 ± 0.027 a | 0.377 ± 0.019 b | 0.473 ± 0.019 b | 0.440 ± 0.037 b | *** |
| Na | g kg−1 FW | 0.059 ± 0.003 | 0.056 ± 0.003 | 0.061 ± 0.002 | 0.070 ± 0.002 | ns |
| Mg | g kg−1 FW | 0.184 ± 0.01 a | 0.146 ± 0.01 b | 0.152 ± 0.00 b | 0.156 ± 0.01 ab | * |
| Mn | mg kg−1 FW | 7.80 ± 0.70 | 7.95 ± 0.35 | 6.86 ± 0.19 | 7.44 ± 0.65 | ns |
| Fe | mg kg−1 FW | 14.38 ± 2.03 | 14.70 ± 2.11 | 12.65 ± 1.02 | 13.66 ± 2.14 | ns |
| Cu | mg kg−1 FW | 0.573 ± 0.031 | 0.598 ± 0.042 | 0.573 ± 0.023 | 0.634 ± 0.014 | ns |
| Chlorophylls | mg g−1 FW | 1.09 ± 0.095 | 1.25 ± 0.030 | 1.29 ± 0.054 | 1.22 ± 0.071 | ns |
| Carotenoids | mg g−1 FW | 0.138 ± 0.015 | 0.159 ± 0.011 | 0.151 ± 0.014 | 0.122 ± 0.018 | ns |
| Flavonoids | mg g−1 FW | 0.585 ± 0.034 | 0.809 ± 0.045 | 0.776 ± 0.144 | 0.695 ± 0.117 | ns |
| Phenols | mg g−1 FW | 1.47 ± 0.12 | 1.67 ± 0.09 | 1.68 ± 0.14 | 1.55 ± 0.22 | ns |
| Antioxidant capacity | mmol Fe (II) kg−1 FW | 6.41 ± 0.84 | 8.13 ± 0.68 | 7.05 ± 0.74 | 7.95 ± 0.93 | ns |
| NO3 | mg kg−1 PF | 2420.6 ± 68.9 | 2269.3 ± 254.0 | 2189.8 ± 321.1 | 2259.6 ± 250.1 | ns |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| u.m. | Control | I.5 | Se.13 | Mix | ANOVA | |
| Fresh weight | g m−2 | 2064.5 ± 185.3 | 2201.2 ± 186.0 | 2198.9 ± 95.8 | 2404.2 ± 267.1 | ns |
| Dry weight | g m−2 | 73.34 ± 9.30 | 81.49 ± 8.53 | 83.39 ± 5.13 | 82.05 ± 7.74 | ns |
| Dry matter content | % FW | 3.52 ± 0.16 | 3.69 ± 0.15 | 3.78 ± 0.08 | 3.43 ± 0.09 | ns |
| N-tot | g kg−1 FW | 2.39 ± 0.06 | 2.49 ± 0.08 | 2.61 ± 0.04 | 2.36 ± 0.04 | ns |
| K | g kg−1 FW | 4.18 ± 0.40 | 3.93 ± 0.38 | 3.85 ± 0.47 | 4.11 ± 0.34 | ns |
| P | g kg−1 FW | 0.313 ± 0.008 ab | 0.332 ± 0.013 ab | 0.359 ± 0.008 a | 0.306 ± 0.014 b | * |
| Ca | g kg−1 FW | 1.181 ± 0.051 ab | 1.014 ± 0.116 ab | 1.299 ± 0.073 a | 0.891 ± 0.022 b | * |
| Na | g kg−1 FW | 0.062 ± 0.002 b | 0.080 ± 0.007 b | 0.063 ± 0.003 b | 0.102 ± 0.004 a | *** |
| Mg | g kg−1 FW | 0.152 ± 0.006 ab | 0.167 ± 0.006 ab | 0.174 ± 0.004 a | 0.148 ± 0.005 b | * |
| Mn | mg kg−1 FW | 3.33 ± 0.07 | 3.45 ± 0.21 | 3.87 ± 0.27 | 3.40 ± 0.17 | ns |
| Fe | mg kg−1 FW | 6.20 ± 0.79 ab | 8.11 ± 0.85 a | 6.21 ± 0.53 ab | 4.65 ± 0.40 b | * |
| Chlorophylls | mg g−1 FW | 1.28 ± 0.065 | 1.23 ± 0.068 | 1.244 ± 0.021 | 1.36 ± 0.053 | ns |
| Carotenoids | mg g−1 FW | 0.117 ± 0.008 | 0.120 ± 0.003 | 0.129 ± 0.005 | 0.118 ± 0.003 | ns |
| Flavonoids | mg g−1 FW | 0.67 ± 0.05 | 0.44 ± 0.09 | 0.51 ± 0.07 | 0.57 ± 0.07 | ns |
| Phenols | mg g−1 FW | 1.40 ± 0.03 | 1.25 ± 0.08 | 1.29 ± 0.10 | 1.26 ± 0.08 | ns |
| Antioxidant capacity | mmol Fe (II) kg−1 FW | 9.08 ± 0.17 | 8.00 ± 0.73 | 8.48 ± 0.74 | 8.33 ± 0.60 | ns |
| NO3 | mg kg−1 PF | 1922.6 ± 58.7 | 2128.1 ± 225.3 | 2152.3 ± 109.9 | 1896.5 ± 69.4 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccinelli, M.; De Padova, A.; Vernieri, P.; Carmassi, G.; Incrocci, L. Response of Aeroponically Cultivated Baby-Leaf Lettuce (Lactuca sativa L.) Plants with Different Zinc, Copper, Iodine, and Selenium Concentrations. Horticulturae 2024, 10, 726. https://doi.org/10.3390/horticulturae10070726
Puccinelli M, De Padova A, Vernieri P, Carmassi G, Incrocci L. Response of Aeroponically Cultivated Baby-Leaf Lettuce (Lactuca sativa L.) Plants with Different Zinc, Copper, Iodine, and Selenium Concentrations. Horticulturae. 2024; 10(7):726. https://doi.org/10.3390/horticulturae10070726
Chicago/Turabian StylePuccinelli, Martina, Andrea De Padova, Paolo Vernieri, Giulia Carmassi, and Luca Incrocci. 2024. "Response of Aeroponically Cultivated Baby-Leaf Lettuce (Lactuca sativa L.) Plants with Different Zinc, Copper, Iodine, and Selenium Concentrations" Horticulturae 10, no. 7: 726. https://doi.org/10.3390/horticulturae10070726






