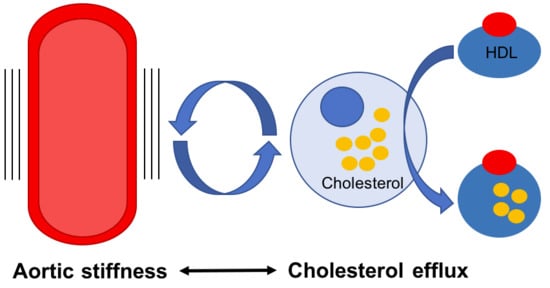
Cholesterol Efflux: Does It Contribute to Aortic Stiffening?
Abstract
:1. Introduction
2. Potential Mechanisms of Aortic Stiffness and Cholesterol Efflux
3. The Role of ABCA1 in Pulse Wave Velocity
4. The Role of ABCA1 in Influencing Cellular Phenotypes
5. ABCA1 as an Anti-Inflammatory Receptor
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Park, S.; Lakatta, E.G. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med. J. 2012, 53, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Mecham, R.P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef] [PubMed]
- Tuna, B.G.; Bakker, E.N.; VanBavel, E. Smooth muscle biomechanics and plasticity: Relevance for vascular calibre and remodelling. Basic Clin. Pharmacol. Toxicol. 2012, 110, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Saphirstein, R.J.; Morgan, K.G. The contribution of vascular smooth muscle to aortic stiffness across length scales. Microcirculation 2014, 21, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Nilsson, P.M.; Blacher, J.; Mimran, A. Pulse pressure, arterial stiffness, and end-organ damage. Curr. Hypertens. Rep. 2012, 14, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Alivon, M.; Beaussier, H.; Boutouyrie, P. Aortic stiffness as a tissue biomarker for predicting future cardiovascular events in asymptomatic hypertensive subjects. Ann. Med. 2012, 44, S93–S97. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.; Rabkin, S.W. The relationship between arterial stiffness and heart failure with preserved ejection fraction: A systemic meta-analysis. Heart Fail. Rev. 2015, 20, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.M.; Chu, C.M.; Chang, S.T.; Cheng, H.W.; Yang, T.Y.; Wan, P.C.; Pan, K.L.; Lin, Y.S.; Hsu, J.T. Quantification of aortic stiffness to predict the degree of left ventricular diastolic function. Am. J. Med. Sci. 2010, 340, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Quinaglia, T.; Bensalah, M.Z.; Bollache, E.; Kachenoura, N.; Soulat, G.; Boutouyrie, P.; Laurent, S.; Mousseaux, E. Differential impact of local and regional aortic stiffness on left ventricular remodeling: A cardiovascular magnetic resonance study. J. Hypertens. 2018, 36, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.X.; Wu, D.F.; Aung, L.H.; Yan, T.T.; Cao, X.L.; Long, X.J.; Miao, L.; Liu, W.Y.; Zhang, L.; Li, M. Several lipid-related gene polymorphisms interact with overweight/obesity to modulate blood pressure levels. Int. J. Mol. Sci. 2012, 13, 12062–12081. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.; Frisdal, E.; Bittar, R.; Le Goff, W.; Bruckert, E.; Lesnik, P.; Guerin, M.; Giral, P. Association of Cholesterol Efflux Capacity With Clinical Features of Metabolic Syndrome: Relevance to Atherosclerosis. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Lucero, D.; Sviridov, D.; Freeman, L.; Lopez, G.I.; Fassio, E.; Remaley, A.T.; Schreier, L. Increased cholesterol efflux capacity in metabolic syndrome: Relation with qualitative alterations in HDL and LCAT. Atherosclerosis 2015, 242, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Ding, D.; Li, X.; Yang, Y.; Li, Q.; Zheng, Y.; Wang, D.; Ling, W. Cholesterol efflux capacity is an independent predictor of all-cause and cardiovascular mortality in patients with coronary artery disease: A prospective cohort study. Atherosclerosis 2016, 249, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Prosser, H.C.; Ng, M.K.; Bursill, C.A. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr. Opin. Lipidol. 2012, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Dubland, J.A.; Francis, G.A. So Much Cholesterol: The unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation. Curr. Opin. Lipidol. 2016, 27, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Ye, P.; Cao, R.; Zhang, Y.; Qi, Y.; Zhao, D. High-density lipoprotein 3 cholesterol is a predictive factor for arterial stiffness: A community-based 4.8-year prospective study. Lipids Health Dis. 2018, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ahn, C.W.; Kang, S.; Ha, J.Y.; Baek, H.; Park, J.S.; Kim, K.R. Association between Apolipoprotein B/Apolipoprotein A-1 and arterial stiffness in metabolic syndrome. Clin. Chim. Acta 2014, 437, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Byfield, F.J.; Tikku, S.; Rothblat, G.H.; Gooch, K.J.; Levitan, I. OxLDL increases endothelial stiffness, force generation, and network formation. J. Lipid Res. 2006, 47, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Shentu, T.P.; Titushkin, I.; Singh, D.K.; Gooch, K.J.; Subbaiah, P.V.; Cho, M.; Levitan, I. oxLDL-induced decrease in lipid order of membrane domains is inversely correlated with endothelial stiffness and network formation. Am. J. Physiol. Cell Physiol. 2010, 299, C218–C229. [Google Scholar] [CrossRef] [PubMed]
- Cuchel, M.; Rader, D.J. Macrophage reverse cholesterol transport: Key to the regression of atherosclerosis? Circulation 2006, 113, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J. Vascular disease: Cholesterol-efflux capacity might be the key to the protective effects of HDL. Nat. Rev. Cardiol. 2011, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Favari, E.; Chroni, A.; Tietge, U.J.; Zanotti, I.; Escola-Gil, J.C.; Bernini, F. Cholesterol efflux and reverse cholesterol transport. Handb. Exp. Pharmacol. 2015, 224, 181–206. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, P.M.; Heinecke, J.W. Cholesterol efflux capacity, macrophage reverse cholesterol transport and cardioprotective HDL. Curr. Opin. Lipidol. 2015, 26, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta 2005, 1735, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.G.; Bortnick, A.E.; Kellner-Weibel, G.; de la Llera-Moya, M.; Phillips, M.C.; Rothblat, G.H. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Fournier, N.; Paul, J.L.; Atger, V.; Cogny, A.; Soni, T.; de la Llera-Moya, M.; Rothblat, G.; Moatti, N. HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y. Rate-limiting factors of cholesterol efflux in reverse cholesterol transport: Acceptors and donors. Clin. Exp. Pharmacol. Physiol. 2010, 37, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldan, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.G.; de la Llera-Moya, M.; Swarnakar, S.; Monzo, P.; Klein, S.M.; Connelly, M.A.; Johnson, W.J.; Williams, D.L.; Rothblat, G.H. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000, 275, 36596–36604. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, B.L.; Demosky, S.J.; Stonik, J.A.; Ghias, M.; Knapper, C.L.; Sampson, M.L.; Dai, C.; Levine, S.J.; Remaley, A.T. Endothelial expression of human ABCA1 in mice increases plasma HDL cholesterol and reduces diet-induced atherosclerosis. J. Lipid Res. 2012, 53, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Munch, G.; Bultmann, A.; Li, Z.; Holthoff, H.P.; Ullrich, J.; Wagner, S.; Ungerer, M. Overexpression of ABCG1 protein attenuates arteriosclerosis and endothelial dysfunction in atherosclerotic rabbits. Heart Int. 2012, 7, e12. [Google Scholar] [CrossRef] [PubMed]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Koetsveld, J.; Yu, S.; Han, S.; Li, R.; Goldberg, I.J.; Welch, C.L.; Tall, A.R. Increased atherosclerosis in mice with vascular ATP-binding cassette transporter G1 deficiency—Brief report. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.J.; Denis, M.; Genest, J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation 2004, 110, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fu, Y.; Hou, Y.; Wang, N.; Guan, Y.; Tang, C.; Shyy, J.Y.; Zhu, Y. Laminar shear stress regulates liver X receptor in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Liao, D.F.; Tang, C.K. ATP-binding membrane cassette transporter A1 (ABCA1): A possible link between inflammation and reverse cholesterol transport. Mol. Med. 2010, 16, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Hamon, Y.; Broccardo, C.; Chambenoit, O.; Luciani, M.F.; Toti, F.; Chaslin, S.; Freyssinet, J.M.; Devaux, P.F.; McNeish, J.; Marguet, D.; et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2000, 2, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ji, L.; Tong, X.; Pan, B.; Han, J.Y.; Huang, Y.; Chen, Y.E.; Pennathur, S.; Zhang, Y.; Zheng, L. Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1. Am. J. Physiol. Cell Physiol. 2011, 301, C739–C748. [Google Scholar] [CrossRef] [PubMed]
- Schror, K.; Hohlfeld, T. Mechanisms of anti-ischemic action of prostaglandin E1 in peripheral arterial occlusive disease. VASA 2004, 33, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Pagler, T.A.; Seimon, T.A.; Thorp, E.; Welch, C.L.; Witztum, J.L.; Tabas, I.; Tall, A.R. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ. Res. 2010, 106, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Kim, C.J. Modulation of renin-angiotensin system and arterial stiffness: Evidence from clinical trials. Curr. Hypertens. Rev. 2014, 10, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Hong, H.C.; Choi, H.Y.; Yoo, H.J.; Cho, G.J.; Hwang, T.G.; Baik, S.H.; Choi, D.S.; Kim, S.M.; Choi, K.M. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin. Endocrinol. 2011, 75, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Ulu, S.M.; Yuksel, S.; Altuntas, A.; Kacar, E.; Ahsen, A.; Altug, A.; Celik, S.; Sezer, M.T. Associations between serum hepcidin level, FGF-21 level and oxidative stress with arterial stiffness in CAPD patients. Int. Urol. Nephrol. 2014, 46, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, J.; Zhang, C.; Zeng, J.; Zhou, S.; Zhang, Z.; Lu, X.; Chen, J.; Feng, W.; Li, X.; et al. Fibroblast growth factor 21 deletion aggravates diabetes-induced pathogenic changes in the aorta in type 1 diabetic mice. Cardiovasc. Diabetol. 2015, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Pan, X.; Wu, F.; Ye, D.; Zhang, Y.; Wang, Y.; Jin, L.; Lian, Q.; Huang, Y.; Ding, H.; et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 2015, 131, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Meng, X.; Dong, M.; Guo, T.; Kong, J.; Zhang, K.; Zhang, Y.; Zhang, C. Endogenous activated angiotensin-(1-7) plays a protective effect against atherosclerotic plaques unstability in high fat diet fed ApoE knockout mice. Int. J. Cardiol. 2015, 184, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Wang, X.; Bian, Y.; Yang, H.; Liu, M.; Bai, R.; Yang, Z.; Xiao, C. Angiotensin-(1-7) upregulates expression of adenosine triphosphate-binding cassette transporter A1 and adenosine triphosphate-binding cassette transporter G1 through the Mas receptor through the liver X receptor alpha signalling pathway in THP-1 macrophages treated with angiotensin-II. Clin. Exp. Pharmacol. Physiol. 2014, 41, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; He, X.L.; Zeng, J.F.; Zhang, H.; Zhao, Y.; Tan, J.K.; Wang, Z. FGF21 increases cholesterol efflux by upregulating ABCA1 through the ERK1/2-PPARγ -LXRalpha pathway in THP1 macrophage-derived foam cells. DNA Cell Biol. 2014, 33, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Engel, T.; Li, Z.; Goepfert, C.; Rust, S.; Zhou, X.; Langer, C.; Schachtrup, C.; Wiekowski, J.; Lorkowski, S.; et al. Polyunsaturated fatty acids and acetoacetate downregulate the expression of the ATP-binding cassette transporter A1. Diabetes 2002, 51, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J. Biol. Chem. 2002, 277, 5692–5697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kris-Etherton, P.M.; Thompson, J.T.; Hannon, D.B.; Gillies, P.J.; Heuvel, J.P. Alpha-linolenic acid increases cholesterol efflux in macrophage-derived foam cells by decreasing stearoyl CoA desaturase 1 expression: Evidence for a farnesoid-X-receptor mechanism of action. J. Nutr. Biochem. 2012, 23, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J. Lipid Res. 2007, 48, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, W.; Wang, Y. ApoA-I or ABCA1 expression suppresses fatty acid synthesis by reducing 27-hydroxycholesterol levels. Biochimie 2014, 103, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, M.P.; Zhang, D.; Lopes, H.F.; Lee, C.G.; Goodfriend, T.L.; Egan, B.M. Hemodynamic effects of lipids in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1674–R1679. [Google Scholar] [CrossRef] [PubMed]
- Udelsmann, A.; Melo Mde, S. Hemodynamic changes with high infusion rates of lipid emulsion. Experimental study in swine. Acta Cir. Bras. 2015, 30, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Royse, C.; Royse, A. The cardiovascular effects of intralipid as a rescue therapy for non-fatal bupivacaine toxicity. J. Perioper. Sci. 2014, 1, 1–6. [Google Scholar]
- Davda, R.K.; Stepniakowski, K.T.; Lu, G.; Ullian, M.E.; Goodfriend, T.L.; Egan, B.M. Oleic acid inhibits endothelial nitric oxide synthase by a protein kinase C-independent mechanism. Hypertension 1995, 26, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.O.; Paradisi, G.; Hook, G.; Crowder, K.; Cronin, J.; Baron, A.D. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 2000, 49, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Avendano, M.S.; Martinez-Revelles, S.; Aguado, A.; Simoes, M.R.; Gonzalez-Amor, M.; Palacios, R.; Guillem-Llobat, P.; Vassallo, D.V.; Vila, L.; Garcia-Puig, J.; et al. Role of COX-2-derived PGE2 on vascular stiffness and function in hypertension. Br. J. Pharmacol. 2016, 173, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, X.; Yuan, Y.; Zhu, H.; Zhou, W.; Huang, H.; Feng, M. Inhibitory effect of apolipoprotein A-I on matrix metalloproteinase-2 expression in vivo and in vitro. Acta Biochim. Biophys. Sin. 2013, 45, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, W.; Wang, Y. Apolipoprotein A-I expression suppresses COX-2 expression by reducing reactive oxygen species in hepatocytes. Biochem. Biophys. Res. Commun. 2014, 454, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Bigazzi, F.; Adorni, M.P.; Puntoni, M.; Sbrana, F.; Lionetti, V.; Pino, B.D.; Favari, E.; Recchia, F.A.; Bernini, F.; Sampietro, T. Analysis of Serum Cholesterol Efflux Capacity in a Minipig Model of Nonischemic Heart Failure. J. Atheroscler. Thromb. 2017, 24, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, H.; Gu, Q.; Li, C. The expression of ATP-binding cassette transporters in hypertensive patients. Hypertens. Res. 2009, 32, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Boisvert, W.A.; Lee, C.H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A PPARγ -LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Ameshima, S.; Golpon, H.; Cool, C.D.; Chan, D.; Vandivier, R.W.; Gardai, S.J.; Wick, M.; Nemenoff, R.A.; Geraci, M.W.; Voelkel, N.F. Peroxisome proliferator-activated receptor gamma (PPARγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ. Res. 2003, 92, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Bochem, A.E.; van Wijk, D.F.; Holleboom, A.G.; Duivenvoorden, R.; Motazacker, M.M.; Dallinga-Thie, G.M.; de Groot, E.; Kastelein, J.J.; Nederveen, A.J.; Hovingh, G.K.; et al. ABCA1 mutation carriers with low high-density lipoprotein cholesterol are characterized by a larger atherosclerotic burden. Eur. Heart J. 2013, 34, 286–291. [Google Scholar] [CrossRef] [PubMed]
- van Dam, M.J.; de Groot, E.; Clee, S.M.; Hovingh, G.K.; Roelants, R.; Brooks-Wilson, A.; Zwinderman, A.H.; Smit, A.J.; Smelt, A.H.; Groen, A.K.; et al. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: An observational study. Lancet 2002, 359, 37–42. [Google Scholar] [CrossRef]
- Favari, E.; Ronda, N.; Adorni, M.P.; Zimetti, F.; Salvi, P.; Manfredini, M.; Bernini, F.; Borghi, C.; Cicero, A.F. ABCA1-dependent serum cholesterol efflux capacity inversely correlates with pulse wave velocity in healthy subjects. J. Lipid Res. 2013, 54, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Mourmoura, E.; Vasilaki, A.; Giannoukas, A.; Michalodimitrakis, E.; Pavlidis, P.; Tsezou, A. Evidence of deregulated cholesterol efflux in abdominal aortic aneurysm. Acta Histochem. 2016, 118, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.O.; Agerholm-Larsen, B.; Wang, N.; Chen, W.; Tall, A.R. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J. Biol. Chem. 2003, 278, 37368–37374. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; Tsujita, M.; Terry, T.L. Apolipoprotein AI efficiently binds to and mediates cholesterol and phospholipid efflux from human but not rat aortic smooth muscle cells. Biochemistry 1999, 38, 16315–16322. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Rahmani, M.; Wong, B.W.; Allahverdian, S.; McManus, B.M.; Pickering, J.G.; Chan, T.; Francis, G.A. ATP-binding cassette transporter A1 expression and apolipoprotein A-I binding are impaired in intima-type arterial smooth muscle cells. Circulation 2009, 119, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Yang, H.; Zhou, L.; Okoro, E.U.; Guo, Z. A novel function of apolipoprotein E: Upregulation of ATP-binding cassette transporter A1 expression. PLoS ONE 2011, 6, e21453. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, D.; Liu, S.L.; Bae, Y.H.; Monslow, J.; Xu, T.; Hawthorne, E.A.; Byfield, F.J.; Castagnino, P.; Rao, S.; Rader, D.J.; et al. Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Rep. 2012, 2, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Reddick, R.L.; Zhang, S.H.; Maeda, N. Atherosclerosis in mice lacking Apo E. Evaluation of lesional development and progression. Arterioscler. Thromb. 1994, 14, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Monti, M.; Arnaboldi, L.; Canavesi, M.; Ainis Buscherini, G.; Calabresi, L.; Corsini, A.; Bellosta, S. ABCA1 and HDL3 are required to modulate smooth muscle cells phenotypic switch after cholesterol loading. Atherosclerosis 2017, 266, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Aiello, R.J.; Brees, D.; Francone, O.L. ABCA1-deficient mice: Insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Bochem, A.E.; van der Valk, F.M.; Tolani, S.; Stroes, E.S.; Westerterp, M.; Tall, A.R. Increased Systemic and Plaque Inflammation in ABCA1 Mutation Carriers With Attenuation by Statins. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Hamon, Y.; Luciani, M.F.; Becq, F.; Verrier, B.; Rubartelli, A.; Chimini, G. Interleukin-1β secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 1997, 90, 2911–2915. [Google Scholar] [PubMed]
- Zhou, X.; Engel, T.; Goepfert, C.; Erren, M.; Assmann, G.; von Eckardstein, A. The ATP binding cassette transporter A1 contributes to the secretion of interleukin 1β from macrophages but not from monocytes. Biochem. Biophys. Res. Commun. 2002, 291, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, Y.; Kessler, P.S.; Vaughan, A.M.; Oram, J.F. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J. Biol. Chem. 2009, 284, 32336–32343. [Google Scholar] [CrossRef] [PubMed]
- Mountain, D.J.; Singh, M.; Menon, B.; Singh, K. Interleukin-1β increases expression and activity of matrix metalloproteinase-2 in cardiac microvascular endothelial cells: Role of PKCalpha/beta1 and MAPKs. Am. J. Physiol. Cell Physiol. 2007, 292, C867–C875. [Google Scholar] [CrossRef] [PubMed]
- Ruhul Amin, A.R.; Senga, T.; Oo, M.L.; Thant, A.A.; Hamaguchi, M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: A role for the dual signalling pathways, Akt and Erk. Genes Cells 2003, 8, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Maki-Petaja, K.M.; Elkhawad, M.; Cheriyan, J.; Joshi, F.R.; Ostor, A.J.; Hall, F.C.; Rudd, J.H.; Wilkinson, I.B. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 2012, 126, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Deane, K.D.; Meditz, A.L.; Kohrt, W.M. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 2013, 230, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Babashamsi, M.M.; Halalkhor, S.; Moradi Firouzjah, H.; Parsian, H.; Jalali, S.F.; Babashamsi, M. Association of ATP-Binding Cassette Transporter A1 (ABCA1)-565 C/T Gene Polymorphism with Hypoalphalipoproteinemia and Serum Lipids, IL-6 and CRP Levels. Avicenna J. Med. Biotechnol. 2017, 9, 38–43. [Google Scholar] [PubMed]
- Li, C.; Guo, R.; Lou, J.; Zhou, H. The transcription levels of ABCA1, ABCG1 and SR-BI are negatively associated with plasma CRP in Chinese populations with various risk factors for atherosclerosis. Inflammation 2012, 35, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Mattace-Raso, F.U.; van der Cammen, T.J.; van der Meer, I.M.; Schalekamp, M.A.; Asmar, R.; Hofman, A.; Witteman, J.C. C-reactive protein and arterial stiffness in older adults: The Rotterdam Study. Atherosclerosis 2004, 176, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Nakamura, M.; Sato, K.; Tanaka, F.; Segawa, T.; Hiramori, K. Association between serum C-reactive protein levels and pulse wave velocity: A population-based cross-sectional study in a general population. Atherosclerosis 2005, 180, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, A.; Joseph, S.B.; Marathe, C.; Mangelsdorf, D.J.; Tontonoz, P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J. Biol. Chem. 2003, 278, 10443–10449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Sukhova, G.; Murphy, C.; Libby, P.; Plutzky, J. Macrophages in human atheroma contain PPARγ: Differentiation-dependent peroxisomal proliferator-activated receptor gamma(PPARγ) expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes in vitro. Am. J. Pathol. 1998, 153, 17–23. [Google Scholar] [CrossRef]
- Laragione, T.; Gulko, P.S. Liver X receptor regulates rheumatoid arthritis fibroblast-like synoviocyte invasiveness, matrix metalloproteinase 2 activation, interleukin-6 and CXCL10. Mol. Med. 2012, 18, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.A.; Lee, H.; Hwang, J.S.; Kang, E.S.; Yoo, T.; Paek, K.S.; Do, J.T.; Park, C.; Oh, J.W.; Kim, J.H.; et al. Activation of peroxisome proliferator-activated receptor delta inhibits angiotensin II-induced activation of matrix metalloproteinase-2 in vascular smooth muscle cells. J. Vasc. Res. 2014, 51, 221–230. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; McLachlan, C.S. Cholesterol Efflux: Does It Contribute to Aortic Stiffening? J. Cardiovasc. Dev. Dis. 2018, 5, 23. https://doi.org/10.3390/jcdd5020023
Liao S, McLachlan CS. Cholesterol Efflux: Does It Contribute to Aortic Stiffening? Journal of Cardiovascular Development and Disease. 2018; 5(2):23. https://doi.org/10.3390/jcdd5020023
Chicago/Turabian StyleLiao, Shutan, and Craig S. McLachlan. 2018. "Cholesterol Efflux: Does It Contribute to Aortic Stiffening?" Journal of Cardiovascular Development and Disease 5, no. 2: 23. https://doi.org/10.3390/jcdd5020023
APA StyleLiao, S., & McLachlan, C. S. (2018). Cholesterol Efflux: Does It Contribute to Aortic Stiffening? Journal of Cardiovascular Development and Disease, 5(2), 23. https://doi.org/10.3390/jcdd5020023