Toxigenic Cyanobacteria and Microcystins in a Large Northern Oligotrophic Lake Onego, Russia
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
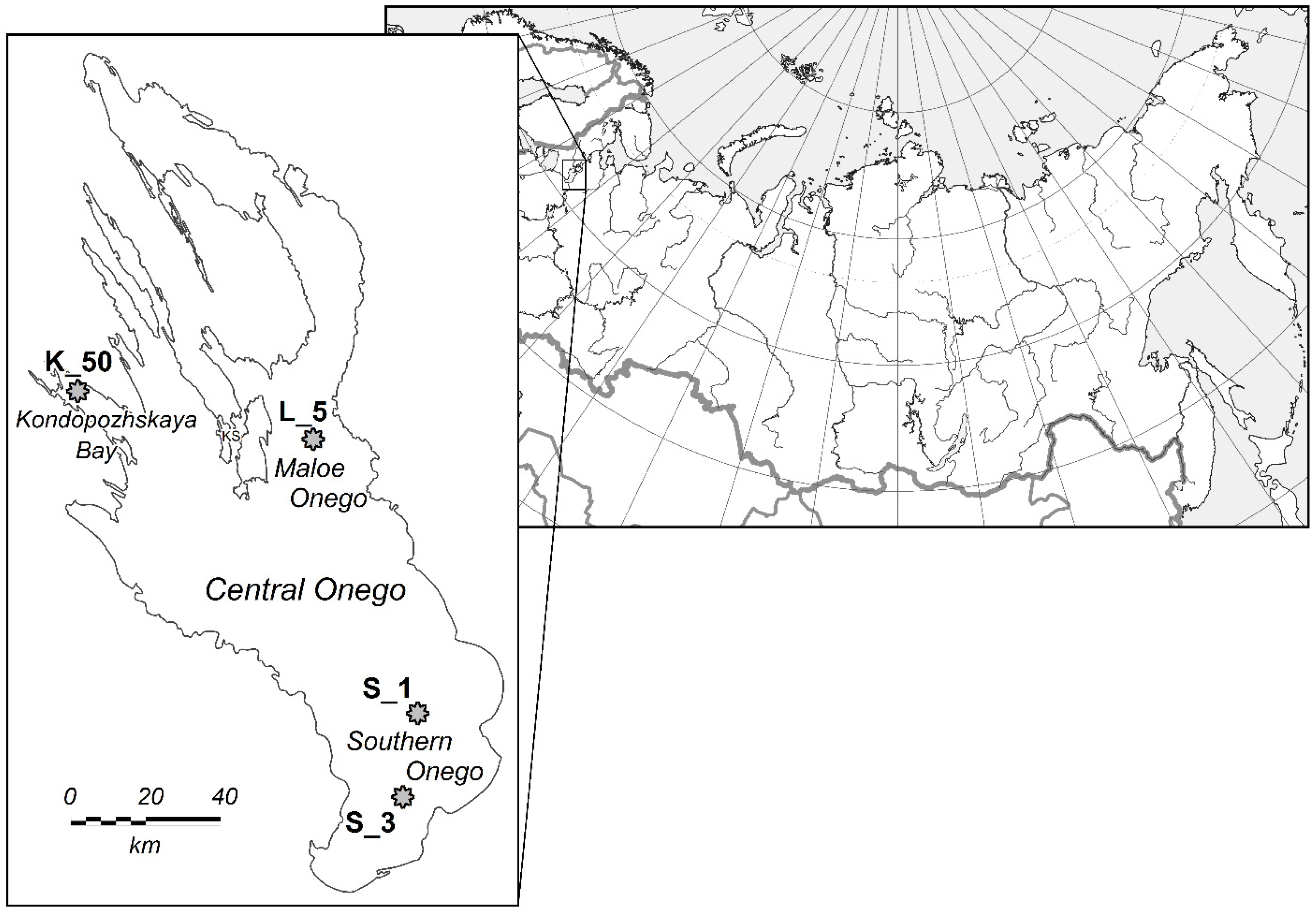
5.1. General Characteristics of the Study Area
5.2. Sampling
5.3. Phytoplankton Analysis
5.4. HPLC–MSHR Analysis of Cyanotoxins
5.5. DNA Extraction and PCR Detection of mcyA Gene
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chorus, I. Cyanotoxins: Occurrence, Causes, Consequences; Springer: Berlin, Germany, 2001. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sukharevich, V.I.; Polak, Y.M. Global occurrence of cyanobacteria: Causes and effects (review). Inland Water Biol. 2020, 4, 566–575. [Google Scholar] [CrossRef]
- Apeldoorn, M.E.; Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.W.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom-forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Fastner, J.; Welker, M. Cyanobacteria and Cyanotoxins in a Changing Environment: Concepts, Controversies, Challenges. Water 2021, 13, 2463. [Google Scholar] [CrossRef]
- Gabyshev, V.; Sidelev, S.; Chernova, E.; Vilnet, A.; Davydov, D.; Barinova, S.; Gabysheva, O.; Zhakovskaya, Z.; Voronov, I. Year-round presence of microcystins and toxin-producing Microcystis in water column and ice cover of a eutrophic lake located in the continuous permafrost zone (Yakutia, Russia). Toxins 2023, 15, 467. [Google Scholar] [CrossRef]
- Przytulska, A.; Bartosiewicz, M.; Vincent, W.F. Increased risk of cyanobacterial blooms in northern high-latitude lakes through climate warming and phosphorus enrichment. Freshw. Biol. 2017, 62, 1986–1996. [Google Scholar] [CrossRef]
- de Magalhães, V.F.; Soares, R.M.; Azevedo, S.M. Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Janeiro, Brazil): Ecological implication and human health risk. Toxicon 2001, 39, 1077–1085. [Google Scholar] [CrossRef]
- Poste, A.E.; Hecky, R.E.; Guildford, S.J. Evaluating microcystin exposure risk through fish consumption. Environ. Sci. Technol. 2011, 45, 5806–5811. [Google Scholar] [CrossRef] [PubMed]
- Onyango, D.M.; Orina, P.S.; Ramkat, R.C.; Kowenje, C.; Githukia, C.M.; Lusweti, D.; Lung’ayia, H.B. Review of current state of knowledge of microcystin and its impacts on fish in Lake Victoria. Lakes Reserv. Res. Manag. 2020, 25, 350–361. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Frenken, T.; Rudman, S.M.; Ibelings, B.W.; Trainer, V.L. Diseases and disorders in fish due to harmful algal blooms. In Climate Change on Diseases and Disorders of Finfish in Cage Culture, 3rd ed.; Woo, P.T.K., Subasinghe, R.P., Eds.; CABI: Wallingford, UK, 2023; pp. 387–429. [Google Scholar] [CrossRef]
- Smith, R.B.; Bass, B.; Sawyer, D.; Depew, D.; Watson, S.B. Estimating the economic costs of algal blooms in the Canadian Lake Erie Basin. Harmful Algae 2019, 87, 101624. [Google Scholar] [CrossRef] [PubMed]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Voyakina, E.; Zhakovskaya, Z. First observation of microcystin- and anatoxin-a-producing cyanobacteria in the easternmost part of the Gulf of Finland (the Baltic Sea). Toxicon 2019, 157, 18–24. [Google Scholar] [CrossRef]
- Voloshko, L.; Kopecky, J.; Safronova, T.; Pljusch, A.; Titova, N.; Hrouzek, P.; Drabkova, V. Toxins and other bioactive compounds produced by cyanobacteria in Lake Ladoga. Est. J. Ecol. 2008, 57, 100–110. [Google Scholar] [CrossRef]
- Litvinchuk, L.F.; Sharov, A.N.; Chernova, E.N.; Smirnov, V.V.; Berezina, N.A. Mutual links between microcystins-producing cyanobacteria and plankton community in clear and brow2n northern lakes. Food Webs 2023, 35, e00279. [Google Scholar] [CrossRef]
- Chernova, E.; Russkikh, I.; Voyakina, E.; Zhakovskaya, Z. Occurrence of microcystins and anatoxin-a in eutrophic lakes of Saint Petersburg, Northwestern Russia. Oceanol. Hydrobiol. Stud. 2016, 45, 466–484. [Google Scholar] [CrossRef]
- Voyakina, E.J.; Russkikh, I.V.; Chernova, E.N.; Zhakovskaya, Z.A. Toxic cyanobacteria and their metabolites in the lakes of the Russian northwest. Theor. Appl. Ecol. 2020, 1, 124–129, In Russian. [Google Scholar] [CrossRef]
- Tekanova, E.V.; Kalinkina, N.M.; Makarova, E.M.; Smirnova, V.S. The current trophic state and water quality of Lake Onego. Inland Water Biol. 2023, 16, 967–973. [Google Scholar] [CrossRef]
- Chernova, E.N.; Russkikh, Y.V.; Podolskaya, E.P.; Zhakovskaya, Z.A. Determination of microcystins and anatoxin-a using liquid chromato-mass-spectrometry of unit resolution. Nauchnoe Priborostr. 2016, 26, 11–25. Available online: http://iairas.ru/mag/2016/full1/Art2.pdf (accessed on 21 October 2024). (In Russian). [CrossRef]
- Rapala, J.; Sivonen, K.; Luukkainen, R.; Niemela, S.I. Anatoxin-a concentration in Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and nontoxic Anabaena-strains—A laboratory study. J. Appl. Phycol. 1993, 5, 581–591. [Google Scholar] [CrossRef]
- Dimitrakopoulos, I.K.; Kaloudis, T.S.; Hiskia, A.E.; Thomaidis, N.S.; Koupparis, M.A. Development of a fast and selective method for the sensitive determination of anatoxin-a in lake waters using liquid chromatography–tandem mass spectrometry and phenylalanine-d5 as internal standard. Anal. Bioanal. Chem. 2010, 397, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Barinova, S.; Chekryzheva, T. Phytoplankton dynamic and bioindication in the Kondopoga Bay, Lake Onego (Northern Russia). J. Limnol. 2014, 73, 282–297. [Google Scholar] [CrossRef]
- Smirnova, V.S.; Tekanova, E.V.; Kalinkina, N.M. Phytoplankton as an indicator of the ecosystem state of the Kondopoga Bay of Lake Onego under cage trout farming. Ecosyst. Transform. 2024, 7, 177–195. [Google Scholar] [CrossRef]
- Chekryzheva, T.A.; Kalinkina, N.M. Structure and seasonal dynamics of phytoplankton communities in the exposed and sheltered littoral zones of Lake Onego (Pinguba Bay, Pukhtinskaya Bay). Trans. Karelian Res. Cent. Russ. Acad. Sci. 2016, 12, 83–95. (In Russian) [Google Scholar] [CrossRef]
- Petrova, N.A. Fitoplankton Litoralnoj Zony Onezhskogo Ozera. In The Vegetation of the Lake Onego; Raspopov, I.M., Ed.; Nauka: Leningrad, Russia, 1975; p. 138144. Available online: https://search.rsl.ru/ru/record/01007196947 (accessed on 21 October 2024). (In Russian)
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 1st ed.; CRC Press: London, UK, 1999. [Google Scholar] [CrossRef]
- Saker, M.L.; Vale, M.; Kramer, D.; Vasconcelos, V.M. Molecular techniques for the early warning of toxic cyanobacteria blooms in freshwater lakes and rivers. Appl. Microbiol. Biotechnol. 2007, 75, 441–449. [Google Scholar] [CrossRef]
- Sidelev, S.I.; Korneva, L.G.; Solovyeva, V.V.; Zubishina, A.A.; Pligin, D.N. Molecular genetic identification and seasonal succession of toxigenic cyanobacteria in phytoplankton of the Rybinsk Reservoir (Russia). Inland Water Biol. 2016, 9, 368–374. [Google Scholar] [CrossRef]
- Baker, J.A.; Entsch, B.; Neilan, B.A.; McKay, D.B. Monitoring changing toxigenicity of a cyanobacterial bloom by molecular methods. Appl. Environ. Microbiol. 2002, 68, 6070–6076. [Google Scholar] [CrossRef]
- Capelli, C.; Ballot, A.; Cerasino, L.; Papini, A.; Salmaso, N. Biogeography of bloom-forming microcystin producing and non-toxigenic populations of Dolichospermum lemmermannii (Cyanobacteria). Harmful Algae 2017, 67, 1–12. [Google Scholar] [CrossRef]
- Harada, K.; Ogawa, K.; Kimura, Y.; Murata, H.; Suzuki, M.; Thorn, P.M.; Evans, W.R.; Carmichael, W.W. Microcystins from Anabaena flosaquae NRC 525-17. Chem. Res. Toxicol. 1991, 4, 535–540. [Google Scholar] [CrossRef]
- Vezie, C.; Brient, L.; Sivonen, K.; Bertru, G.; Lefeuvre, J.-C.; Salkinoja-Salonen, M. Variation of microcystin content of cyanobacterial blooms and isolated strains in Lake Grand-Lieu (France). Microb. Ecol. 1998, 35, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Rukhovets, L.; Filatov, N. Ladoga and Onego—Great European Lakes. In Observations and Modeling; Springer: Berlin/Heidelberg, Germany, 2010; Available online: https://www.springer.com/gp/book/9783540681441 (accessed on 21 October 2024).
- Sterner, R.W.; Reinl, K.L.; Lafrancois, B.M.; Brovold, S.; Miller, T.R. A first assessment of cyanobacterial blooms in oligotrophic Lake Superior. Limnol. Oceanogr. 2020, 65, 2984–2998. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: London, UK, 2021. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Bhavsar, S.P.; Almirall, X.O.; Marklevitz, S.A.; Rudman, S.M.; Sibley, P.K. Lake Erie fish safe to eat yet afflicted by algal hepatotoxins. Sci. Total Environ. 2023, 861, 160474. [Google Scholar] [CrossRef] [PubMed]
- Shahmohamadloo, R.S.; Bhavsar, S.P.; Almirall, X.O.; Marklevitz, S.A.; Rudman, S.M.; Sibley, P.K. Cyanotoxins accumulate in Lake St. Clair fish yet their fillets are safe to eat. Sci. Total Environ. 2023, 874, 162381. [Google Scholar] [CrossRef]
- Callieri, C.; Bertoni, R.; Contesini, M.; Bertoni, F. Lake level fluctuations boost toxic cyanobacterial “oligotrophic blooms”. PLoS ONE 2014, 9, e109526. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Hadas, O.; Summers, M.L.; Rücker, J.; Sukenik, A. Akinetes: Dormant cells of cyanobacteria. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin, Germany, 2010; pp. 5–27. Available online: https://link.springer.com/book/10.1007/978-3-642-12422-8 (accessed on 21 October 2024).
- Zobkov, M.; Zobkova, M.; Galakhina, N.; Efremova, T.; Efremenko, N.; Kulik, N. Data on the chemical composition of Lake Onego water in 2019–2021. Data Brief 2022, 42, e108079. [Google Scholar] [CrossRef] [PubMed]
- Legrand, B.; Lamarque, A.; Sabart, M.; Latour, D. Characterization of akinetes from cyanobacterial strains and lake sediment: A study of their resistance and toxic potential. Harmful Algae 2016, 59, 42–50. [Google Scholar] [CrossRef]
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; De Senerpont Domis, L.N.; Atkins, K.S.; Isles, P.D.F.; Mesman, J.P.; et al. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshw. Biol. 2021, 66, 1846–1859. [Google Scholar] [CrossRef]
- Belykh, O.I.; Fedorova, G.A.; Kuzmin, A.V.; Tikhonova, I.V.; Timoshkin, O.A.; Sorokovikova, E.G. Microcystins in cyanobacterial biofilms from the littoral zone of Lake Baikal. Mosc. Univ. Biol. Sci. Bull. 2017, 72, 225–231. [Google Scholar] [CrossRef]
- Belykh, O.I.; Gladkikh, A.S.; Sorokovikova, E.G.; Tikhonova, I.V.; Butina, T.V. Identification of Toxic Cyanobacteria in Lake Baikal. Dokl. Biochem. Biophys. 2015, 463, 220–224. [Google Scholar] [CrossRef]
- Bartlett, S.L.; Brunner, S.L.; Val Klump, J.; Houghton, E.M.; Miller, T.R. Spatial analysis of toxic or otherwise bioactive cyanobacterial peptides in Green Bay, Lake Michigan. J. Great Lakes Res. 2018, 44, 924–933. [Google Scholar] [CrossRef]
- Litinsky, P.Y. Onego Lake catchment ecosystem GIS as a tool for estimating components of the water and carbon balance. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2019, 9, 136–144. (In Russian) [Google Scholar] [CrossRef]
- Galakhina, N.; Zobkov, M.; Zobkova, M. Current chemistry of Lake Onego and its spatial and temporal changes for the last three decades with special reference to nutrient concentrations. Environ. Nanotechnol. Monit. Manag. 2022, 17, e100619. [Google Scholar] [CrossRef]
- Sabylina, A.V.; Lozovik, P.A.; Zobkov, M.B. Water chemistry in Onega Lake and its tributaries. Water Resour. 2010, 37, 842–853. [Google Scholar] [CrossRef]
- Tekanova, E.V.; Litvinova, I.A. Organic matter destruction in the Kodopoga Bay, Onego Lake, under changing anthropogenic load. Water Res. 2022, 49, 1009–1016. [Google Scholar] [CrossRef]
- Timakova, T.M.; Kulikova, T.P.; Litvinova, I.A.; Polyakova, T.N.; Syarki, M.T.; Tekanova, E.V.; Chekryzheva, T.A. Changes in biocenoses of Kondopoga Bay, Lake Onego, under the effect of effluents from a pulp and paper mill. Water Res. 2014, 41, 78–86. [Google Scholar] [CrossRef]
- Clark, W.J.; Sigler, W.F. Method of concentrating phytoplankton samples using membrane filters. Limnol. Oceanogr. 1963, 8, 127–129. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Counting slides. In Phytoplankton Manual, Monographs on Oceanographic Methodology; Sournia, A., Ed.; UNESCO Publishing: Paris, France, 1978; Volume 6, pp. 182–189. [Google Scholar]
- LeGresley, M.; McDermott, G. Counting chamber methods for quantitative phytoplankton analysis—Haemocytometer, Palmer-Maloney cell and Sedgewick-Rafter cell. In Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; Karlson, B., Cusack, C., Bresnan, E., Eds.; UNESCO: Paris, France, 2010; pp. 25–30. [Google Scholar]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. Helcom Balt. Sea Environ. Proc. 2006, 106, 144. Available online: https://helcom.fi/wp-content/uploads/2019/08/BSEP106.pdf (accessed on 21 October 2024).
- Okhapkin, A.; Sharagina, E.; Kulizin, P.; Startseva, N.; Vodeneeva, E. Phytoplankton community structure in highly mineralized small gypsum karst lake (Russia). Microorganisms 2022, 10, 386. [Google Scholar] [CrossRef]
- Ger, K.A.; Hansson, L.-A.; Lürling, M. Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshw. Biol. 2014, 59, 1783–1798. [Google Scholar] [CrossRef]
- Komarek, J. Cyanoprokaryota. 3. Heterocytous genera. In Süswasserflora von Mitteleuropa/Freshwater Flora of Central Europe; Büdel, B., Gartner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany, 2013; 1130p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. T. 1. Chroococcales; Gustav Fischer Verlag: Jena, Germany, 1998; 548p, Available online: https://www.springer.com/gp/book/9783827421111 (accessed on 21 October 2024).
- Report of SCOR-Unesco Working Group 17. Determination of photosynthetic pigments in sea-water. In Monographs Onocéanographie Methodology; UNESCO: Paris, France, 1966; pp. 9–18. [Google Scholar]
- Kurmayer, R.; Sivonen, K.; Wilmotte, A.; Salmaso, N. Molecular Tools for Detection and Quantification of Toxigenic Cyanobacteria; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Lee, J.; Choi, J.; Fatka, M.; Swanner, E.; Ikuma, K.; Liang, X.; Leung, T.; Howe, A. Improved detection of mcyA genes and their phylogenetic origins in harmful algal blooms. Water Res. 2020, 176, e115730. [Google Scholar] [CrossRef] [PubMed]
- Sidelev, S.I. Detection of microcystin-producing cyanobacteria Microcystis, Planktothrix and Dolichospermum using multiprimer amplification of mcy genes. Microbiology 2022, 91, 792–795. [Google Scholar] [CrossRef]



| Station | Ntot, 106 cells L−1 | Btot, mg L−1 | Chl a, µg L−1 |
|---|---|---|---|
| S_1 | 129.29 | 36.42 | 31 |
| S_3 | 84.52 | 19.38 | 33 |
| L_5 | 24.32 | 6.82 | 47 |
| K50 | 12.23 | 0.69 | 11 |
| Sampling Station | Species | Share of the Species in Abundance, % | Share of the Species in Biomass, % |
|---|---|---|---|
| S_1 | Aphanizomenon flos-aquae | 0.003 | 0.04 |
| Dolichospermum lemmermannii | 57 | 74.9 | |
| Dolichospermum spiroides | 43 | 25.0 | |
| S_3 | Aphanizomenon flos-aquae | 0.05 | 0.79 |
| Aphanothece minutissima | 1 | 0.04 | |
| Aphanocapsa grevillei | 1 | 0.06 | |
| Dolichospermum circinale | 49 | 31.2 | |
| Dolichospermum lemmermannii | 46 | 67.7 | |
| Microcystis aeruginosa | 2 | 0.12 | |
| L_5 | Aphanothece nidularis | 8 | 0.3 |
| Dolichospermum circinale | 44 | 28 | |
| Dolichospermum lemmermannii | 49 | 71.7 | |
| K50 | Aphanizomenon flos-aquae | 0.007 | 16.5 |
| Dolichospermum flos-aquae | 56 | 52.6 | |
| Dolichospermum spiroides | 12 | 11.5 | |
| Dolichospermum lemmermannii | 4 | 8.7 | |
| Microcystis aeruginosa | 26 | 10.6 |
| MC Congeners and Detection of mcyA Gene | S_1 | K50 | S_3 | L_50 |
|---|---|---|---|---|
| [D-Asp3]MC-LR | ‒ 1 | 2 | 8 | ‒ |
| MC-LR | ‒ | 8 | ‒ | ‒ |
| [D-Asp3]MC-RR | ‒ | 125 | ‒ | ‒ |
| MC-RR | ‒ | 14 | ‒ | ‒ |
| MC-YR | ‒ | 4 | 4 | ‒ |
| Total MCs concentration | ‒ | 153 | 12 | ‒ |
| Dolichospermum-specific mcyA | ‒ | + 2 | + | NA |
| Microcystis-specific mcyA | + | + | + | NA |
| Characteristics | Pelagic zone of Lake Onego | Kondopozhskaya Bay Off-Shore | Kondopozhskaya Bay In-Shore |
|---|---|---|---|
| Conductivity, μS cm−1 | 50.7 | 48.5 | 53.0 |
| pH | 7.3 | 7.2 | 7.0 |
| TOC, mg L−1 | 6.4 | 7.6 | 12.3 |
| Color, mg Pt-Co L−1 | 31.0 | 35.6 | 51.7 |
| TP, µg L−1 | 8.1 | 22.0 | 68.5 |
| PO4-P, µg L−1 | 0 | 0 | 19.0 |
| NO3-N, mg L−1 | 0.15 | 0.16 | 0.10 |
| NH4-N, mg L−1 | 0.01 | 0.01 | 0.08 |
| TN, mg L−1 | 0.36 | 0.38 | 0.56 |
| Chlorophyll a, µg L−1 | 3.2 | 4.6 | 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekanova, E.; Sidelev, S.; Kalinkina, N.; Chernova, E.; Barinova, S.; Sharov, A.; Smirnova, V. Toxigenic Cyanobacteria and Microcystins in a Large Northern Oligotrophic Lake Onego, Russia. Toxins 2024, 16, 457. https://doi.org/10.3390/toxins16110457
Tekanova E, Sidelev S, Kalinkina N, Chernova E, Barinova S, Sharov A, Smirnova V. Toxigenic Cyanobacteria and Microcystins in a Large Northern Oligotrophic Lake Onego, Russia. Toxins. 2024; 16(11):457. https://doi.org/10.3390/toxins16110457
Chicago/Turabian StyleTekanova, Elena, Sergey Sidelev, Nataliia Kalinkina, Ekaterina Chernova, Sophia Barinova, Andrey Sharov, and Valeria Smirnova. 2024. "Toxigenic Cyanobacteria and Microcystins in a Large Northern Oligotrophic Lake Onego, Russia" Toxins 16, no. 11: 457. https://doi.org/10.3390/toxins16110457
APA StyleTekanova, E., Sidelev, S., Kalinkina, N., Chernova, E., Barinova, S., Sharov, A., & Smirnova, V. (2024). Toxigenic Cyanobacteria and Microcystins in a Large Northern Oligotrophic Lake Onego, Russia. Toxins, 16(11), 457. https://doi.org/10.3390/toxins16110457







