Regional and Longitudinal Dynamics of Cyanobacterial Blooms/Cyanobiome and Cyanotoxin Production in the Great Lakes Area
Abstract
:1. Introduction
2. Results
2.1. Quality Control Analysis
2.2. Trends in Microcystin and Cyanotoxin Distributions
2.3. Identification of Microcystin Congeners and Cyanotoxins
2.4. Relationship Between Microcystin and Cyanobacteria/Cyanotoxin Gene Markers
2.5. Microbiome and Cyanobiome Diversity Analysis
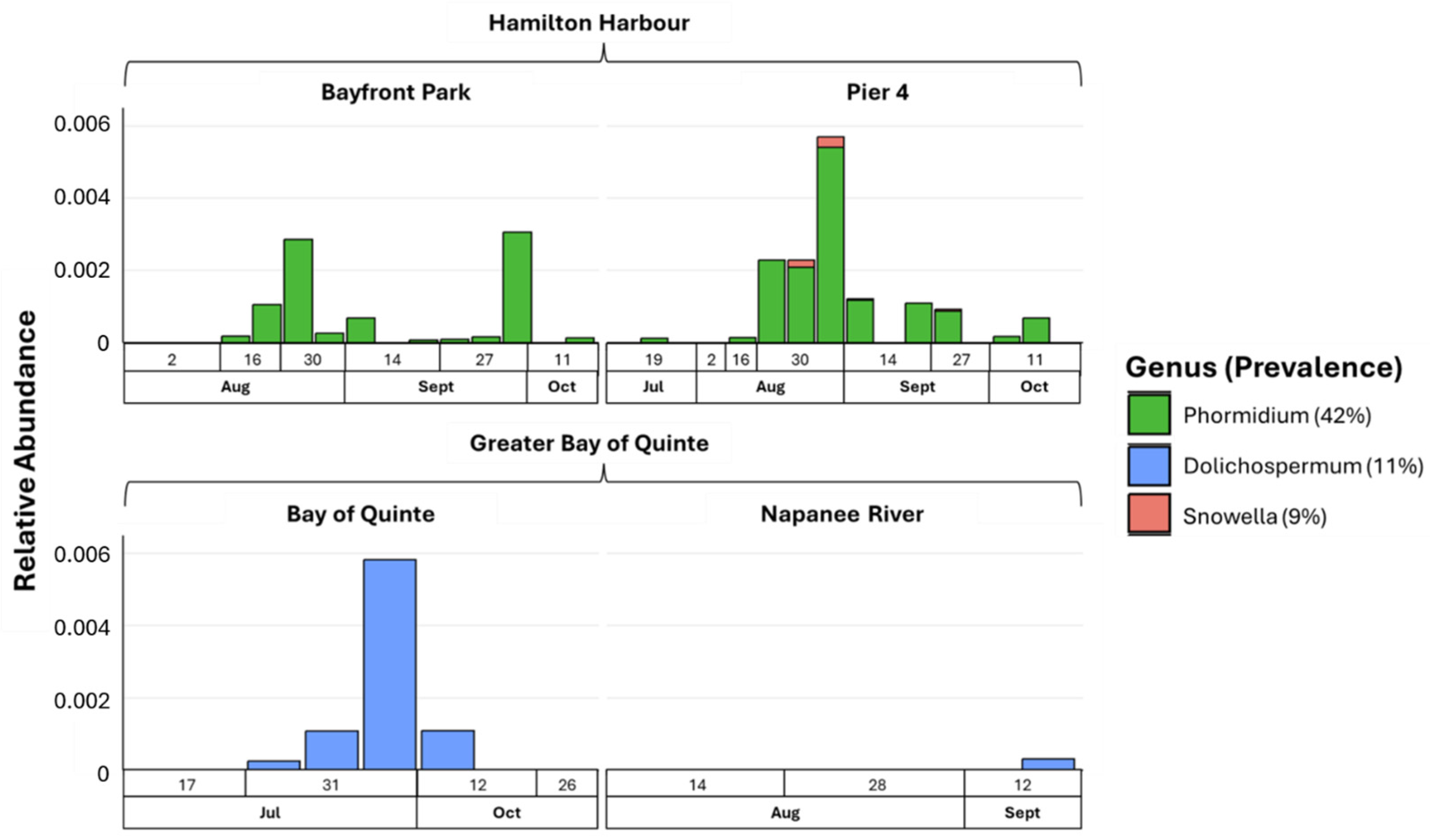
2.6. Whole Microbiome and Cyanobiome Dynamics
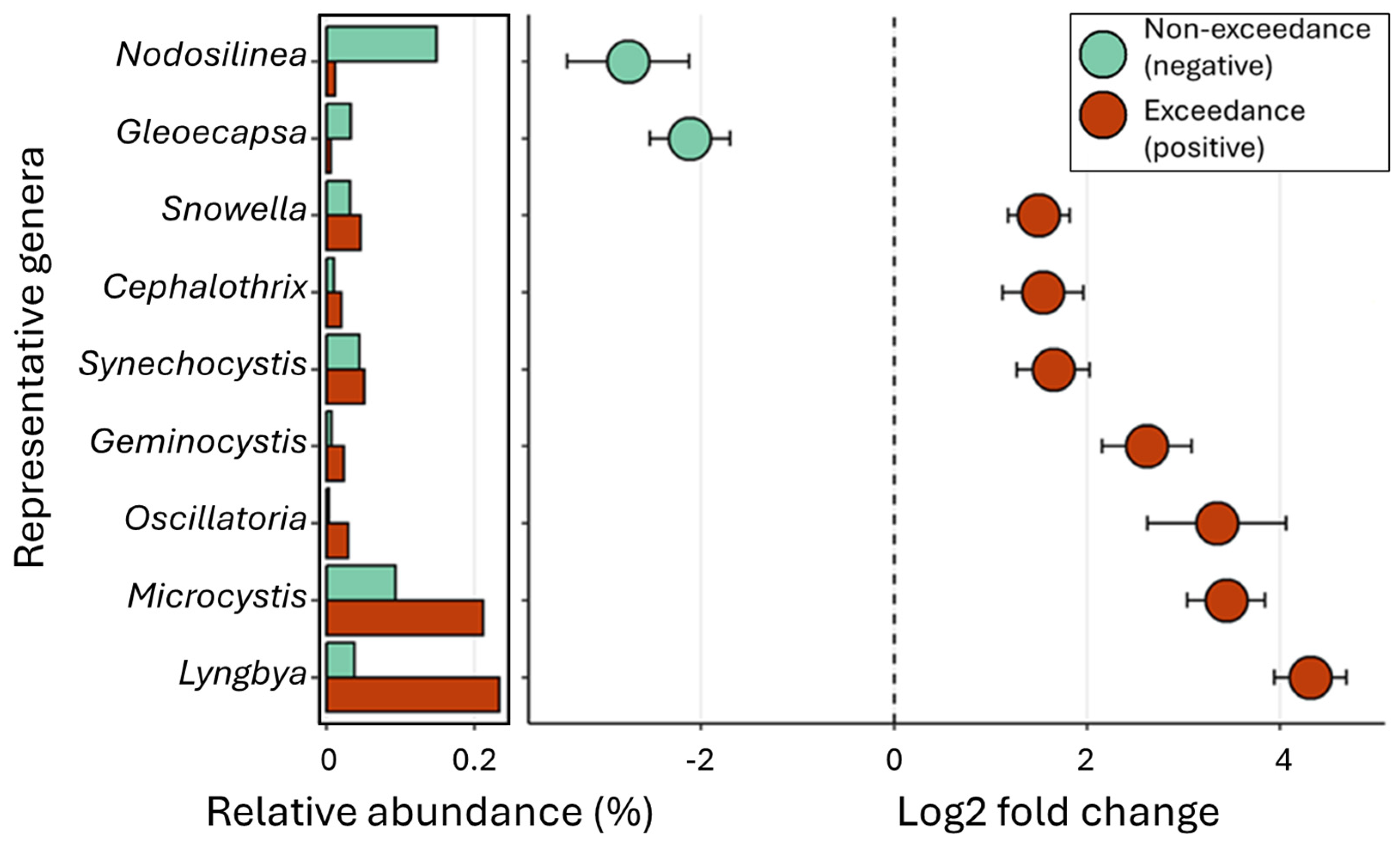
2.7. Characterization of Microcystin-Producing Cyanobiome
3. Discussion
4. Conclusions
- Smaller inland lakes can have different environmental conditions than larger lake systems, leading to regional or environmentally specific taxonomic and molecular profiles.
- Generic cyanobacterial toxin detection technologies may not fully assess the whole spectrum of microcystin congeners and other toxins, which may lead to an underestimation of cyanotoxin production.
- Microcystin concentration may not be an effective predictor/indicator of other cyanotoxins, including saxitoxin, underscoring the importance of incorporating site-specific molecular testing strategies in environmental monitoring programs.
- Rivers and associated receiving lake waters can have similar microbial profiles, indicating a continuum of cyanobacterial seeding into lake ecosystems.
- Dolichospermum, Pseudanabaena, Nodosilinea, and Cyanobium show a regionally specific relationship with the saxitoxin gene levels, indicating their site-specific role in toxin production.
- Microcystis and Planktothrix were consistently detected in all the tested sites among cHAB species, suggesting their dominance in bloom formation for the Great Lakes.
- The taxonomic and functional cyanobacterial trends identified in this study can augment current recreational water monitoring programs for site/region-specific cHAB testing.
5. Materials and Methods
5.1. Study Design
5.2. Sample Preprocessing and Nucleic Acid Extraction
5.3. Enzyme-Linked Immunosorbent Assay for Microcystin Quantification
5.4. Analyses of Cyanotoxins and Other Bioactive Secondary Metabolites
5.5. Quantitative/Real-Time PCR for Cyanotoxins
5.6. Primer Design and Metabarcoding Sequencing Library Preparation
5.7. Data Analysis and Bioinformatics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bekker, A.; Holland, H.D.; Wang, P.-L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, P. Effects of rising atmospheric CO2 levels on physiological response of cyanobacteria and cyanobacterial bloom development: A review. Sci. Total Environ. 2021, 754, 141889. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, R.U.; Hu, Z.; Zhang, Y.; Lu, J. Cyanobacterial community succession and associated cyanotoxin production in hypereutrophic and eutrophic freshwaters. Environ. Pollut. 2021, 290, 118056. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal Bloom and Its Economic Impact. European Commission; Joint Research Centre Institute for Environment and Sustainability: Ispra, Italy, 2016; p. 51. [Google Scholar]
- Hudnell, H.K. (Ed.) Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Louati, I.; Pascault, N.; Debroas, D.; Bernard, C.; Humbert, J.F.; Leloup, J. Structural diversity of bacterial communities associated with bloom-forming freshwater cyanobacteria differs according to the cyanobacterial genus. PLoS ONE 2015, 10, e0140614. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada (ECCC). Great Lakes Protection. 2019. Available online: https://www.canada.ca/en/environment-climate-change/services/great-lakes-protection.html (accessed on 1 January 2024).
- Great Lakes Commission (GLC). Great Lakes Commission Federal Priorities. 2017. Available online: https://www.canada.ca/en/environment-climatechange/ (accessed on 1 January 2024).
- Steffen, M.M.; Belisle, B.S.; Watson, S.B.; Boyer, G.L.; Wilhelm, S.W. Status, causes and controls of cyanobacterial blooms in Lake Erie. J. Great Lakes Res. 2014, 40, 215–225. [Google Scholar] [CrossRef]
- Bartlett, S.L.; Brunner, S.L.; Klump, J.V.; Houghton, E.M.; Miller, T.R. Spatial analysis of toxic or otherwise bioactive cyanobacterial peptides in Green Bay, Lake Michigan. J. Great Lakes Res. 2018, 44, 924–933. [Google Scholar] [CrossRef]
- Makarewicz, J.C.; Boyer, G.L.; Lewis, T.W.; Guenther, W.; Atkinson, J.; Arnold, M. Spatial and temporal distribution of the cyanotoxin microcystin-LR in the Lake Ontario ecosystem: Coastal embayments, rivers, nearshore and offshore, and upland lakes. J. Great Lakes Res. 2009, 35, 83–89. [Google Scholar] [CrossRef]
- Sterner, R.W.; Reinl, K.L.; Lafrancois, B.M.; Brovold, S.; Miller, T.R. A first assessment of cyanobacterial blooms in oligotrophic Lake Superior. Limnol. Oceanogr. 2020, 65, 2984–2998. [Google Scholar] [CrossRef]
- Health Canada. Guidelines for Canadian Recreational Water Quality-Cyanobacteria and Their Toxins. 2022. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidance-canadian-recreational-water-quality-cyanobacteria-toxins.html#a6.4 (accessed on 1 January 2024).
- May, N.W.; Olson, N.E.; Panas, M.; Axson, J.L.; Tirella, P.S.; Kirpes, R.M.; Craig, R.L.; Gunsch, M.J.; China, S.; Laskin, A.; et al. Aerosol emissions from great lakes harmful algal blooms. Environ. Sci. Technol. 2018, 52, 397–405. [Google Scholar] [CrossRef]
- Qian, S.S.; Chaffin, J.D.; DuFour, M.R.; Sherman, J.J.; Golnick, P.C.; Collier, C.D.; Nummer, S.A.; Margida, M.G. Quantifying and reducing uncertainty in estimated microcystin concentrations from the ELISA method. Environ. Sci. Technol. 2015, 49, 14221–14229. [Google Scholar] [CrossRef] [PubMed]
- Zastepa, A.; Pick, F.R.; Blais, J.M.; Saleem, A. Analysis of intracellular and extracellular microcystin variants in sediments and pore waters by accelerated solvent extraction and high performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 872, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Jiang, J.L.; Atrache, R.; Paschos, A.; Edge, T.A.; Schellhorn, H.E. Cyanobacterial Algal bloom monitoring: Molecular methods and technologies for freshwater Ecosystems. Microorganisms 2023, 11, 851. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The diversity of cyanobacterial toxins on structural characterization, distribution and identification: A systematic review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef]
- Guedes, I.A.; da Costa Leite, D.M.; Manhães, L.A.; Bisch, P.M.; Azevedo, S.M.; Pacheco, A.B.F. Fluctuations in microcystin concentrations, potentially toxic Microcystis and genotype diversity in a cyanobacterial community from a tropical reservoir. Harmful Algae 2014, 39, 303–309. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Botts, S.R.; Paschos, A.; Schellhorn, H.E. Temporal and spatial changes in bacterial diversity in mixed use watersheds of the Great Lakes region. J. Great Lakes Res. 2019, 45, 109–118. [Google Scholar] [CrossRef]
- Gale, J.; Sweeney, C.; Paver, S.; Coleman, M.L.; Thompson, A.W. Diverse and variable community structure of picophytoplankton across the Laurentian Great Lakes. Limnol. Oceanogr. 2023, 68, 2327–2345. [Google Scholar] [CrossRef]
- Li, H.; Alsanea, A.; Barber, M.; Goel, R. High-throughput DNA sequencing reveals the dominance of pico-and other filamentous cyanobacteria in an urban freshwater Lake. Sci. Total Environ. 2019, 661, 465–480. [Google Scholar] [CrossRef]
- Alves-de-Souza, C.; Benevides, T.S.; Santos, J.B.; Von Dassow, P.; Guillou, L.; Menezes, M. Does environmental heterogeneity explain temporal β diversity of small eukaryotic phytoplankton? Example from a tropical eutrophic coastal lagoon. J. Plankton Res. 2017, 39, 698–714. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X.; Chen, F.; Yang, Z.; Yu, Y. The underlying causes and effects of phytoplankton seasonal turnover on resource use efficiency in freshwater lakes. Ecol. Evol. 2021, 11, 8897–8909. [Google Scholar] [CrossRef]
- Crevecoeur, S.; Edge, T.A.; Watson, L.C.; Watson, S.B.; Greer, C.W.; Ciborowski, J.J.; Diep, N.; Dove, A.; Drouillard, K.G.; Frenken, T.; et al. Spatio-temporal connectivity of the aquatic microbiome associated with cyanobacterial blooms along a Great Lake riverine-lacustrine continuum. Front. Microbiol. 2023, 14, 1073753. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Borges, H.; Puddick, J.; Biessy, L.; Atalah, J.; Hawes, I.; Dietrich, D.R.; Hamilton, D.P. Contrasting cyanobacterial communities and microcystin concentrations in summers with extreme weather events: Insights into potential effects of climate change. Hydrobiologia 2017, 785, 71–89. [Google Scholar] [CrossRef]
- Pineda-Mendoza, R.M.; Zúñiga, G.; Martínez-Jerónimo, F. Microcystin production in Microcystis aeruginosa: Effect of type of strain, environmental factors, nutrient concentrations, and N: P ratio on mcyA gene expression. Aquat. Ecol. 2016, 50, 103–119. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, F.; Zeng, J.; Huang, R.; Yu, Z.; Wu, Q.L. Network analysis reveals seasonal variation of co-occurrence correlations between Cyanobacteria and other bacterioplankton. Sci. Total Environ. 2016, 573, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Litchman, E. Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia 2016, 763, 357–369. [Google Scholar] [CrossRef]
- Ryan, C.N.; Thomas, M.K.; Litchman, E. The effects of phosphorus and temperature on the competitive success of an invasive cyanobacterium. Aquat. Ecol. 2017, 51, 463–472. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Yu, Y.; Shi, X. Temperature triggers the annual cycle of Microcystis, comparable results from the laboratory and a large shallow lake. Chemosphere 2020, 260, 127543. [Google Scholar] [CrossRef]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610, 786–795. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Westrick, J.A.; Furr, E.; Birbeck, J.A.; Reitz, L.A.; Stanislawczyk, K.; Li, W.; Weber, P.K.; Bridgeman, T.B.; Davis, T.W.; et al. Quantification of microcystin production and biodegradation rates in the western basin of Lake Erie. Limnol. Oceanogr. 2022, 67, 1470–1483. [Google Scholar] [CrossRef]
- Parveen, B.; Mary, I.; Vellet, A.; Ravet, V.; Debroas, D. Temporal dynamics and phylogenetic diversity of free-living and particle-associated Verrucomicrobia communities in relation to environmental variables in a mesotrophic lake. FEMS Microbiol. Ecol. 2013, 83, 189–201. [Google Scholar] [CrossRef]
- Ghai, R.; Mizuno, C.M.; Picazo, A.; Camacho, A.; Rodriguez-Valera, F. Key roles for freshwater A ctinobacteria revealed by deep metagenomic sequencing. Mol. Ecol. 2014, 23, 6073–6090. [Google Scholar] [CrossRef]
- Hudon, C.; De Sève, M.; Cattaneo, A. Increasing occurrence of the benthic filamentous cyanobacterium Lyngbya wollei: A symptom of freshwater ecosystem degradation. Freshw. Sci. 2014, 33, 606–618. [Google Scholar] [CrossRef]
- Zastepa, A.; Chemali, C. Bloom announcement: Late season cyanobacterial blooms co-dominated by Microcystis flos-aquae, Lyngbya birgei, and Aphanizomenon flos-aquae complex in Hamilton Harbour (Lake Ontario), an area of concern impacted by industrial effluent and residential wastewater. Data Brief 2021, 35, 106800. [Google Scholar]
- Bridgeman, T.B.; Penamon, W.A. Lyngbya wollei in western Lake Erie. J. Great Lakes Res. 2010, 36, 167–171. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Vijayavel, K.; Sadowsky, M.J.; Ferguson, J.A.; Kashian, D.R. The establishment of the nuisance cyanobacteria Lyngbya wollei in Lake St. Clair and its potential to harbor fecal indicator bacteria. J. Great Lakes Res. 2013, 39, 560–568. [Google Scholar] [CrossRef]
- Dreher, T.W.; Collart, L.P.; Mueller, R.S.; Halsey, K.H.; Bildfell, R.J.; Schreder, P.; Sobhakumari, A.; Ferry, R. Anabaena/Dolichospermum as the source of lethal microcystin levels responsible for a large cattle toxicosis event. Toxicon X 2019, 1, 100003. [Google Scholar] [CrossRef]
- Kramer, B.J.; Hem, R.; Gobler, C.J. Elevated CO2 significantly increases N2 fixation, growth rates, and alters microcystin, anatoxin, and saxitoxin cell quotas in strains of the bloom-forming cyanobacteria, Dolichospermum. Harmful Algae 2022, 120, 102354. [Google Scholar] [CrossRef]
- Zaffiro, A.; Rosenblum, L.; Wendelken, S.C.; Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by Adda EnzymeLinked Immunosorbent Assay. United States Environmental Protection Agency. 2016. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/method-546-determinationtotal-microcystins-nodularins-drinking-water-ambient-water-adda-enzyme-linkedimmunosorbent-assay.pdf (accessed on 1 January 2024).
- Zastepa, A.; Westrick, J.A.; Liang, A.; Birbeck, J.A.; Furr, E.; Watson, L.C.; Stockdill, J.L.; Ramakrishna, B.S.; Crevecoeur, S. Broad screening of toxic and bioactive metabolites in cyanobacterial and harmful algal blooms in Lake of the Woods (Canada and USA), 2016–2019. J. Great Lakes Res. 2023, 49, 134–146. [Google Scholar] [CrossRef]
- Zastepa, A.; Adzija, H.; Westrick, J.A.; Crevecoeur, S. Unravelling the microbial and chemical ecology of deep chlorophyll layers in Lake Ontario: Insights from microscopy, high-throughput DNA sequencing and high-resolution mass spectrometry. J. Aquat. Ecosyst. Health Manag. (AEHM), 2024; in press. [Google Scholar]
- Lu, J.; Struewing, I.; Wymer, L.; Tettenhorst, D.R.; Shoemaker, J.; Allen, J. Use of qPCR and RT-qPCR for monitoring variations of microcystin producers and as an early warning system to predict toxin production in an Ohio inland lake. Water Res. 2020, 170, 115262. [Google Scholar] [CrossRef]
- Schwaber, J.; Andersen, S.; Nielsen, L. Shedding light: The importance of reverse transcription efficiency standards in data interpretation. Biomol. Detect. Quantif. 2020, 17, 100077. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, A.-D.; Neilan, B.A. Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria. Arch. Microbiol. 2006, 185, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- McDonald, D.; Jiang, Y.; Balaban, M.; Cantrell, K.; Zhu, Q.; Gonzalez, A.; Morton, J.T.; Nicolaou, G.; Parks, D.H.; Karst, S.M.; et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 2024, 42, 715–718. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.M.; Sienkiewicz, N.; Struewing, I.; Stelzer, E.A.; Graham, J.L.; Lu, J. Metagenomic mapping of cyanobacteria and potential cyanotoxin producing taxa in large rivers of the United States. Sci. Rep. 2023, 13, 2806. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Sun, Y.; Ip, L.Y.T.; Wang, L.; Chan, F.K.L.; Miao, Y.; Ng, S.C. Prevotella species in the human gut is primarily comprised of Prevotella copri, Prevotella stercorea and related lineages. Sci. Rep. 2022, 12, 9055. [Google Scholar] [CrossRef]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Introduction to the Microbiome R Package. 2018. Available online: https://microbiome.github.io/tutorials (accessed on 1 January 2023).
- Yazici, B.; Yolacan, S. A comparison of various tests of normality. J. Stat. Comput. Simul. 2007, 77, 175–183. [Google Scholar] [CrossRef]
- Zhang, X.; Mallick, H.; Yi, N. Zero-inflated negative binomial regression for differential abundance testing in microbiome studies. J. Bioinform. Genom. 2015, 2. [Google Scholar] [CrossRef]
- Hawinkel, S.; Mattiello, F.; Bijnens, L.; Thas, O. A broken promise: Microbiome differential abundance methods do not control the false discovery rate. Brief. Bioinform. 2019, 20, 210–221. [Google Scholar] [CrossRef]



| Microcystin Congener/ Cyanotoxin | Hamilton Harbour (Detection Frequency) | Bay of Quinte (Detection Frequency) | Three Mile Lake (Detection Frequency) |
|---|---|---|---|
| MC-RR | 95% | 30% | ND |
| MC-YR | 79% | ND | ND |
| MC-HtyR | ND | ND | ND |
| MC-LR | 89% | 30% | ND |
| MC-HilR | ND | ND | ND |
| MC-WR | ND | ND | ND |
| MC-LA | 5% | 10% | ND |
| MC-LY | ND | ND | ND |
| MC-LW | ND | ND | ND |
| MC-LF | ND | ND | ND |
| Anabaenopeptin B | 26% | ND | 6% |
| Anabaenopeptin A | 37% | ND | ND |
| Oscillamide Y | 37% | ND | ND |
| Anatoxin A | 63% | ND | ND |
| Cylindrospermopsin | ND | ND | ND |
| Cyanobacteria/Cyanotoxin Metrics | Correlation Coefficient | p-Value | |
|---|---|---|---|
| Microcystin | mcyE Gene Copies | 0.71 | 7.9−7 |
| Cyanobacteria 16S | 0.50 | 1.4−3 | |
| sxtA Gene Copies | 0.23 | 0.34 | |
| mcyE Transcripts | 0.52 | 4.4−3 | |
| mcyE Transcripts | mcyE Gene Copies | 0.62 | 5.8−4 |
| Cyanobacteria 16S | 0.31 | 0.08 | |
| sxtA Gene Copies | 0.05 | 0.72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, F.; Jiang, J.L.; Li, E.; Tran, K.; Boere, A.; Rahman, M.; Paschos, A.; Westrick, J.A.; Zastepa, A.; Edge, T.A.; et al. Regional and Longitudinal Dynamics of Cyanobacterial Blooms/Cyanobiome and Cyanotoxin Production in the Great Lakes Area. Toxins 2024, 16, 471. https://doi.org/10.3390/toxins16110471
Saleem F, Jiang JL, Li E, Tran K, Boere A, Rahman M, Paschos A, Westrick JA, Zastepa A, Edge TA, et al. Regional and Longitudinal Dynamics of Cyanobacterial Blooms/Cyanobiome and Cyanotoxin Production in the Great Lakes Area. Toxins. 2024; 16(11):471. https://doi.org/10.3390/toxins16110471
Chicago/Turabian StyleSaleem, Faizan, Jennifer L. Jiang, Enze Li, Kevin Tran, Adam Boere, Mahbuba Rahman, Athanasios Paschos, Judy A. Westrick, Arthur Zastepa, Thomas A. Edge, and et al. 2024. "Regional and Longitudinal Dynamics of Cyanobacterial Blooms/Cyanobiome and Cyanotoxin Production in the Great Lakes Area" Toxins 16, no. 11: 471. https://doi.org/10.3390/toxins16110471
APA StyleSaleem, F., Jiang, J. L., Li, E., Tran, K., Boere, A., Rahman, M., Paschos, A., Westrick, J. A., Zastepa, A., Edge, T. A., & Schellhorn, H. E. (2024). Regional and Longitudinal Dynamics of Cyanobacterial Blooms/Cyanobiome and Cyanotoxin Production in the Great Lakes Area. Toxins, 16(11), 471. https://doi.org/10.3390/toxins16110471






