In Situ Modification of Reverse Osmosis Membrane Elements for Enhanced Removal of Multiple Micropollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
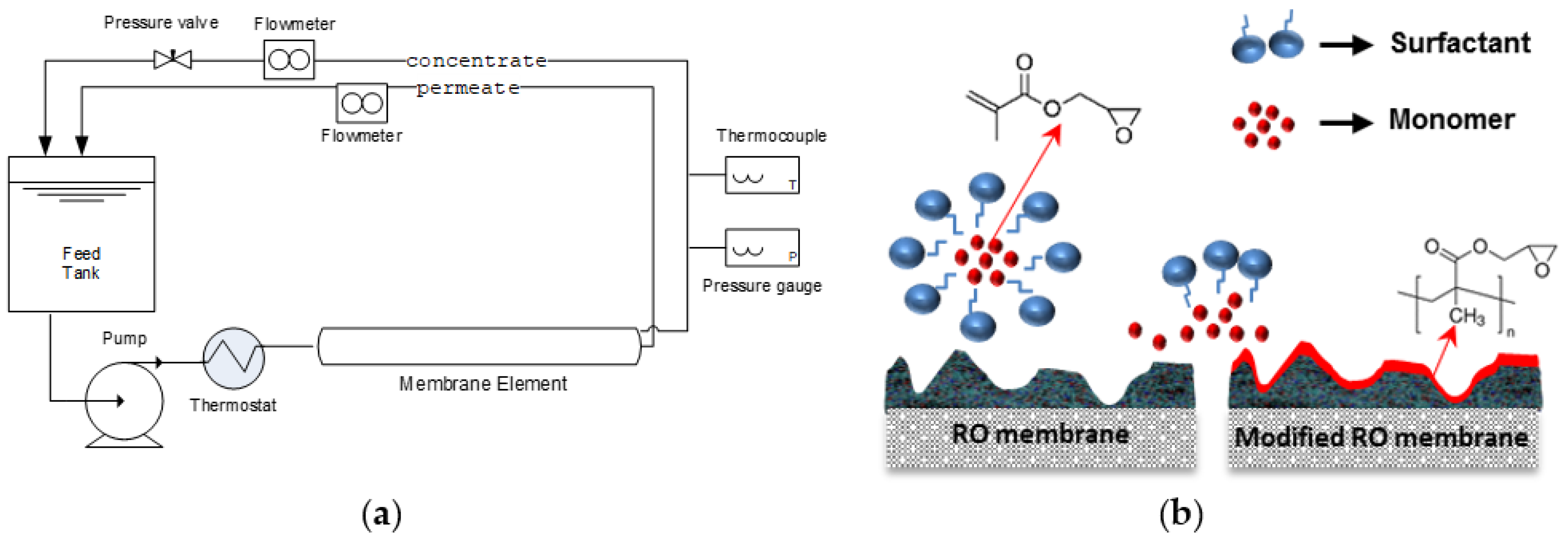
2.2. Membrane Modification
2.3. Membrane Characterization
2.4. Membrane Testing
3. Results and Discussion
3.1. Membrane Surface Characteristics, Morphology, and Uniformity
3.2. Membrane Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oulton, R.L.; Kohn, T.; Cwiertny, D.M. Pharmaceuticals and personal care products in effluent matrices: A survey of transformation and removal during wastewater treatment and implications for wastewater management. J. Environ. Monit. 2010, 12, 1956–1978. [Google Scholar] [CrossRef] [PubMed]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Plakas, K.V.; Karabelas, A.J. Removal of pesticides from water by NF and RO membranes—A review. Desalination 2012, 287, 255–265. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Kimura, K.; Toshima, S.; Amy, G.; Watanabe, Y. Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical active compounds (PhACs) by RO membranes. J. Membr. Sci. 2004, 245, 71–78. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Colborn, T.; Saal, F.S.V.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Kreuzinger, N. Carbamazepine as a possible anthropogenic marker in the aquatic environment: Investigations on the behaviour of carbamazepine in wastewater treatment and during groundwater infiltration. Water Res. 2004, 38, 947–954. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I. Critical risk points of nanofiltration and reverse osmosis processes in water recycling applications. Desalination 2006, 187, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Mamo, J.; García-Galán, M.J.; Stefani, M.; Rodríguez-Mozaz, S.; Barceló, D.; Monclús, H.; Rodriguez-Roda, I.; Comas, J. Fate of pharmaceuticals and their transformation products in integrated membrane systems for wastewater reclamation. Chem. Eng. J. 2018, 331, 450–461. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Kong, F.-X.; Wang, Z.; Yang, H.-W.; Wang, X.-M.; Xie, Y.F.; Waite, T.D. Role of membrane and compound properties in affecting the rejection of pharmaceuticals by different RO/NF membranes. Front. Environ. Sci. Eng. 2017, 11, 20. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Drewes, J.E.; Bellona, C.; Oedekoven, M.; Xu, P.; Kim, T.U.; Amy, G. Rejection of wastewater-derived micropollutants in high-pressure membrane applications leading to indirect potable reuse. Environ. Prog. 2005, 24, 400–409. [Google Scholar] [CrossRef]
- Drewes, J.E.; Heberer, T.; Reddersen, K. Fate of pharmaceuticals during indirect potable reuse. Water Sci. Technol. 2002, 46, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Amy, G.; Drewes, J.E.; Heberer, T.; Kim, T.U.; Watanabe, Y. Rejection of organic micropollutants (disinfection by-products, endocrine disrupting compounds, and pharmaceutically active compounds) by NF/RO membranes. J. Membr. Sci. 2003, 227, 113–121. [Google Scholar] [CrossRef]
- Kiso, Y.; Nishimura, Y.; Kitao, T.; Nishimura, K. Rejection properties of non-phenylic pesticides with nanofiltration membranes. J. Membr. Sci. 2000, 171, 229–237. [Google Scholar] [CrossRef]
- Kiso, Y.; Mizuno, A.; Othman, R.A.A.B.; Jung, Y.J.; Kumano, A.; Ariji, A. Rejection properties of pesticides with a hollow fiber NF membrane (HNF-1). Desalination 2002, 143, 147–157. [Google Scholar] [CrossRef]
- Kimura, K.; Amy, G.; Drewes, J.; Watanabe, Y. Adsorption of hydrophobic compounds onto NF/RO membranes: An artifact leading to overestimation of rejection. J. Membr. Sci. 2003, 221, 89–101. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A. Adsorption and Transport of Trace Contaminant Estrone in NF/RO Membranes. Environ. Eng. Sci. 2002, 19, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Nghiem, L.D.; Schafer, A.I.; Elimelech, M. Pharmaceutical retention mechanisms by nanofiltration membranes. Environ. Sci. Technol. 2005, 39, 7698–7705. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Removal of natural hormones by nanofiltration membranes: Measurements, modeling, and mechanisms. Environ. Sci. Technol. 2004, 38, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, A.; Bason, S.; Jopp, J.; Oren, Y.; Freger, V. Partitioning of organic solutes between water and polyamide layer of RO and NF membranes: Correlation to rejection. J. Membr. Sci. 2006, 281, 480–490. [Google Scholar] [CrossRef]
- Ben-David, A.; Bernstein, R.; Oren, Y.; Belfer, S.; Dosoretz, C.; Freger, V. Facile surface modification of nanofiltration membranes to target the removal of endocrine-disrupting compounds. J. Membr. Sci. 2010, 357, 152–159. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Dielectric exclusion of ions from membranes. Adv. Colloid Interface Sci. 2000, 85, 193–230. [Google Scholar] [CrossRef]
- Fridman-Bishop, N.; Tankus, K.A.; Freger, V. Permeation mechanism and interplay between ions in nanofiltration. J. Membr. Sci. 2018, 548, 449–458. [Google Scholar] [CrossRef]
- Lanteri, Y.; Fievet, P.; Szymczyk, A. Evaluation of the steric, electric, and dielectric exclusion model on the basis of salt rejection rate and membrane potential measurements. J. Colloid Interface Sci. 2009, 331, 148–155. [Google Scholar] [CrossRef]
- Levchenko, S.; Freger, V. Breaking the symmetry: Mitigating scaling in tertiary treatment of waste effluents using a positively charged nanofiltration membrane. Environ. Sci. Technol. Lett. 2016, 3, 339–343. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Non-steric mechanisms of nanofiltration: Superposition of Donnan and dielectric exclusion. Sep. Purif. Technol. 2001, 22–23, 143–158. [Google Scholar] [CrossRef]
- Kabay, N.; Bryjak, M.; Hilal, N.; Yoshizuka, K.; Nishihama, S. Boron Separation Processes; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Prats, D.; Chillon-Arias, M.F.; Rodriguez-Pastor, M. Analysis of the influence of pH and pressure on the elimination of boron in reverse osmosis. Desalination 2000, 128, 269–273. [Google Scholar] [CrossRef]
- Bernstein, R.; Belfer, S.; Freger, V. Toward improved boron removal in RO by membrane modification: Feasibility and challenges. Environ. Sci. Technol. 2011, 45, 3613–3620. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, A.; León, F.A.; Ramos-Martín, A. Different boron rejection behavior in two RO membranes installed in the same full-scale SWRO desalination plant. Desalination 2019, 449, 131–138. [Google Scholar] [CrossRef]
- Bernstein, R.; Belfer, S.; Freger, V. Surface modification of dense membranes using radical graft polymerization enhanced by monomer filtration. Langmuir 2010, 26, 12358–12365. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, P.K.; Lee, C.H.; Kwon, H.H. Surface modification of nanofiltration membranes to improve the removal of organic micro-pollutants (EDCs and PhACs) in drinking water treatment: Graft polymerization and cross-linking followed by functional group substitution. J. Membr. Sci. 2008, 321, 190–198. [Google Scholar] [CrossRef]
- Guo, H.; Deng, Y.; Tao, Z.; Yao, Z.; Wang, J.; Lin, C.; Zhang, T.; Zhu, B.; Tang, C.Y. Does Hydrophilic Polydopamine Coating Enhance Membrane Rejection of Hydrophobic Endocrine-Disrupting Compounds? Environ. Sci. Technol. Lett. 2016, 3, 332–338. [Google Scholar] [CrossRef]
- Bernstein, R.; Belfer, S.; Freger, V. Improving performance of spiral wound RO elements by in situ concentration polarization-enhanced radical graft polymerization. J. Membr. Sci. 2012, 405–406, 79–84. [Google Scholar] [CrossRef]
- Baransi-Karkaby, K.; Bass, M.; Levchenko, S.; Eitan, S.; Freger, V. Facile modification of reverse osmosis membranes by surfactant-assisted acrylate grafting for enhanced selectivity. Environ. Sci. Technol. 2017, 51, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.lenntech.com/Data-sheets/Hydranautics-ESPA-2521-L.pdf (accessed on 12 February 2019).
- Staples, C.A.; Dom, P.B.; Klecka, G.M.; Sandra, T.O.; Harris, L.R. A review of the environmental fate, effects, and exposures of Bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; de la Nuez-Pestana, I. A computational tool for designing BWRO systems with spiral wound modules. Desalination 2018, 426, 69–77. [Google Scholar] [CrossRef]
- Schock, G.; Miquel, A. Mass transfer and pressure loss in spiral wound modules. Desalination 1987, 64, 339–352. [Google Scholar] [CrossRef]
- Sutzkover, I.; Hasson, D.; Semiat, R. Simple technique for measuring the concentration polarization level in a reverse osmosis system. Desalination 2000, 131, 117–127. [Google Scholar] [CrossRef]
- Avlonitis, S.; Hanbury, W.T.; Boudinar, M.B. Spiral Wound Modules Performance an Analytical Solution: Part II. Desalination 1993, 89, 227–246. [Google Scholar] [CrossRef]
- Bason, S.; Kedem, O.; Freger, V. Determination of concentration-dependent transport coefficients in nanofiltration: Experimental evaluation of coefficients. J. Membr. Sci. 2009, 326, 197–204. [Google Scholar] [CrossRef]
- Ben-David, A.; Oren, Y.; Freger, V. Thermodynamic factors in partitioning and rejection of organic compounds by polyamide composite membranes. Environ. Sci. Technol. 2006, 40, 7023–7028. [Google Scholar] [CrossRef]
- Bass, M.; Freger, V. Facile evaluation of coating thickness on membranes using ATR-FTIR. J. Membr. Sci. 2015, 492, 348–354. [Google Scholar] [CrossRef]
- Verliefde, A.; Cornelissen, E.; Amy, G.; van der Bruggen, B.; van Dijk, H. Priority organic micropollutants in water sources in Flanders and the Netherlands and assessment of removal possibilities with nanofiltration. Environ. Pollut. 2007, 146, 281–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Causserand, C.; Aimar, P.; Cravedi, J.P. Removal of bisphenol A by a nanofiltration membrane in view of drinking water production. Water Res. 2006, 40, 3793–3799. [Google Scholar] [CrossRef] [Green Version]
- Drazevic, E.; Bason, S.; Kosutic, K.; Freger, V. Enhanced partitioning and transport of phenolic micropollutants within polyamide composite membranes. Environ. Sci. Technol. 2012, 46, 3377–3383. [Google Scholar] [CrossRef]
- Williams, M.E.; Hestekin, J.A.; Smothers, C.N.; Bhattacharyya, D. Separation of Organic Pollutants by Reverse Osmosis and Nanofiltration Membranes: Mathematical Models and Experimental Verification. Ind. Eng. Chem. Res. 1999, 38, 3683–3695. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Flux decline during nanofiltration of organic components in aqueous solution. Environ. Sci. Technol. 2001, 35, 3535–3540. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Drewes, J.E.; Heil, D. Beneficial use of co-produced water through membrane treatment: Technical-economic assessment. Desalination 2008, 225, 139–155. [Google Scholar] [CrossRef]
- Redondo, J.; Busch, M.; de Witte, J.P. Boron removal from seawater using FILMTEC high rejection SWRO membranes. Desalination 2003, 156, 229–238. [Google Scholar] [CrossRef]
- Murray-Gulde, C.; Heatley, J.E.; Karanfil, T.; Rodgers, J.H.; Myers, J.E. Performance of a hybrid reverse osmosis-constructed wetland treatment system for brackish oil field produced water. Water Res. 2003, 37, 705–713. [Google Scholar] [CrossRef]






| Characteristics | Bisphenol-A Plastic Additive, EDC | Carbamazepine Drug, PhAC | Acetaminophen Drug, PhAC |
|---|---|---|---|
| Abbreviation | BPA | CBZ | ACN |
| Molecular weight | 228 | 236.3 | 151 |
| Solubility in water (mg/L) * | 120 | 18 | 14,000 |
| Log Kow * | 3.32 | 2.45 | 0.46 |
| pKa | 9.6–10.2 ** | 13.9 *** | 9.4 *** |
| Chemical structure |  |  |  |
| Before/After Modificaton | Solute Passage, % | Hydraulic Permeability Lp, L/m2 h bar | |||
|---|---|---|---|---|---|
| CBZ | BPA | ACN | DW | CBZ/BPA/ACN Solution | |
| Before modification | 0.33 ± 0.06 | 0.34 ± 0.05 | 0.7 ± 0.06 | 6.5 ± 0.05 | 1.8 ± 0.3 |
| After modification | n.d. | 0.09 ± 0.007 | 0.41 ± 0.03 | 3.5 ± 0.1 | 1.4 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baransi-Karkaby, K.; Bass, M.; Freger, V. In Situ Modification of Reverse Osmosis Membrane Elements for Enhanced Removal of Multiple Micropollutants. Membranes 2019, 9, 28. https://doi.org/10.3390/membranes9020028
Baransi-Karkaby K, Bass M, Freger V. In Situ Modification of Reverse Osmosis Membrane Elements for Enhanced Removal of Multiple Micropollutants. Membranes. 2019; 9(2):28. https://doi.org/10.3390/membranes9020028
Chicago/Turabian StyleBaransi-Karkaby, Katie, Maria Bass, and Viatcheslav Freger. 2019. "In Situ Modification of Reverse Osmosis Membrane Elements for Enhanced Removal of Multiple Micropollutants" Membranes 9, no. 2: 28. https://doi.org/10.3390/membranes9020028
APA StyleBaransi-Karkaby, K., Bass, M., & Freger, V. (2019). In Situ Modification of Reverse Osmosis Membrane Elements for Enhanced Removal of Multiple Micropollutants. Membranes, 9(2), 28. https://doi.org/10.3390/membranes9020028





