Isothermal Sections of the Ni-Cr-Ta Ternary System at 1200 °C and 1300 °C
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Microstructure
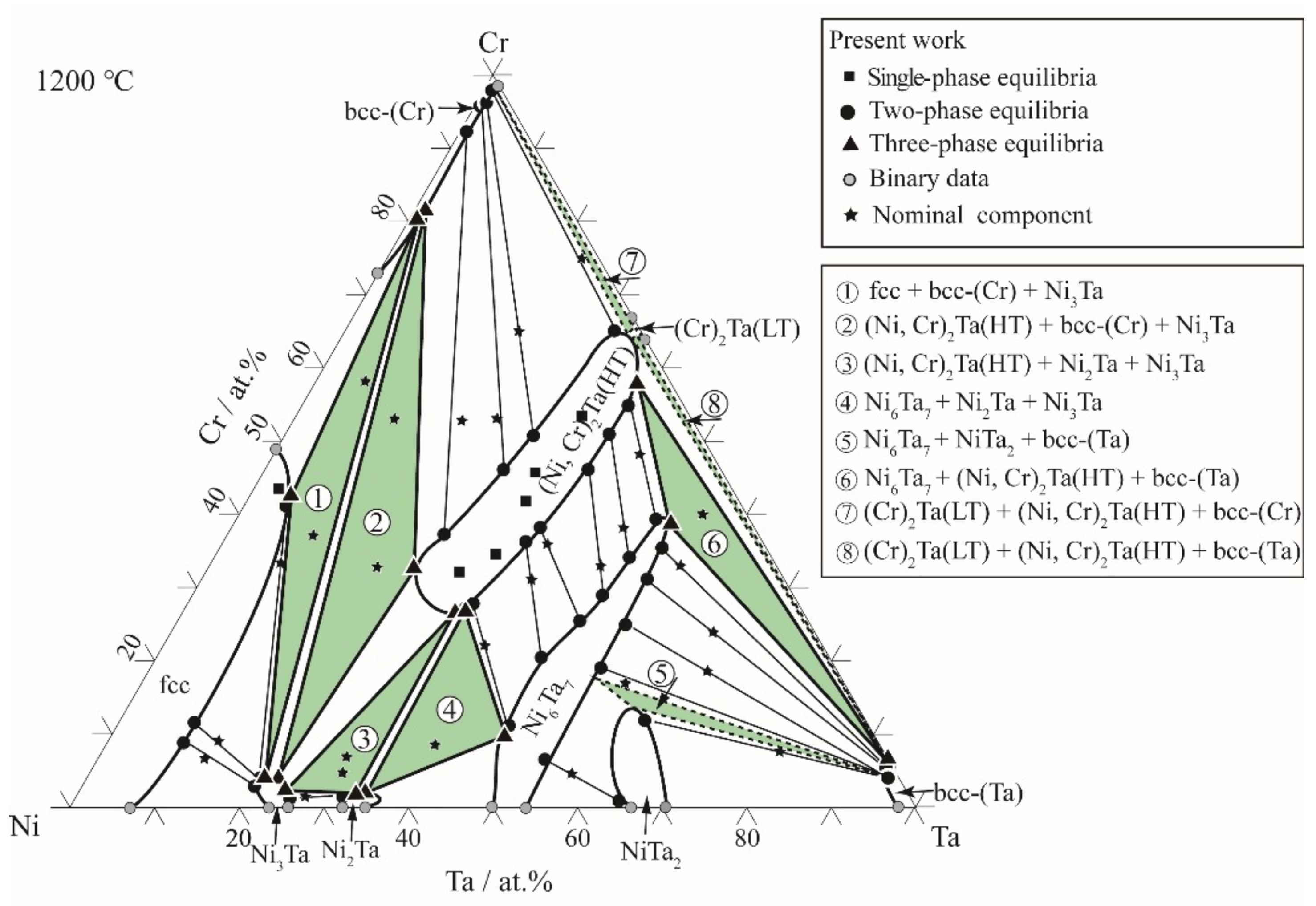
3.2. Isothermal Sections
3.3. The Liquid Region
4. Conclusions
- (1)
- The solubility of Cr in Ni6Ta7 phase was about 41.6 at. % at 1300 °C, and no ternary compound was found at two sections.
- (2)
- The high temperature (Ni, Cr)2Ta(HT) (MgZn2-type) phase with a large composition range was determined at both two temperatures, which was stabilized by the Ni addition to Cr–Ta alloys against low temperature, and its solubility increased as temperature raise from 1200 °C to 1300 °C.
- (3)
- A small liquid region was confirmed at 1300 °C, while it disappeared at 1200 °C. The results indicate that the addition of Ta reduced the melting point of the Ni–Cr alloys.
Author Contributions
Funding
Conflicts of Interest
References
- Erickson, G.L. A new, third-generation, single-crystal, casting superalloy. JOM 1995, 47, 36–39. [Google Scholar] [CrossRef]
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, Microstructure and Properties. J. Propul. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Jena, A.K.; Chaturvedi, M.C. The role of alloying elements in the design of nickel-base superalloys. J. Matrl. Sci. 1984, 19, 3121–3139. [Google Scholar] [CrossRef]
- Xu, X.J.; Wu, Q.; Gong, S.K.; Li, S.S. Effect of Cr and Re on the oxidation resistance of Ni3Al-base single crystal alloy IC21 at 1100 °C. High Perform. Struct. Mater. 2013, 748, 582–587. [Google Scholar]
- Zheng, L.; Zhang, G.; Lee, T.L.; Gorley, M.J.; Wang, Y.; Xiao, C.; Li, Z. The effects of Ta on the stress rupture properties and microstructural stability of a novel Ni-base superalloy for land-based high temperature applications. Mater. Des. 2014, 61, 61–69. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, Z.; Noebe, R.D.; Seidman, D.N. The effects of refractory elements on Ni-excesses and Ni-depletions at γ(fcc)/γ′(L12) interfaces in model Ni-based superalloys: Atom-probe tomographic experiments and first-principles calculations. Acta Mater. 2016, 121, 288–298. [Google Scholar] [CrossRef]
- Eliaz, N.; Shemesh, G.; Latanision, R.M. Hot corrosion in gas turbine components. Eng. Failure Anal. 2002, 9, 31–43. [Google Scholar] [CrossRef]
- Okazaki, M. High-temperature strength of Ni-base superalloy coatings. Sci. Technol. Adv. Mater. 2001, 2, 357–366. [Google Scholar] [CrossRef]
- Hayes, J.R.; Gray, J.J.; Szmodis, A.W.; Orme, C.A. Influence of chromium and molybdenum on the corrosion of nickel-based alloys. Corros. Sci. 2006, 62, 491–500. [Google Scholar] [CrossRef]
- Yang, S.W. Effect of Ti and Ta on the oxidation of a complex superalloy. Oxid. Met. 1981, 15, 375–397. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, S.-M.; Yoo, Y.-S.; Jeong, H.-W.; Jang, H. Effects of Al and Ta on the high temperature oxidation of ni-based superalloys. Corros. Sci. 2015, 90, 305–312. [Google Scholar] [CrossRef]
- Acharya, M.V.; Fuchs, G.E. The effect of long-term thermal exposures on the microstructure and properties of CMSX-10 single crystal Ni-base superalloys. Mater. Sci. Eng. A 2004, 381, 143–153. [Google Scholar] [CrossRef]
- Pigrova, G.D. TCP-phases in nickel-base alloys with elevated chromium content. Met. Sci. Heat Treat. 2005, 47, 544–551. [Google Scholar] [CrossRef]
- Nash, P. The Cr-Ni (chromium-nickel) system. Bull. Alloy Phase Diagrams 1986, 7, 466–476. [Google Scholar] [CrossRef]
- Lee, B.-J. On the stability of Cr carbides. Calphad 1992, 16, 121–149. [Google Scholar] [CrossRef]
- Zhu, N.; Li, J.; Lu, X.-G.; He, Y.; Zhang, J. Experimental and Computational study of diffusion mobilities for fcc Ni-Cr-Mo alloys. Metall. Mater. Trans. A 2015, 46, 5444–5455. [Google Scholar] [CrossRef]
- Kripyakevich, P.I.; Pylaeva, E.N. Crystal structure of the compound Ta2Ni. J. Struct. Chem. 1962, 3, 35–37. [Google Scholar] [CrossRef]
- Giessen, B.C.; Grant, N.J. The crystal structure of TaNi3 and its change on cold working. Acta Metall. 1967, 15, 871–877. [Google Scholar] [CrossRef]
- Larson, J.M.; Taggart, R.; Polonis, D.H. Ni8Ta in Nickel-rich Ni-Ta alloys. Metall. Mater. Trans. B 1970, 1, 485–489. [Google Scholar] [CrossRef]
- Saburi, T.; Nakamura, M.; Nenno, S. Crystal structure and twin lamellae of Ni3Ta. J. Less-Common Met. 1975, 41, 135–139. [Google Scholar] [CrossRef]
- Nash, P.; West, D.R.F. Ni-Al and Ni-Ta phase diagrams. Met. Sci. 1983, 17, 99–100. [Google Scholar] [CrossRef]
- Nash, A.; Nash, P. The Ni-Ta system. J. Phase Equilib. 1984, 5, 444–445. [Google Scholar] [CrossRef]
- Kaufman, L. Coupled thermochemical and phase diagram data for tantalum based binary alloys. Calphad 1991, 15, 243–259. [Google Scholar] [CrossRef]
- Ansara, I.; Selleby, M. Thermodynamic analysis of the Ni-Ta system. Calphad 1994, 18, 99–107. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, Z. Experimental study and reassessment of the Ni-Ta binary system. Z. Metallkd. 1999, 90, 233–241. [Google Scholar]
- Pan, X.; Jin, Z. Experimental determination and Re-optimization of Ni-Ta binary system. Trans. Nonferrous Met. Soc. China 2002, 12, 748–753. [Google Scholar]
- Zhou, S.H.; Wang, Y.; Chen, L.Q.; Liu, Z.K.; Napolitano, R.E. Solution-based thermodynamic modeling of the Ni-Ta and Ni-Mo-Ta systems using first-principle calculations. Calphad 2009, 33, 631–641. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, B.; Ma, Y.; Melnik, R.; Liu, X. First-principles studies of Ni-Ta intermetallic compounds. J. Solid State Chem. 2012, 187, 211–218. [Google Scholar] [CrossRef]
- Kosorukova, T.; Firstov, G.; Noel, H.; Ivanchenko, V. Crystal structure changes in the Ni3Ta intermetallic compound. Chem. Met. Alloys 2013, 6, 196–199. [Google Scholar]
- Zhou, C.; Guo, C.; Li, C.; Du, Z. Thermodynamic optimization of the Ni-Ta system supported by the key experiments. Thermochim. Acta 2018, 666, 135–147. [Google Scholar] [CrossRef]
- Pavlů, J.; Vřešt’ál, J.; Šob, M. Re-modeling of Laves phases in the Cr-Nb and Cr-Ta systems using first-principles results. Calphad 2009, 33, 179–186. [Google Scholar] [CrossRef]
- Venkatraman, M.; Neumann, J.P. The Cr-Ta (chromium-tantalum) system. J. Phase Equilib. 1987, 8, 112–116. [Google Scholar] [CrossRef]
- Okamoto, H. Cr-Ta (chromium-tantalum). J. Phase Equilib. 1996, 17, 457–457. [Google Scholar] [CrossRef]
- Dupin, N.; Ansara, I. Thermodynamic assessment of the Cr-Ta system. J. Phase Equilib. 1993, 14, 451–456. [Google Scholar] [CrossRef]
- Nash, P.; West, D.R.F. Nickel-rich region of Ni-Cr-Ta system at 1523 and 1273 K. Met. Sci. 1980, 14, 273–276. [Google Scholar] [CrossRef]
- Schittny, S.U.; Lugscheider, E.; Knotek, O. Melting behaviour and phase equilibria in the system nickel-chromium-tantalum. Thermochim. Acta 1985, 85, 167–170. [Google Scholar] [CrossRef]
- Nikolaev, S.V.; Balykova, Y.V.; Kerimov, E.Y.; Slyusarenko, E.M. Phase equilibria at 1375 K in three-component system of nickel-chromium-tantalum. Moscow Univ. Chem. Bull. 2013, 68, 23–28. [Google Scholar] [CrossRef]
- Dupin, N.; Ansara, I. Theermodynamic assessment of the Cr–Ni–Ta system. Z. Metallkd. 1996, 87, 555–561. [Google Scholar]






| System | Phase | Pearson Symbol | Prototye | Space Group | Strukturbericht | Ref. |
|---|---|---|---|---|---|---|
| Ni-Cr | fcc | cF4 | Cu | Fm-3m | A1 | [14] |
| bcc-(Cr) | cI2 | W | Im-3m | A2 | [14] | |
| Ni2Cr | oP6 | Pt2Mo | [14] | |||
| Ni-Ta | fcc | cF4 | Cu | Fm-3m | A1 | [30] |
| bcc-(Ta) | cI2 | W | Im-3m | A2 | [30] | |
| Ni3Ta | tI8 | TiAl3 | I4/mmm | D022 | [30] | |
| mP16 | NbPt3 | P21/m | [30] | |||
| oP8 | Cu3Ti | Pmmn | D0a | [30] | ||
| Ni2Ta | tI6 | MoSi2 | I4/mmm | C11b | [30] | |
| Ni6Ta7 | hP13 | Fe7W6 | R-3m | D85 | [30] | |
| NiTa2 | tI12 | Al2Cu | I4/mcm | C16 | [30] | |
| Cr-Ta | bcc-(Cr) | cI2 | W | Im-3m | A2 | [31] |
| bcc-(Ta) | cI2 | W | Im-3m | A2 | [31] | |
| Cr2Ta(HT) | hP12 | MgZn2 | P63/mmc | C14 | [31] | |
| Cr2Ta(LT) | cF24 | MgCu2 | Fd-3m | C15 | [31] |
| Nominal Alloys (at. %) | Phase Equilibrium | Composition (at. %) | |||||
|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | ||||
| Cr | Ta | Cr | Ta | Cr | Ta | ||
| Ni54Cr43.5Ta2.5 | fcc | 43.4 | 53.9 | ||||
| Ni80.5Cr7Ta12.5 | fcc/Ni3Ta | 8.5 | 10 | 3.3 | 19.9 | ||
| Ni59Cr33Ta8 | fcc/Ni3Ta | 41.5 | 5.0 | 3.0 | 21.7 | ||
| Ni14Cr65Ta21 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 97.0 | 0.4 | 50.8 | 30.0 | ||
| Ni46Cr33Ta21 | bcc-(Cr)/(Ni, Cr)2Ta(HT)/Ni3Ta | 81.5 | 0.9 | 33 | 23.4 | 3.4 | 22.4 |
| Ni65Cr5Ta30 | Ni3Ta/Ni2Ta/(Ni, Cr)2Ta(HT) | 0.6 | 25.1 | 1.6 | 37.3 | 26.5 | 31.9 |
| Ni40Cr22Ta38 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 10.8 | 45 | 27.8 | 34.8 | ||
| Ni30Cr31Ta39 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 20.7 | 45.2 | 36.3 | 35.8 | ||
| Ni21Cr33Ta46 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 28.4 | 48 | 44.8 | 38.3 | ||
| Ni39Cr4Ta57 | Ni6Ta7/NiTa2 | 6.5 | 52.7 | 0.2 | 64.5 | ||
| Ni13Cr7Ta80 | NiTa2/bcc-(Ta) | 12 | 62.2 | 4.0 | 95.2 | ||
| Ni33Cr34Ta33 | (Ni, Cr)2Ta(HT) | 34.5 | 33.0 | ||||
| Ni78Cr9Ta13 | fcc/Ni3Ta | 11.4 | 8.3 | 1.9 | 21.9 | ||
| Ni53Cr37Ta10 | fcc/Ni3Ta/bcc-(Cr) | 41.9 | 4.6 | 3.6 | 22.5 | 80.3 | 0.6 |
| Ni38Cr32Ta30 | (Ni, Cr)2Ta(HT) | 31.9 | 29.5 | ||||
| Ni25Cr37Ta38 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 15.5 | 48.5 | 38.6 | 35.6 | ||
| Ni64Cr7Ta29 | Ni3Ta/Ni2Ta/(Ni, Cr)2Ta(HT) | 0.5 | 24.9 | 1.8 | 37.1 | 26.6 | 32.1 |
| Ni53Cr8Ta39 | Ni6Ta7/Ni2Ta/(Ni, Cr)2Ta(HT) | 9.6 | 46.1 | 0.8 | 33.5 | 26.8 | 33.2 |
| Ni2Cr75Ta23 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 98.6 | 0.7 | 66.7 | 30.8 | ||
| Ni9Cr48Ta43 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 40.1 | 50 | 54.1 | 39.6 | ||
| Ni27Cr41Ta32 | (Ni, Cr)2Ta(HT) | 40.9 | 32.1 | ||||
| Ni26Cr57Ta17 | Ni6Ta7/bcc-(Ta) | 19.1 | 53.8 | 6.2 | 93.7 | ||
| Ni35Cr53Ta12 | bcc-(Cr)/(Ni, Cr)2Ta(HT)/Ni3Ta | 81.3 | 0.9 | 33.5 | 23.7 | 3.2 | 22.5 |
| Ni27Cr53Ta20 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 92.8 | 0.2 | 36.9 | 25.9 | ||
| Ni23Cr53Ta24 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 96.3 | 0.5 | 45.3 | 28.9 | ||
| Ni22Cr45Ta33 | (Ni, Cr)2Ta(HT) | 45.2 | 32.9 | ||||
| Ni13Cr53Ta34 | (Ni, Cr)2Ta(HT) | 53.1 | 34 | ||||
| Ni16Cr38Ta46 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 33.3 | 49.8 | 51 | 38.9 | ||
| Ni12Cr33Ta55 | Ni6Ta7/bcc-(Ta) | 35.6 | 52.1 | 6.8 | 93.1 | ||
| Ni15Cr19Ta66 | Ni6Ta7/bcc-(Ta) | 24.4 | 55.1 | 6.4 | 93.5 | ||
| Ni36Cr58Ta6 | fcc/Ni3Ta/bcc-(Cr) | 41.8 | 4.5 | 3.4 | 22.3 | 80.1 | 0.5 |
| Ni5Cr40Ta55 | Ni6Ta7/(Ni, Cr)2Ta(HT)/bcc-(Ta) | 39.2 | 51.5 | 58.7 | 38.5 | 6.3 | 93.5 |
| Ni10Cr25Ta65 | Ni6Ta7/bcc-(Ta) | 30.5 | 52.4 | 5.4 | 93.2 | ||
| Nominal Alloys (at%) | Phase Equilibrium | Composition (at. %) | |||||
|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | ||||
| Cr | Ta | Cr | Ta | Cr | Ta | ||
| Ni54Cr43.5Ta2.5 | fcc | 46.0 | 2.4 | ||||
| Ni59Cr33Ta8 * | fcc/Ni3Ta/L | 34.3 | 6.6 | 3.7 | 22.4 | 37.5 | 11.7 |
| Ni14Cr65Ta21 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 95.5 | 0.6 | 51.6 | 29.6 | ||
| Ni46Cr33Ta21 * | L/(Ni, Cr)2Ta(HT)/Ni3Ta | 35.1 | 15.9 | 33.0 | 24.6 | 3.3 | 23.8 |
| Ni65Cr5Ta30 | Ni3Ta/Ni2Ta/(Ni, Cr)2Ta(HT) | 0.6 | 23.6 | 1.0 | 31.3 | 22.4 | 31.9 |
| Ni40Cr22Ta38 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 11.6 | 45.5 | 22.9 | 35.2 | ||
| Ni30Cr31Ta39 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 18.9 | 46.7 | 36.5 | 35.6 | ||
| Ni21Cr33Ta46 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 27.7 | 48.3 | 45.1 | 38.2 | ||
| Ni39Cr4Ta57 | Ni6Ta7/NiTa2 | 0.5 | 65.5 | 7.2 | 55 | ||
| Ni13Cr7Ta80 | NiTa2/bcc-(Ta) | 12.0 | 58.2 | 4.7 | 94.5 | ||
| Ni33Cr34Ta33 | (Ni, Cr)2Ta(HT) | 34.5 | 33.1 | ||||
| Ni78Cr9Ta13 | fcc | 9.2 | 12.3 | ||||
| Ni53Cr37Ta10 * | fcc/L | 38.4 | 6.5 | 36.9 | 13.5 | ||
| Ni38Cr32Ta30 | (Ni, Cr)2Ta(HT) | 32.2 | 30.2 | ||||
| Ni25Cr37Ta38 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 19.6 | 47.6 | 39.3 | 36.6 | ||
| Ni64Cr7Ta29 | Ni3Ta/Ni2Ta/(Ni, Cr)2Ta(HT) | 0.7 | 23.4 | 1.2 | 31.1 | 22.3 | 31.6 |
| Ni53Cr8Ta39 | Ni6Ta7/Ni2Ta/(Ni, Cr)2Ta(HT) | 8.5 | 45.4 | 0.2 | 32.7 | 17.4 | 36.5 |
| Ni2Cr75Ta23 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 98.1 | 0.8 | 65.2 | 32.3 | ||
| Ni9Cr48Ta43 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 42.3 | 50.2 | 54.3 | 40.6 | ||
| Ni27Cr41Ta32 | (Ni, Cr)2Ta(HT) | 41.0 | 31.8 | ||||
| Ni26Cr57Ta17 | Ni6Ta7/bcc-(Ta) | 19.8 | 54.0 | 5.1 | 94.3 | ||
| Ni35Cr53Ta12 * | bcc-(Cr)/(Ni, Cr)2Ta(HT)/L | 82.8 | 0.9 | 37.5 | 24.4 | 39.8 | 12.4 |
| Ni27Cr53Ta20 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 89.2 | 0.5 | 43.6 | 27.0 | ||
| Ni23Cr53Ta24 | bcc-(Cr)/(Ni, Cr)2Ta(HT) | 93.5 | 0.5 | 45.5 | 26.8 | ||
| Ni22Cr45Ta33 | (Ni, Cr)2Ta(HT) | 45.3 | 32.5 | ||||
| Ni13Cr53Ta34 | (Ni, Cr)2Ta(HT) | 53.5 | 33.7 | ||||
| Ni16Cr38Ta46 | Ni6Ta7/(Ni, Cr)2Ta(HT) | 35.7 | 48.8 | 49.8 | 39.4 | ||
| Ni51Cr28Ta21 * | Ni3Ta/(Ni, Cr)2Ta(HT)/L | 3.1 | 23.4 | 33.0 | 24.5 | 34.8 | 16.2 |
| Ni12Cr33Ta55 | Ni6Ta7/bcc-(Ta) | 36.9 | 51.0 | 5.4 | 94.1 | ||
| Ni15Cr19Ta66 | Ni6Ta7/bcc-(Ta) | 26.8 | 53.3 | 5.3 | 93.8 | ||
| Ni36Cr58Ta6 * | fcc/bcc-(Cr)/L | 40.1 | 6.1 | 80.7 | 0.5 | 39.7 | 11.8 |
| Ni78Cr12Ta10 | fcc/Ni3Ta | 13.4 | 9.7 | 3.2 | 21.5 | ||
| Ni5Cr40Ta55 | Ni6Ta7/(Ni, Cr)2Ta(HT)/(Ta) | 41.6 | 51.1 | 53.5 | 42.1 | 6.3 | 93.5 |
| Ni10Cr25Ta65 | Ni6Ta7/bcc-(Ta) | 29.8 | 54.6 | 6.8 | 93.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liang, Y.; Yang, S.; Yang, M.; Li, L.; Han, J.; Lu, Y.; Liu, X. Isothermal Sections of the Ni-Cr-Ta Ternary System at 1200 °C and 1300 °C. Metals 2019, 9, 770. https://doi.org/10.3390/met9070770
Wang C, Liang Y, Yang S, Yang M, Li L, Han J, Lu Y, Liu X. Isothermal Sections of the Ni-Cr-Ta Ternary System at 1200 °C and 1300 °C. Metals. 2019; 9(7):770. https://doi.org/10.3390/met9070770
Chicago/Turabian StyleWang, Cuiping, Yuhui Liang, Shuiyuan Yang, Mujin Yang, Lingling Li, Jiajia Han, Yong Lu, and Xingjun Liu. 2019. "Isothermal Sections of the Ni-Cr-Ta Ternary System at 1200 °C and 1300 °C" Metals 9, no. 7: 770. https://doi.org/10.3390/met9070770
APA StyleWang, C., Liang, Y., Yang, S., Yang, M., Li, L., Han, J., Lu, Y., & Liu, X. (2019). Isothermal Sections of the Ni-Cr-Ta Ternary System at 1200 °C and 1300 °C. Metals, 9(7), 770. https://doi.org/10.3390/met9070770






