Antioxidant Capacity and Cytotoxic Effects of Catechins and Resveratrol Oligomers Produced by Enzymatic Oxidation against T24 Human Urinary Bladder Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
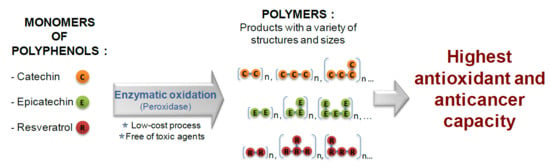
2.2. Synthesis of Oligomers of Catechin, Epicatechin and Resveratrol Through Enzymatic Oxidation
2.2.1. UV-Vis Absorption Spectra of Reaction Substrates and Products
2.2.2. Determination of the Consumption of Substrate and Formation of Product through Reversed Phase (RP)-HPLC
2.2.3. Characterization of Oligomers by Mass Spectrometry
2.2.4. Evaluation of the Total Phenolic Content (TPC) in the Reaction Solutions
2.2.5. Determination of the Antioxidant Capacity by the ORAC Method
2.2.6. Evaluation of the Fe2+ and Cu2+ ions’ Chelating Capacity
2.3. Evaluation of Cytotoxic Effects of Oligomers of Catechin, Epicatechin and Resveratrol in a Human Urinary Bladder Cancer Cell Culture
2.3.1. Cell Culture
2.3.2. Cell Viability Assessed by MTT Reduction
2.4. Statistical Analysis
3. Results
3.1. Enzymatic Oxidation of Catechin, Epicatechin, and Resveratrol
3.2. Evaluation of Molecular Weight Profile in the ER
3.3. Capacity of the Oligomers of Catechin, Epicatechin and Resveratrol to Scavenge Reactive Oxygen Species (ROS)
3.4. Chelating Capacity of Oligomers of Catechin, Epicatechin, and Resveratrol
3.5. Anti-Viability Activity of the Oligomers of Catechin, Epicatechin and Resveratrol
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miksits, M.; Wlcek, K.; Svoboda, M.; Kunert, O.; Haslinger, E.; Thalhammer, T.; Szekeres, T.; Jäger, W. Antitumor Activity of Resveratrol and its Sulfated Metabolites Against Human Breast Cancer Cells. Planta Med. 2009, 75, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.J.; Coyle, C.H.; Morrisroe, S.N.; Chancellor, M.B.; Yoshimura, N. Induction of Apoptosis in Human Bladder Cancer Cells by Green Tea Catechins. Biomed. Res. 2009, 30, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ma, J.; Xiao, J.; Lv, X.; Li, X.; Yang, H.; Liu, Y.; Feng, S.; Zhang, Y. Arctigenin Anti-Tumor Activity in Bladder Cancer T24 Cell Line Through Induction of Cell-Cycle Arrest and Apoptosis. Anat. Rec. 2012, 295, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic Uses of Epicatechin in Diabetes and Cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Sanusi, J.; Rajarajeswaran, J. White Tea (Camellia Sinensis) Inhibits Proliferation of the Colon Cancer Cell Line, HT-29, Activates Caspases and Protects DNA of Normal Cells Against Oxidative Damage. Food Chem. 2015, 169, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Avtanski, D.; Poretsky, L. Phyto-Polyphenols as Potential Inhibitors of Breast Cancer Metastasis. Mol. Med. 2018, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gajera, H.P.; Gevariya, S.N.; Hirpara, D.G.; Patel, S.V.; Golakiya, B.A. Antidiabetic and Antioxidant Functionality Associated with Phenolic Constituents from Fruit Parts of Indigenous Black Jamun (Syzygium Cumini L.) Landraces. J. Food Sci. Technol. 2017, 54, 3180–3191. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Choi, H.J.; Zhu, B.T. Mechanism for the Protective Effect of Resveratrol Against Oxidative Stress-Induced Neuronal Death. Free Radic. Biol. Med. 2010, 49, 800–813. [Google Scholar] [CrossRef]
- Xueyan, R.; Jia, Y.; Xuefeng, Y.; Lidan, T.; Qingjun, K. Isolation and Purification of Five Phenolic Compounds from the Xinjiang Wine Grape (Vitis Vinifera) and Determination of their Antioxidant Mechanism at Cellular Level. Eur. Food Res. Technol. 2018, 244, 1569–1579. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Prasain, J.K.; Jones, K.; Moore, R.; Barnes, S.; Leahy, M.; Roderick, R.; Juliana, M.M.; Grubbs, C.J. Effect of Cranberry Juice Concentrate on Chemically-Induced Urinary Bladder Cancers. Oncol. Rep. 2008, 19, 1565–1570. [Google Scholar] [PubMed]
- Shingai, Y.; Fujimoto, A.; Nakamura, M.; Masuda, T. Structure and Function of the Oxidation products of Polyphenols and Identification of Potent Lipoxygenase inhIbitors from Fe-Catalyzed Oxidation of Resveratrol. J. Agric. Food Chem. 2011, 59, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Oh, S.; Kim, E.; Imm, J.Y. α-Glucosidase Inhibiton and Antiglycation Activity of Laccase-Catalyzed Catechin Polymers. J. Agric. Food Chem. 2013, 61, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts and Figures. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf (accessed on 13 June 2019).
- Stocco, B.; Toledo, K.; Salvador, M.; Paulo, M.; Koyama, N.; Torqueti Toloi, M.R. Dose-Dependent Effect of Resveratrol on Bladder Cancer Cells: Chemoprevention and Oxidative Stress. Maturitas 2012, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- López-Serrano, M.; Ros Barceló, A. Comparative Study of the Products of the Peroxidase-Catalyzed and the Polyphenoloxidase-Catalyzed (+)-Catechin Oxidation. Their Possible Implications in Strawberry (Fragaria X Ananassa) Browning Reactions. J. Agric. Food Chem. 2002, 50, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; De Los Mozos-Pascual, M.; Tarantilis, P.; Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol Composition and Antioxidant and Metal Chelating Activities of the Solid Residues from the Essential Oil Industry. Ind. Crop. Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT Assay to Evaluate the Cytotoxic Potential of a Drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; García-Molina, F.; Molina-Alarcón, M.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Kinetic Characterization of the Enzymatic and Chemical Oxidation of the Catechins in Green Tea. J. Agric. Food Chem. 2008, 56, 9215–9224. [Google Scholar] [CrossRef]
- Hosny, M.; Rosazza, J.P.N. Novel Oxidations of (+)-Catechin by Horseradish Peroxidase and Laccase. J. Agric. Food Chem. 2002, 50, 5539–5545. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Nicholson, R.L. Chemical properties of phenolic compounds. In Phenolic Compound Biochemistry; Vermerris, W., Nicholson, R.L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 35–62. [Google Scholar]
- Reihmann, M.; Ritter, H. Synthesis of phenol polymers using peroxidases. In Enzyme-Catalyzed Synthesis of Polymers; Kobayashi, S., Ritter, H., Kaplan, D., Eds.; Springer: Berlin, Germany, 2005; Volume 194, pp. 1–49. [Google Scholar]
- Niki, E. Assessment of Antioxidant Capacity In Vitro and In Vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Santos, M.R.; Caroҫo, G.; Rocha, R.; Justino, A.; Mira, L. Structure-Antioxidantactivity Relationships of Flavonoids: A Re-Examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Rhouma, G.B.; Chekir-Ghedira, L.; Ghoul, M. Enzymatic polymerization of rutin and esculin and evaluation of the antioxidant capacity of polyrutin and polyesculin. In Biotechnology; Ekinci, D., Ed.; IntechOpen: London, UK, 2015; pp. 117–133. [Google Scholar]
- Nicotra, S.; Cramarossa, M.R.; Mucci, A.; Pagnoni, U.M.; Riva, S.; Forti, L. Biotransformation of Resveratrol: Synthesis of Trans-Dehydrodimers Catalyzed by Laccases from Myceliophtora Thermophyla and from Trametes Pubescens. Tetrahedron 2004, 60, 595–600. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Kim, Y.J.; Uyama, H.; Kobayashi, S. Amplification of Antioxidant Activity and Xanthine Oxidase Inhibition of Catechin by Enzymatic Polymerization. Biomacromolecules 2003, 4, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Favreau, N.; Fossey, S.; Grella, A.R.; Ndou, T.; Bruno, F.F. Antioxidant Potency of Highly Purified Polyepicatechin Fractions. Food Chem. 2012, 130, 902–907. [Google Scholar] [CrossRef]
- Yu, B.B.; Han, X.Z.; Lou, H.X. Oligomers of Resveratrol and Ferulic acid Prepared by Peroxidase-Catalyzed Oxidation and their Protective Effects on Cardiac Injury. J. Agric. Food Chem. 2007, 55, 7753–7757. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Letelier, M.E.; Sánchez-Jofré, S.; Peredo-Silva, L.; Cortés-Troncoso, J.; Aracena-Parks, P. Mechanisms Underlying Iron and Copper Ions Toxicity in Biological Systems: Pro-Oxidant Activity and Protein-Binding Effects. Chem. Biol. Interact. 2010, 188, 220–227. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.L.; Beal, M.F.; Gibson, G.E. Dietary Supplementation with Resveratrol Reduces Plaque Pathology in a Transgenic Model of Alzheimer’s Disease. Neurochem. Int. 2009, 54, 111–118. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.B.H. Neuroprotective Molecular Mechanisms of (-)-Epigallocatechin-3-Gallate: A Reflective Outcome of its Antioxidant, Iron Chelating and Neuritogenic Properties. Genes Nutr. 2009, 4, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Peguero, J.G.; Arenas, I.; Lamas, G.A. Chelation Therapy and Cardiovascular Disease: Connecting Scientific Silos to Benefit Cardiac Patients. Trends Cardiovas. Med. 2014, 24, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.L.; Weiss, J.M.; Lavau, C.P.; Wechsler, D.S. Iron Deprivation in Cancer—Potential Therapeutic Implications. Nutrients 2013, 5, 2836–2859. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Nagarajan, R.; Braunhut, S.J.; Bruno, F.; McIntosh, D.; Samuelson, L.; Kumar, J. Biocatalytically Oligomerized Epicatechin with Potent and Specific Anti-Proliferative Activity for Human Breast Cancer Cells. Molecules 2008, 13, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Akao, Y.; Yi, H.; Ohguchi, K.; Matsumoto, K.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Antitumor Effect of Resveratrol Oligomers Against Human Cancer Cell Lines and the Molecular Mechanism of Apoptosis Induced by Vaticanol C. Carcinogenesis 2003, 24, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Medina-Campos, O.N.; Hernández-Pando, R.; Negrette-Guzmán, M.; Huerta-Yepez, S.; Pedraza-Chaverri, J. C-Phycocyanin Prevents Cisplatin-Induced Nephrotoxicity through Inhibition of Oxidative Stress. Food Funct. 2014, 5, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.Q.; Di, J.M.; Luo, Y.; Cheng, K.J.; Wei, X.; Shi, Z. Resveratrol Oligomers for the Prevention and Treatment of Cancers. Oxid. Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Y.; Bai, Y.; Yang, K.; Mao, Q.; Lin, Y.; Kong, D.; Zheng, X.; Xie, L. A Component of Green Tea, (-)-Epigallocatechin-3-Gallate, Promotes Apoptosis in T24 Human Bladder Cancer Cells via Modulation of the PI3K/Akt Pathway and Bcl-2 Family Proteins. Mol. Med. Rep. 2012, 6, 1040–1044. [Google Scholar] [CrossRef]




| Products and Standard. | Fe2+ Chelating Activity IC50 * (μM CE) | Cu2+ Chelating Activity IC50 (μM CE) |
|---|---|---|
| Control reaction: | ||
| Catechin | 1484.3 ± 3.2 b | 889.8 ± 7.2 d |
| Epicatechin | 1633.9 ± 32.5 a | 1105.8 ± 36.9 c |
| Resveratrol | 1484.4 ± 42.9 b | 937.1 ± 25.8 d |
| Enzymatic reaction: | ||
| Catechin | 0.4 ± 0.02 g | 1.8 ± 0.02 g |
| Epicatechin | 0.5 ± 0.00 g | 1.7 ± 0.06 g |
| Resveratrol | 0.5 ± 0.02 g | 20.9 ± 0.3 g |
| Standard: | ||
| EDTA | 125.2 ± 1.4 e (μM EDTA) | 367 ± 6.1 f (μM EDTA) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses-Gutiérrez, C.L.; Hernández-Damián, J.; Pedraza-Chaverri, J.; Guerrero-Legarreta, I.; Téllez, D.I.; Jaramillo-Flores, M.E. Antioxidant Capacity and Cytotoxic Effects of Catechins and Resveratrol Oligomers Produced by Enzymatic Oxidation against T24 Human Urinary Bladder Cancer Cells. Antioxidants 2019, 8, 214. https://doi.org/10.3390/antiox8070214
Meneses-Gutiérrez CL, Hernández-Damián J, Pedraza-Chaverri J, Guerrero-Legarreta I, Téllez DI, Jaramillo-Flores ME. Antioxidant Capacity and Cytotoxic Effects of Catechins and Resveratrol Oligomers Produced by Enzymatic Oxidation against T24 Human Urinary Bladder Cancer Cells. Antioxidants. 2019; 8(7):214. https://doi.org/10.3390/antiox8070214
Chicago/Turabian StyleMeneses-Gutiérrez, Claudia Lizet, Jacqueline Hernández-Damián, José Pedraza-Chaverri, Isabel Guerrero-Legarreta, Dario Iker Téllez, and María Eugenia Jaramillo-Flores. 2019. "Antioxidant Capacity and Cytotoxic Effects of Catechins and Resveratrol Oligomers Produced by Enzymatic Oxidation against T24 Human Urinary Bladder Cancer Cells" Antioxidants 8, no. 7: 214. https://doi.org/10.3390/antiox8070214