Cell–Cell Adhesion and Myosin Activity Regulate Cortical Actin Assembly in Mammary Gland Epithelium on Concaved Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Image Acquisition
2.3. Drug Treatment
2.4. Immunofluorescence and Staining of F-Actin and the Nucleus
2.5. Concaved Channel Fabrication
2.5.1. Negative Mold Fabrication
2.5.2. Concaved Channel Molding
2.6. Imaging Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Epithelial Cells Formed Continuous Epithelium on Curved Surfaces
3.2. Curvature-Dependent Cortical Actin Increase Is Regulated by Myosin II Phosphorylation
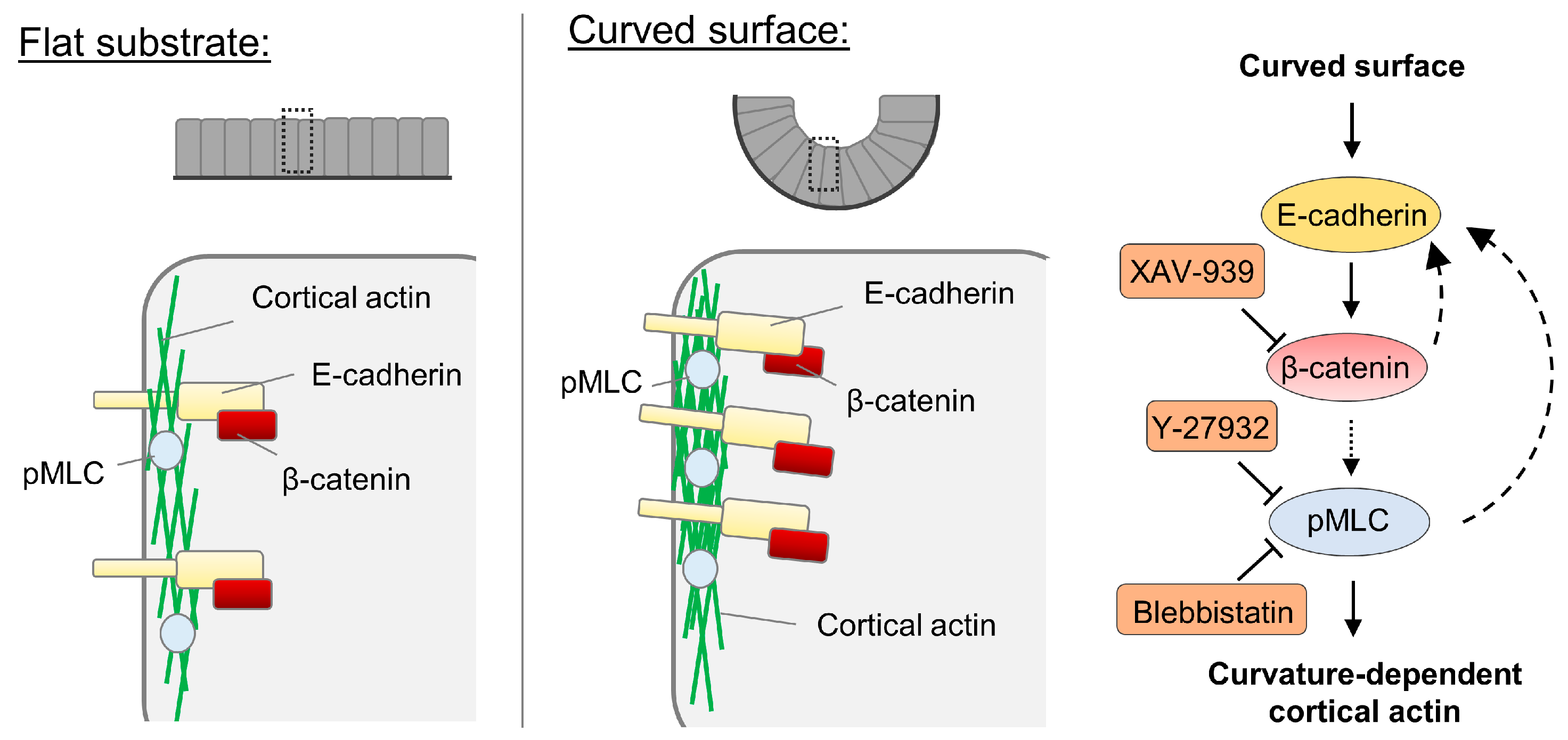
3.3. Cell-Cell Adhesion Is Required in the Curvature-Dependent Regulation of Cortical Actin Assembly
3.4. β-Catenin Is Involved in the Curvature-Dependent Regulation of Cortical Actin Assembly
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.H.; Chi, M.; Yi, B.; Kim, S.H.; Oh, S.; Kim, Y.; Park, S.; Sung, J.H. Three-dimensional intestinal villi epithelium enhances protection of human intestinal cells from bacterial infection by inducing mucin expression. Integr. Biol. 2014, 6, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Abdeen, A.A.; Wycislo, K.L.; Fan, T.M.; Kilian, K.A. Interfacial geometry dictates cancer cell tumorigenicity. Nat. Mater. 2016, 15, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Boghaert, E.; Gleghorn, J.P.; Lee, K.; Gjorevski, N.; Radisky, D.C.; Nelson, C.M. Host epithelial geometry regulates breast cancer cell invasiveness. Proc. Natl. Acad. Sci. USA 2012, 109, 19632–19637. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Kidoaki, S. Manipulation of cell mechanotaxis by designing curvature of the elasticity boundary on hydrogel matrix. Biomaterials 2015, 41, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bade, N.D.; Xu, T.; Kamien, R.D.; Assoian, R.K.; Stebe, K.J. Gaussian Curvature Directs Stress Fiber Orientation and Cell Migration. Biophys. J. 2018, 114, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Sonam, S.; Beng Saw, T.; Ladoux, B.; Teck Lim, C. Emergent patterns of collective cell migration under tubular confinement. Nat. Commun. 2017, 8, 1517. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, A.; Cheddadi, I.; Chen, T.; Pereira, T.; Ong, H.T.; Bertocchi, C.; Brugues, A.; Jacinto, A.; Kabla, A.J.; Toyama, Y.; et al. Gap geometry dictates epithelial closure efficiency. Nat. Commun. 2015, 6, 7683. [Google Scholar] [CrossRef] [PubMed]
- Antonny, B. Mechanisms of Membrane Curvature Sensing. Annu. Rev. Biochem. 2011, 80, 101–123. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Gallop, J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.G.; Evans, P.R.; McMahon, H.T. BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef]
- Hannezo, E.; Scheele, C.L.G.J.; Moad, M.; Drogo, N.; Heer, R.; Sampogna, R.V.; van Rheenen, J.; Simons, B.D. A Unifying Theory of Branching Morphogenesis. Cell 2017, 171, 242–255.e27. [Google Scholar] [CrossRef] [PubMed]
- Broaders, K.E.; Cerchiari, A.E.; Gartner, Z.J. Coupling between apical tension and basal adhesion allow epithelia to collectively sense and respond to substrate topography over long distances. Integr. Biol. (Camb.) 2015, 7, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Yevick, H.G.; Duclos, G.; Bonnet, I.; Silberzan, P. Architecture and migration of an epithelium on a cylindrical wire. Proc. Natl. Acad. Sci. USA 2015, 112, 5944–5949. [Google Scholar] [CrossRef] [PubMed]
- Bade, N.D.; Kamien, R.D.; Assoian, R.K.; Stebe, K.J. Curvature and Rho activation differentially control the alignment of cells and stress fibers. Sci. Adv. 2017, 3, e1700150. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Blanquer, S.B.G.; Haimi, S.P.; Korus, G.; Dunlop, J.W.C.; Duda, G.N.; Grijpma, D.W.; Petersen, A. Surface Curvature Differentially Regulates Stem Cell Migration and Differentiation via Altered Attachment Morphology and Nuclear Deformation. Adv. Sci. 2017, 4, 1600347. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Blacher, S.; Gérard, C.; Gallez, A.; Foidart, J.-M.; Noël, A.; Péqueux, C. Quantitative Assessment of Mouse Mammary Gland Morphology Using Automated Digital Image Processing and TEB Detection. Endocrinology 2016, 157, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Emerman, J.T.; Vogl, A.W. Cell Size and Shape Changes in the Myoepithelium of the Mammary Gland during Differentiation. Anat. Rec. 1986, 216, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Mythreye, K.; O’Brien, E.T.; Berchuck, A.; Blobe, G.C.; Superfine, R.; Tim O’Brien, E.; Berchuck, A.; Blobe, G.C.; Superfine, R.; et al. Mechanical Stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011, 71, 5075–5080. [Google Scholar] [CrossRef]
- Salbreux, G.; Charras, G.; Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012, 22, 536–545. [Google Scholar] [CrossRef]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Kovács, M.; Tóth, J.; Hetényi, C.; Málnási-Csizmadia, A.; Sellers, J.R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004, 279, 35557–35563. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, R.; de Matos Simoes, S.; Röper, J.-C.; Eaton, S.; Zallen, J.A. Myosin II Dynamics Are Regulated by Tension in Intercalating Cells. Dev. Cell 2009, 17, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Gjorevski, N.; Inman, J.L.; Bissell, M.J.; Nelson, C.M. Self-organization of engineered epithelial tubules by differential cellular motility. Proc. Natl. Acad. Sci. USA 2009, 106, 14890–14895. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, T. Mechanics of cell spreading: role of myosin II. J. Cell Sci. 2003, 116, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, Y.; Yumura, S. Molecular dynamics and forces of a motile cell simultaneously visualized by TIRF and force microscopies. Biotechniques 2008, 44, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, J.L.; Cohen, D.M.; Yang, M.T.; Sniadecki, N.J.; Ruiz, S.A.; Nelson, C.M.; Chen, C.S. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA 2010, 107, 9944–9949. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, T.; Xu, L.-C.; Siedlecki, C.; Brown, J.L. Substrate curvature sensing through Myosin IIa upregulates early osteogenesis. Integr. Biol. 2013, 5, 1407. [Google Scholar] [CrossRef]
- Han, S.J.; Rodriguez, M.L.; Sniadecki, N.J.; Ting, L.H.; Jahn, J.R.; Jung, J.I.; Shuman, B.R.; Feghhi, S. Flow mechanotransduction regulates traction forces, intercellular forces, and adherens junctions. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2220–H2229. [Google Scholar]
- Chen, T.; Callan-Jones, A.; Fedorov, E.; Ravasio, A.; Brugués, A.; Ong, H.T.; Toyama, Y.; Low, B.C.; Trepat, X.; Shemesh, T.; et al. Large-scale curvature sensing by directional actin flow drives cellular migration mode switching. Nat. Phys. 2019, 15, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Biton, Y.Y.; Safran, S.A. The cellular response to curvature-induced stress. Phys. Biol. 2009, 6, 046010. [Google Scholar] [CrossRef] [PubMed]
- Tarle, V.; Ravasio, A.; Hakim, V.; Gov, N.S. Modeling the finger instability in an expanding cell monolayer. Integr. Biol. 2015, 7, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chadha, G.K.; Feygin, A.; Ivanov, A.I. F-actin binding protein, anillin, regulates integrity of intercellular junctions in human epithelial cells. Cell. Mol. Life Sci. 2015, 72, 3185–3200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ng, M.R.; Besser, A.; Brugge, J.S.; Danuser, G. Mapping the dynamics of force transduction at cell-cell junctions of epithelial clusters. Elife 2014, 3, e03282. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, A.; Le, A.P.; Saw, T.B.; Tarle, V.; Ong, H.T.; Bertocchi, C.; Mège, R.-M.; Lim, C.T.; Gov, N.S.; Ladoux, B. Regulation of epithelial cell organization by tuning cell–substrate adhesion. Integr. Biol. 2015, 7, 1228–1241. [Google Scholar] [CrossRef]
- Maruthamuthu, V.; Sabass, B.; Schwarz, U.S.; Gardel, M.L. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc. Natl. Acad. Sci. USA 2011, 108, 4708–4713. [Google Scholar] [CrossRef]
- Sim, J.Y.; Moeller, J.; Hart, K.C.; Ramallo, D.; Vogel, V.; Dunn, A.R.; Nelson, W.J.; Pruitt, B.L. Spatial distribution of cell–cell and cell–ECM adhesions regulates force balance while main-taining E-cadherin molecular tension in cell pairs. Mol. Biol. Cell 2015, 26, 2456–2465. [Google Scholar] [CrossRef]
- Huang, S.-M.A.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Tian, X.-H.; Hou, W.-J.; Fang, Y.; Fan, J.; Tong, H.; Bai, S.-L.; Chen, Q.; Xu, H.; Li, Y. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/β-catenin signaling pathway. J. Exp. Clin. Cancer Res. 2013, 32, 100. [Google Scholar] [CrossRef]
- Li, C.; Zheng, X.; Han, Y.; Lv, Y.; Lan, F.; Zhao, J. XAV939 inhibits the proliferation and migration of lung adenocarcinoma A549 cells through the WNT pathway. Oncol. Lett. 2018, 15, 8973–8982. [Google Scholar] [CrossRef] [PubMed]
- Stakheev, D.; Taborska, P.; Strizova, Z.; Podrazil, M.; Bartunkova, J.; Smrz, D. The WNT/β-catenin signaling inhibitor XAV939 enhances the elimination of LNCaP and PC-3 prostate cancer cells by prostate cancer patient lymphocytes in vitro. Sci. Rep. 2019, 9, 4761. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Shen, F.; Xiao, W.; Chen, J.; Pan, F. Wnt inhibitor XAV939 suppresses the viability of small cell lung cancer NCI-H446 cells and induces apoptosis. Oncol. Lett. 2017, 14, 6585–6591. [Google Scholar] [CrossRef]
- Jang, J.; Jung, Y.; Chae, S.; Bae, T.; Kim, S.-M.; Shim, Y.J.; Chung, S.-I.; Yoon, Y. XAV939, a Wnt/β-catenin pathway modulator, has inhibitory effects on LPS-induced inflammatory response. Immunopharmacol. Immunotoxicol. 2019, 41, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Shen, F.; Yang, L.; Zhang, L.; Guo, W.; Tian, J. Inhibitory effects of XAV939 on the proliferation of small-cell lung cancer H446 cells and Wnt/β-catenin signaling pathway in vitro. Oncol. Lett. 2018, 16, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, H.; Sun, Z.; Xiang, Z.; Ge, Y.; Ni, C.; Luo, Z.; Qian, W.; Han, X. Inhibition of Wnt/β-catenin signaling promotes epithelial differentiation of mesenchymal stem cells and repairs bleomycin-induced lung injury. Am. J. Physiol. Physiol. 2014, 307, C234–C244. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Robitaille, A.M.; Berndt, J.D.; Davidson, K.C.; Fischer, K.A.; Mathieu, J.; Potter, J.C.; Ruohola-Baker, H.; Moon, R.T. Wnt/β-catenin signaling promotes self-renewal and inhibits the primed state transition in naïve human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6382–E6390. [Google Scholar] [CrossRef] [PubMed]
- Bastakoty, D.; Saraswati, S.; Cates, J.; Lee, E.; Nanney, L.B.; Young, P.P. Inhibition of Wnt/β-catenin pathway promotes regenerative repair of cutaneous and cartilage injury. FASEB J. 2015, 29, 4881–4892. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef]
- Yamada, S.; Pokutta, S.; Drees, F.; Weis, W.I.; Nelson, W.J. Deconstructing the Cadherin-Catenin-Actin Complex. Cell 2005, 123, 889–901. [Google Scholar] [CrossRef]
- Imamura, Y.; Itoh, M.; Maeno, Y.; Tsukita, S.; Nagafuchi, A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 1999, 144, 1311–1322. [Google Scholar] [CrossRef]
- Pacquelet, A.; Rørth, P. Regulatory mechanisms required for DE-cadherin function in cell migration and other types of adhesion. J. Cell Biol. 2005, 170, 803–812. [Google Scholar] [CrossRef]
- Peng, X.; Cuff, L.E.; Lawton, C.D.; DeMali, K.A. Vinculin regulates cell-surface E-cadherin expression by binding to -catenin. J. Cell Sci. 2010, 123, 567–577. [Google Scholar] [CrossRef]
- Sunyer, R.; Conte, V.; Escribano, J.; Elosegui-Artola, A.; Labernadie, A.; Valon, L.; Navajas, D.; García-Aznar, J.M.; Muñoz, J.J.; Roca-Cusachs, P.; et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 2016, 353, 1157–1161. [Google Scholar] [CrossRef]
- Zschiesche, W.; Schönborn, I.; Behrens, J.; Herrenknecht, K.; Hartveit, F.; Lilleng, P.; Birchmeier, W. Expression of E-cadherin and catenins in invasive mammary carcinomas. Anticancer Res. 1997, 17, 561–567. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.-H.; Elawad, K.; Kang, S.H.; Chen, Y. Cell–Cell Adhesion and Myosin Activity Regulate Cortical Actin Assembly in Mammary Gland Epithelium on Concaved Surface. Cells 2019, 8, 813. https://doi.org/10.3390/cells8080813
Jung W-H, Elawad K, Kang SH, Chen Y. Cell–Cell Adhesion and Myosin Activity Regulate Cortical Actin Assembly in Mammary Gland Epithelium on Concaved Surface. Cells. 2019; 8(8):813. https://doi.org/10.3390/cells8080813
Chicago/Turabian StyleJung, Wei-Hung, Khalid Elawad, Sung Hoon Kang, and Yun Chen. 2019. "Cell–Cell Adhesion and Myosin Activity Regulate Cortical Actin Assembly in Mammary Gland Epithelium on Concaved Surface" Cells 8, no. 8: 813. https://doi.org/10.3390/cells8080813
APA StyleJung, W.-H., Elawad, K., Kang, S. H., & Chen, Y. (2019). Cell–Cell Adhesion and Myosin Activity Regulate Cortical Actin Assembly in Mammary Gland Epithelium on Concaved Surface. Cells, 8(8), 813. https://doi.org/10.3390/cells8080813





