Anti-Oxidant Activity of Gallotannin-Enriched Extract of Galla Rhois Can Associate with the Protection of the Cognitive Impairment through the Regulation of BDNF Signaling Pathway and Neuronal Cell Function in the Scopolamine-Treated ICR Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purification of GEGR
2.2. Free Radical Scavenging Activity of GEGR
2.3. ROS Levels Analyses in B35 Cells and Brain Tissues
2.4. Design of Animal Experiment
2.5. Passive Avoidance Test
2.6. Determination of AChE Activity
2.7. Analysis of Superoxide Dismutase (SOD) Activity
2.8. Analysis of Catalase (CAT) Activity
2.9. Quantitative Real Time-PCR Analysis
2.10. Western Blot
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results
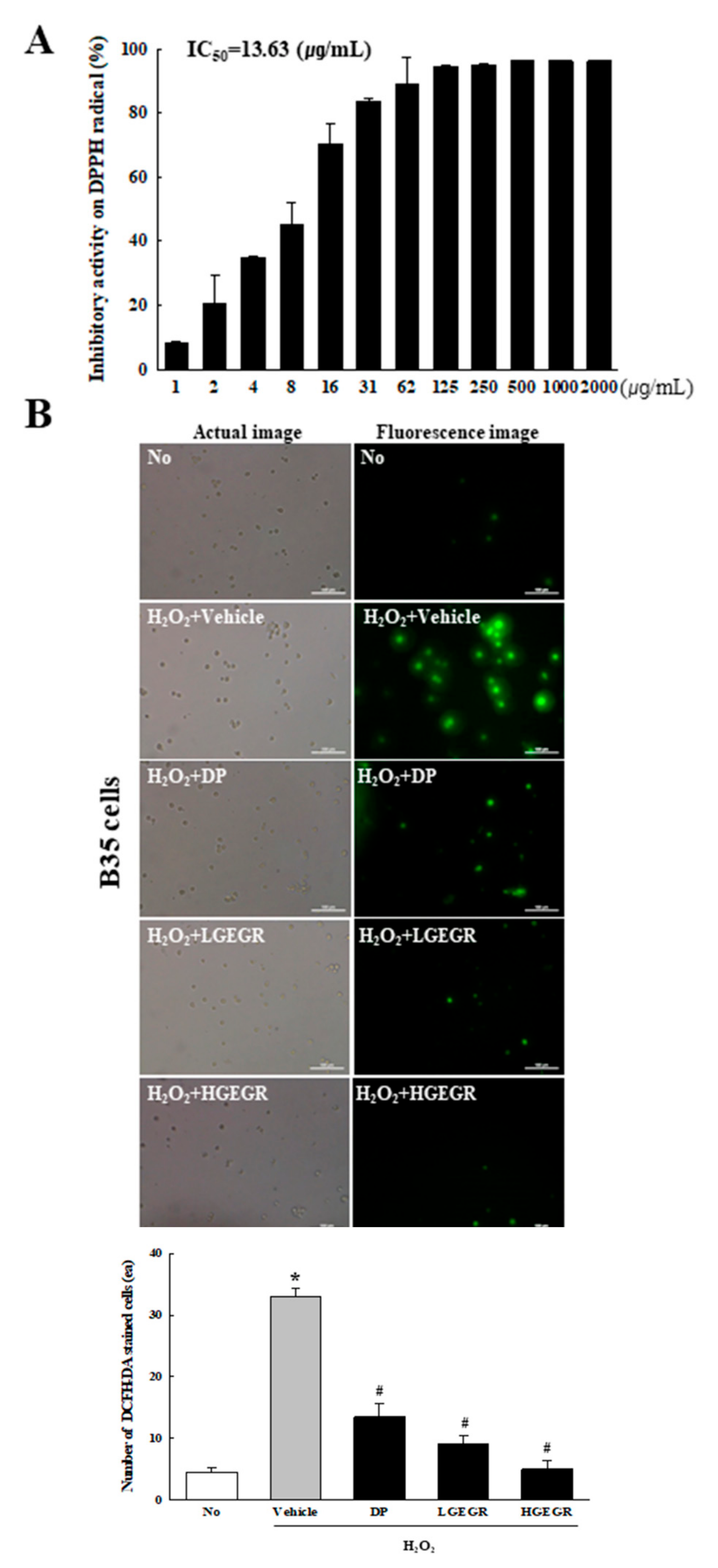
3.1. Anti-Oxidant Activity and BDNF Recovery Effects of GEGR in B35 Cells
3.2. Anti-Oxidant Activity of GEGR in SP-Induced Memory Impairment Model
3.3. Protective Effects of GEGR against Memory Impairment
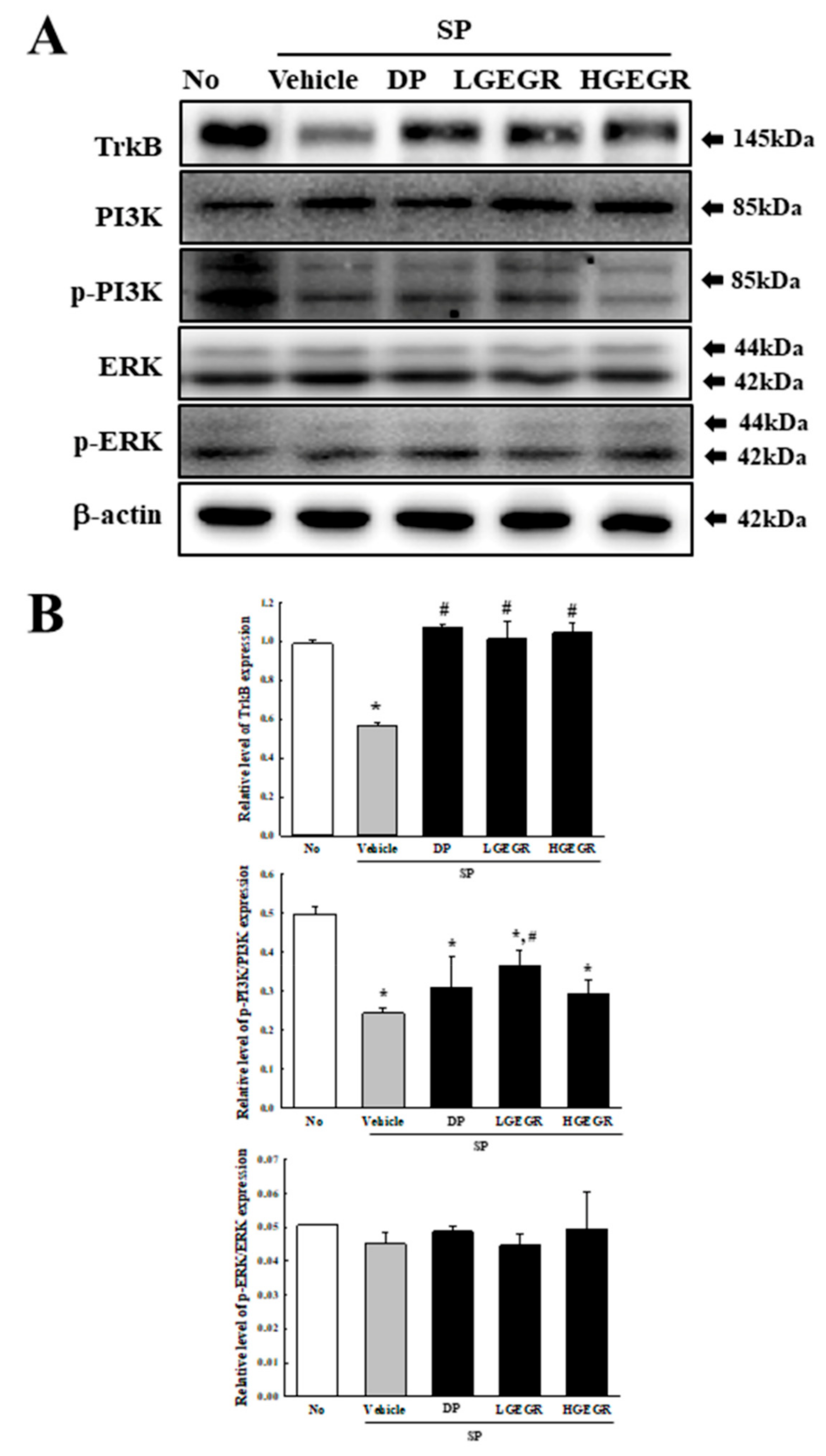
3.4. Effect of GEGR on BDNF Secretion and Their Receptor Signaling Pathway
3.5. Effects of GEGR on the Survival and Function of Neuronal Cells
3.6. Effects of GEGR on the Regulation of Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of Oxidative Stress in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial Abnormalities in Alzheimer Brain: Mechanistic Implications. Ann. Neurol. 2005, 57, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M.; Delaney, P. Antioxidant Strategies for Alzheimer’s Disease. Proc. Nutr. Soc. 2002, 61, 191–202. [Google Scholar] [CrossRef]
- Sylla, T.; Pouysegu, L.; Da Costa, G.; Deffieux, D.; Monti, J.P.; Quideau, S. Gallotannins and Tannic Acid: First Chemical Syntheses and In Vitro Inhibitory Activity on Alzheimer’s Amyloid β-Peptide Aggregation. Angew. Chem. 2015, 127, 8335–8339. [Google Scholar] [CrossRef]
- Mori, T.; Rezai-Zadeh, K.; Koyama, N.; Arendash, G.W.; Yamaguchi, H.; Kakuda, N.; Horikoshi-Sakuraba, Y.; Tan, J.; Town, T. Tannic Acid Is a Natural β-Secretase Inhibitor That Prevents Cognitive Impairment and Mitigates Alzheimer-like Pathology in Transgenic Mice. J. Biol. Chem. 2012, 287, 6912–6927. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Puig, K.L.; Combs, C.K. Pomegranate Polyphenols and Extract Inhibit Nuclear Factor of Activated T-cell Activity and Microglial Activation In Vitro and in a Transgenic Mouse Model of Alzheimer Disease. J. Nutr. 2013, 143, 597–605. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic Activity of Tannic Acid and Its Activity to Destabilize Alzheimer’s β-amyloid Fibrils In Vitro. Biochim. Biophys. Acta 2004, 1690, 193–202. [Google Scholar] [CrossRef]
- Bhakta, H.K.; Park, C.H.; Yokozawa, T.; Tanaka, T.; Jung, H.A.; Choi, J.S. Potential Anti-cholinesterase and β-site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibitory Activities of Cornuside and Gallotannins from Cornus officinalis Fruits. Arch. Pharmacal Res. 2017, 40, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Tomoo, K.; Yao, T.M.; Minoura, K.; Hiraoka, S.; Sumida, M.; Taniguchi, T.; Ishida, T. Possible Role of Each Repeat Structure of the Microtubule-Binding Domain of the Tau Protein in In Vitro Aggregation. J. Biochem. 2005, 138, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Gao, X.; Sun, W.; Yao, T.; Shi, S.; Ji, L. Molecular Hairpin: A Possible Model for Inhibition of Tau Aggregation by Tannic Acid. Biochemistry 2013, 52, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Kim, J.E.; Koh, E.K.; Song, S.H.; Sung, J.E.; Lee, H.A.; Lee, Y.H.; Lim, Y.; Hong, J.T.; Hwang, D.Y. Protective Effect of Gallotannin-Enriched Extract Isolated from Galla Rhois against CCl₄-Induced Hepatotoxicity in ICR Mice. Nutrients 2016, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Lu, B. BDNF and Activity-Dependent Synaptic Modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Borodinova, A.A.; Salozhin, S.V. Differences in the Biological Functions of BDNF and proBDNF in the Central Nervous System. Neurosci. Behav. Physiol. 2017, 47, 251–265. [Google Scholar] [CrossRef]
- Gibon, J.; Barker, P.A.; Séguéla, P. Opposing Presynaptic Roles of BDNF and ProBDNF in the Regulation of Persistent Activity in the Entorhinal Cortex. Mol. Brain 2016, 9, 23. [Google Scholar] [CrossRef]
- Pan, W.; A Banks, W.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of Brain-derived Neurotrophic Factor Across the Blood–brain Barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The Involvement of BDNF, NGF and GDNF in Aging and Alzheimer’s Disease. Aging Dis. 2015, 6, 331–341. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Go, J.; Kim, J.E.; Koh, E.K.; Song, S.H.; Kang, H.G.; Lee, Y.H.; Kim, H.D.; Hong, J.T. Hepatoprotective Effect of Gallotannin-enriched Extract Isolated from Gall on Hydrogen Peroxide-induced Cytotoxicity in HepG2 Cells. Pharmacogn. Mag. 2017, 13, 294–S300. [Google Scholar] [CrossRef]
- Oh, H.; Ko, E.K.; Kim, D.H.; Jang, K.K.; Park, S.E.; Lee, H.S.; Kim, Y.C. Secoiridoid Glucosides with Free Radical Scavenging Activity from the Leaves of Syringa dilatata. Phytother. Res. 2003, 17, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Kim, J.E.; Sung, J.E.; Yun, W.B.; Kim, D.S.; Lee, H.S.; Hong, J.T.; Hwang, D.Y. Asparagus cochinchinensis Stimulates Release of Nerve Growth Factor and Abrogates Oxidative Stress in the Tg2576 Model for Alzheimer’s Disease. BMC Complement Altern Med. 2018, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Takayama, S.; Iwasaki, K.; Tabuchi, M.; Yamaguchi, T.; Sekiguchi, K.; Ikarashi, Y.; Kudo, Y.; Kase, Y.; Arai, H.; et al. Traditional Japanese Medicine, Ameliorates Memory Disturbance and Abnormal Social Interaction with Anti-aggregation Effect of Cerebral Amyloid β Proteins in Amyloid Precursor Protein Transgenic Mice. Neuroscience 2011, 180, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-time Quantitative PCR and the 2(-∆∆C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Westendarp, H. Effects of Tannins in Animal Nutrition. DTW. Dtsch. Tierarztl. Wochenschr. 2006, 113, 264–268. [Google Scholar]
- Ferreira, D.; Gross, G.G.; Hagerman, A.E.; Kolodziej, H.; Yoshida, T. Tannins and Related Polyphenols: Perspectives on Their Chemistry, Biology, Ecological Effects, and Human Health Protection. Phytochemistry 2008, 69, 3006–3008. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Tong, F.; Zhang, J.; Liu, L.; Gao, X.; Cai, Q.; Wei, C.; Dong, J.; Hu, Y.; Wu, G.; Dong, X. Corilagin Attenuates Radiation-induced Brain Injury in Mice. Mol. Neurobiol. 2016, 53, 6982–6996. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.B.; Kim, H.S.; Park, E.K.; et al. Inhibitory Effect of Punicalagin on Lipopolysaccharide-induced Neuroinflammation, Oxidative Stress and Memory Impairment via Inhibition of Nuclear Factor-kappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Gol, M.; Ghorbanian, D.; Soltanpour, N.; Faraji, J.; Pourghasem, M. Protective Effect of Raisin (currant) Against Spatial Memory Impairment and Oxidative Stress in Alzheimer Disease Model. Nutr. Neurosci. 2017, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Jara-Moreno, D.; Castro-Torres, R.D.; Ettcheto, M.; Auladell, C.; Kogan, M.J.; Folch, J.; Verdaguer, E.; Cano, A.; Busquets, O.; Delporte, C.; et al. The Ethyl Acetate Extract of Leaves of Ugni molinae Turcz. Improves Neuropathological Hallmarks of Alzheimer’s Disease in Female APPswe/PS1dE9 Mice Fed with a High Fat Diet. J. Alzheimers. Dis. 2018, 66, 175–1191. [Google Scholar] [CrossRef] [PubMed]
- Sarahroodi, S.; Esmaeili, S.; Mikaili, P.; Hemmati, Z.; Saberi, Y. The Effects of Green Ocimum basilicum Hydroalcoholic Extract on Retention and Retrieval of Memory in Mice. Anc. Sci. Life 2012, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Golechha, M.; Bhatia, J.; Arya, D.S. Studies on Effects of Emblica officinalis (Amla) on Oxidative Stress and Cholinergic Function in Scopolamine Induced Amnesia in Mice. J. Environ. Biol. 2012, 33, 95–100. [Google Scholar]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the Detection of Reactive Oxygen Species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Kehrer, I.; Kozlov, A.V.; Haller, M.; Redi, H.; Hermann, M.; Grimm, M.; Troppmair, J. Mitochondrial ROS Production under Cellular Stress: Comparison of Different Detection Methods. Anal. Bioanal. Chem. 2011, 400, 2383–2390. [Google Scholar] [CrossRef]
- Wang, H.; A Joseph, J. Quantifying Cellular Oxidative Stress by Dichlorofluorescein Assay using Microplate Reader. Free. Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalvanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J.; Vina, J.; et al. Even Free Radicals Should Follow Some Rules: A Guide to Free Radical Research Terminology and Methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- Zielonka, J.; Kalyanaraman, B. “ROS-generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis”—A Critical Commentary. Free. Radic. Biol. Med. 2008, 45, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Patel, K.B.; Wardman, P.; Wrona, M. Kinetics of Reaction of Nitrogen Dioxide with Dihydrorhodamine and the Reaction of the Dihydrorhodamine Radical with Oxygen: Implications for Quantifying Peroxynitrite Formation in Cells. Arch. Biochem. Biophys. 2009, 484, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.Y.; Buettner, G.R. Iron and Dioxygen Chemistry is an Important Route to Initiation of Biological Free Radical Oxidations: An Electron Paramagnetic Resonance Spin Trapping Study. Free. Radic. Biol. Med. 1999, 26, 1447–1456. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin Signal Transduction in the Nervous System. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived Neurotrophic Factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. Trk Receptors: Mediators of Neurotrophin Action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Segal, R.A. Selectivity in Neurotrophin Signaling: Theme and Variations. Annu. Rev. Neurosci. 2003, 26, 299–330. [Google Scholar] [CrossRef]
- DeChant, G.; Barde, Y.-A. The Neurotrophin Receptor p75NTR: Novel Functions and Implications for Diseases of the Nervous System. Nat. Neurosci. 2002, 5, 1131–1136. [Google Scholar] [CrossRef]
- Stringer, T.P.; Guerrieri, D.; Vivar, C.; Van Praag, H. Plant-derived Flavanol (-)Epicatechin Mitigates Anxiety in Association with Elevated Hippocampal Monoamine and BDNF Levels, but Does Not Influence Pattern Separation in Mice. Transl. Psychiatry 2015, 5, e493. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal Memory Enhancing Activity of Pine Needle Extract against Scopolamine-induced Amnesia in a Mouse. Model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef]
- Lee, J.E.; Song, H.S.; Park, M.N.; Kim, S.H.; Shim, B.S.; Kim, B. Ethanol Extract of Oldenlandia diffusa Herba Attenuates Scopolamine-Induced Cognitive Impairments in Mice via Activation of BDNF, P-CREB and Inhibition of Acetylcholinesterase. Int. J. Mol. Sci. 2018, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pang, P.T.; Woo, N.H. The Yin and Yang of Neurotrophin Action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; An, S.C.; Xu, C.; Ma, X.M. Role of proBDNF and BDNF in Dendritic Spine Plasticity and Depressive-like Behaviors Induced by an Animal Model of Depression. Brain Res. 2017, 1663, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, Y.; Ramya, E.; Navya, K.; Kumar, G.P.; Anilakumar, K. Antidepressant Like Effects of Hydrolysable Tannins of Terminalia Catappa Leaf Extract via Modulation of Hippocampal Plasticity and Regulation of Monoamine Neurotransmitters Subjected to Chronic Mild Stress (CMS). Biomed. Pharmacother. 2017, 86, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Rong, S.; Xie, B.; Sun, Z.; Zhang, L.; Wu, H.; Yao, P.; Zhang, X.; Zhang, Y.; Liu, L. Rejuvenation of Antioxidant and Cholinergic Systems Contributes to the Effect of Procyanidins Extracted from the Lotus Seedpod Ameliorating Memory Impairment in Cognitively Impaired Aged Rats. Eur. Neuropsychopharmacol. 2009, 19, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Jolitha, A.B.; Ishii, N. Grape Seed Proanthocyanidin Extract (GSPE) and Antioxidant Defense in the Brain of Adult Rats. Med Sci. Monit. 2006, 12, 124–129. [Google Scholar]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.W.; Kim, J.E.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Kim, S.H.; Jung, Y.S.; Hong, J.T.; Hwang, D.Y. Anti-Oxidant Activity of Gallotannin-Enriched Extract of Galla Rhois Can Associate with the Protection of the Cognitive Impairment through the Regulation of BDNF Signaling Pathway and Neuronal Cell Function in the Scopolamine-Treated ICR Mice. Antioxidants 2019, 8, 450. https://doi.org/10.3390/antiox8100450
Park JW, Kim JE, Kang MJ, Choi HJ, Bae SJ, Kim SH, Jung YS, Hong JT, Hwang DY. Anti-Oxidant Activity of Gallotannin-Enriched Extract of Galla Rhois Can Associate with the Protection of the Cognitive Impairment through the Regulation of BDNF Signaling Pathway and Neuronal Cell Function in the Scopolamine-Treated ICR Mice. Antioxidants. 2019; 8(10):450. https://doi.org/10.3390/antiox8100450
Chicago/Turabian StylePark, Ji Won, Ji Eun Kim, Mi Ju Kang, Hyeon Jun Choi, Su Ji Bae, Sou Hyun Kim, Young Suk Jung, Jin Tae Hong, and Dae Youn Hwang. 2019. "Anti-Oxidant Activity of Gallotannin-Enriched Extract of Galla Rhois Can Associate with the Protection of the Cognitive Impairment through the Regulation of BDNF Signaling Pathway and Neuronal Cell Function in the Scopolamine-Treated ICR Mice" Antioxidants 8, no. 10: 450. https://doi.org/10.3390/antiox8100450
APA StylePark, J. W., Kim, J. E., Kang, M. J., Choi, H. J., Bae, S. J., Kim, S. H., Jung, Y. S., Hong, J. T., & Hwang, D. Y. (2019). Anti-Oxidant Activity of Gallotannin-Enriched Extract of Galla Rhois Can Associate with the Protection of the Cognitive Impairment through the Regulation of BDNF Signaling Pathway and Neuronal Cell Function in the Scopolamine-Treated ICR Mice. Antioxidants, 8(10), 450. https://doi.org/10.3390/antiox8100450





