Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects
Abstract
:1. Introduction
2. Advantages for Using CNT Composite Materials for Bone Tissue Regeneration and Engineering
2.1. Morphological Features
2.2. Mechanical Properties
2.3. Chemical Properties
2.4. Electrical and Magnetic Properties
3. Concerns and Current Solutions of CNTs as Nanomaterials for Bone Tissue Regeneration and Engineering
3.1. Toxicity and Dispersity
3.2. Current Solutions for Biosafety
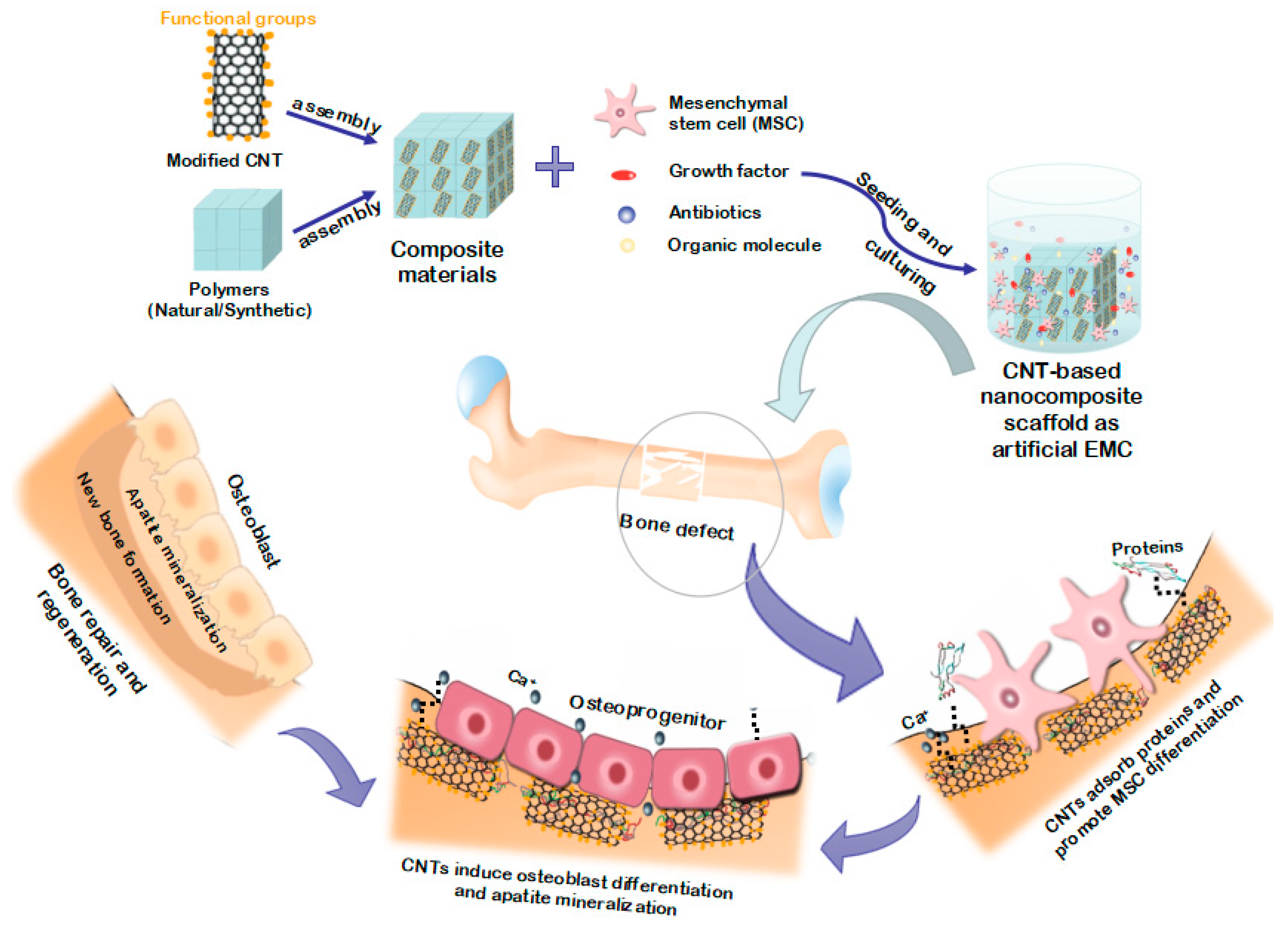
4. Advancements in CNT-Based Scaffolds or Implants for Bone Tissue Regeneration and Engineering
4.1. Synthesis Strategies for CNT-Based Scaffolds or Implants
4.2. CNTs with Calcium Phosphate Materials
4.3. CNTs with Natural Biopolymers
4.4. CNTs with Synthetic Biopolymers
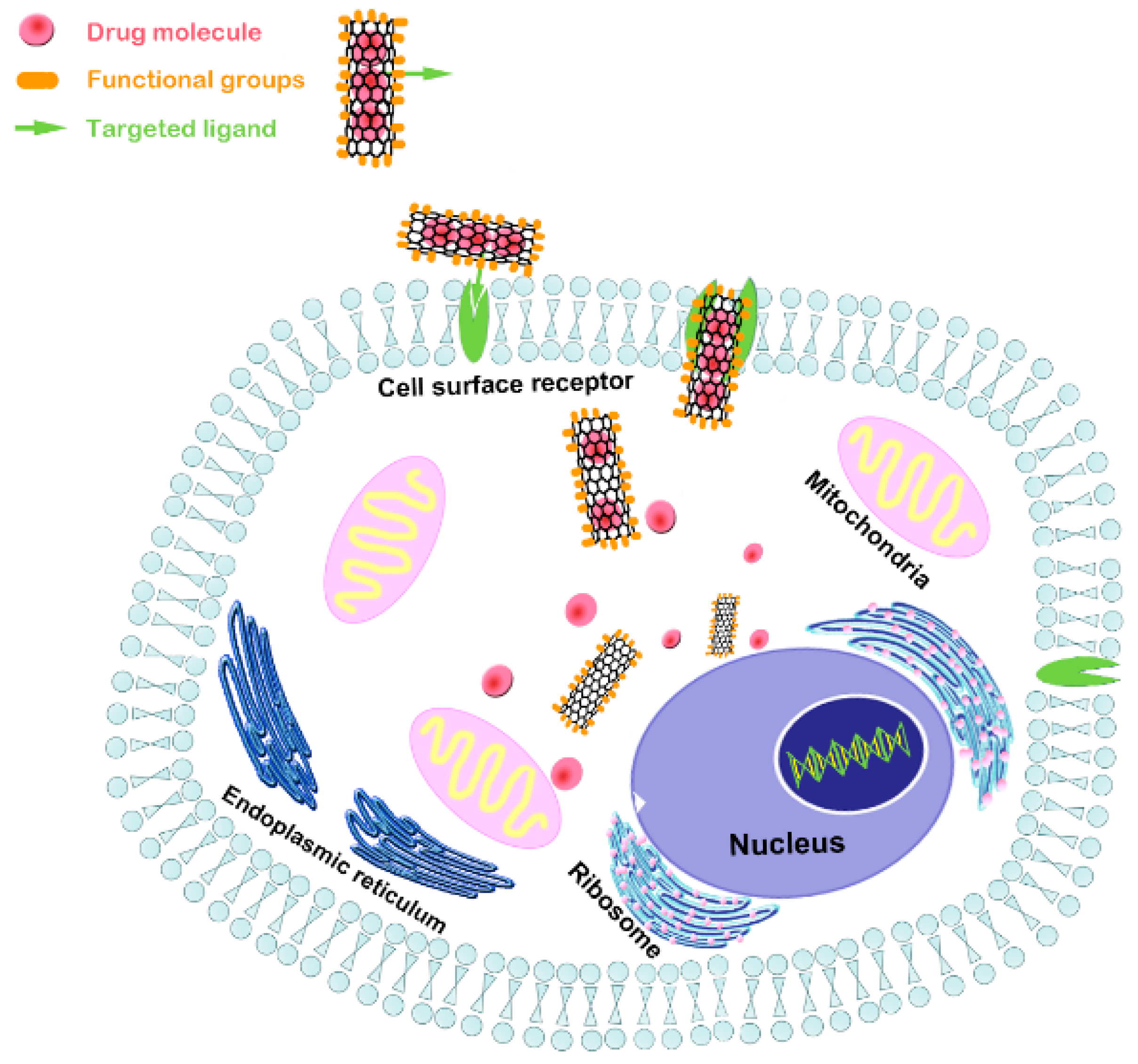
5. Advancements of CNT Composite as Nanocarriers for Bone Tissue Regeneration and Engineering
5.1. CNTs as Nanocarriers for Osteogenic Drugs
5.2. CNTs as Nanocarriers for Proteins, Peptides, and Genes
6. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNT | carbon nanotube |
| HA | hydroxyapatite |
| ECM | extracellular matrix |
| ALP | alkaline phosphatase |
| TCP | tricalcium phosphate |
| β-TCP | Beta-tricalcium phosphate |
| CPC | calcium phosphate cement |
| BMP | bone morphogenetic protein |
| rhBMP-2 | recombinant human bone morphogenetic protein-2 |
| rhBMP-9 | recombinant human bone morphogenetic protein-9 |
| ASC | human adipose-derived stem cell |
| MSC | mesenchymal stem cell |
| BMSC | bone marrow stem cell |
| BSA | bovine serum albumin |
| PCL | polycaprolactone |
| PMMA | polymethyl methacrylate |
| PLGA | poly(lactide-co-glycolide) |
| PLA | polylactic acid |
| PLLA | poly-L-lactic acid |
| PVA | polyvinyl alcohol |
| PGA | poly glycolic acid |
| PEEK | poly(etheretherketone) |
| PPF | polyanhydrides poly(propylene fumarate) |
| DEX | dexamethasone |
| Zol | zoledronic acid |
| MG132 | Z-Leu-Leu-Leu-al |
| CP3 | pro-apoptotic protein caspase-3 |
References
- Gao, C.; Feng, P.; Peng, S.; Shuai, C. Carbon nanotube, graphene and boron nitride nanotube reinforced bioactive ceramics for bone repair. Acta Biomater. 2017, 61, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Bonjour, J.P.; Coxam, V.; Goltzman, D.; Kanis, J.A.; Lappe, J.; Rejnmark, L.; Sahni, S.; Weaver, C. Benefits and safety of dietary protein for bone health—An expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Fo. Maturitas 2018, 79, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Olmos, J.M.; Hernández, J.L.; Martínez, J.; Pariente, E.; Castillo, J.; Prieto-Alhambra, D.; González-Macías, J. Relationship between spinal osteoarthritis and vertebral fractures in men older than 50 years: Data from the Camargo Cohort Study. J. Bone Mineral. Metab. 2018, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cimatti, B.; Santos, M.A.D.; Brassesco, M.S.; Okano, L.T.; Barboza, W.M.; Nogueira-Barbosa, M.H.; Engel, E.E. Safety, osseointegration, and bone ingrowth analysis of PMMA-based porous cement on animal metaphyseal bone defect model. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.D.; Liu, Y.; Xia, D.; Maria, O.M.; Khalili, S.; Wang, R.W.; Quan, V.H.; Hu, S.; Seuntjens, J. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PLoS ONE 2013, 8, e61632. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, M.; Holmes, L., Jr.; Gesheff, M.G.; Conway, J.D. Treatment Options for Nonunion With Segmental Bone Defects: Systematic Review and Quantitative Evidence Synthesis. J. Orthop. Trauma 2017, 31, 111–119. [Google Scholar] [CrossRef]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef]
- Toogood, P.; Miclau, T. Critical-Sized Bone Defects: Sequence and Planning. J. Orthop. Trauma 2017, 31, S23–S26. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1329–1343. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, Q.; Pei, X. Graphene Family Materials in Bone Tissue Regeneration: Perspectives and Challenges. Nanoscale Res. Lett. 2018, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, T.; Rybka, J.D.; Richter, M.; Kaczmarczyk, J.; Ramalingam, M.; Giersig, M. Cells and Nanomaterial-Based Tissue Engineering Techniques in the Treatment of Bone and Cartilage Injuries. J. Nanosci. Nanotechnol. 2016, 16, 8948–8952. [Google Scholar] [CrossRef]
- Spin-Neto, R.; Landazuri Del Barrio, R.A.; Pereira, L.A.; Marcantonio, R.A.; Marcantonio, E.; Marcantonio, E., Jr. Clinical similarities and histological diversity comparing fresh frozen onlay bone blocks allografts and autografts in human maxillary reconstruction. Clin. Implant Dent. Relat. Res. 2013, 15, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Amorosa, L.F.; Lee, C.H.; Aydemir, A.B.; Nizami, S.; Hsu, A.; Patel, N.R.; Gardner, T.R.; Navalgund, A.; Kim, D.G.; Park, S.H.; et al. Physiologic load-bearing characteristics of autografts, allografts, and polymer-based scaffolds in a critical sized segmental defect of long bone: An experimental study. Int. J. Nanomed. 2013, 8, 1637–1643. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Fernandez-Bances, I.; Perez-Basterrechea, M.; Perez-Lopez, S.; Nunez Batalla, D.; Fernandez Rodriguez, M.A.; Alvarez-Viejo, M.; Ferrero-Gutierrez, A.; Menendez-Menendez, Y.; Garcia-Gala, J.M.; Escudero, D.; et al. Repair of long-bone pseudoarthrosis with autologous bone marrow mononuclear cells combined with allogenic bone graft. Cytotherapy 2013, 15, 571–577. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef]
- Igwe, J.; Amini, A.; Mikael, P.; Laurencin, C.; Nukavarapu, S. Nanostructured Scaffolds for Bone Tissue Engineering. In Active Implants and Scaffolds for Tissue Regeneration; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–192. [Google Scholar]
- Sahoo, N.G.; Pan, Y.Z.; Li, L.; He, C.B. Nanocomposites for bone tissue regeneration. Nanomedicine 2013, 8, 639–653. [Google Scholar] [CrossRef]
- Yang, C.; Unursaikhan, O.; Lee, J.S.; Jung, U.W.; Kim, C.S.; Choi, S.H. Osteoconductivity and biodegradation of synthetic bone substitutes with different tricalcium phosphate contents in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 80–88. [Google Scholar] [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2015, 71, 354–361. [Google Scholar] [CrossRef]
- Torres-Costa, V.; Martínez-Muñoz, G.; Sánchez-Vaquero, V.; Muñoz-Noval, Á.; González-Méndez, L.; Punzón-Quijorna, E.; Gallach-Pérez, D.; Manso-Silván, M.; Climent-Font, A.; García-Ruiz, J.P. Engineering of silicon surfaces at the micro-and nanoscales for cell adhesion and migration control. Int. J. Nanomed. 2012, 7, 623–630. [Google Scholar]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the micro-and nanoscale to control cell function. Angew. Chem. 2010, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Gogolides, E.; Ellinas, K.; Tserepi, A. Hierarchical micro and nano structured, hydrophilic, superhydrophobic and superoleophobic surfaces incorporated in microfluidics, microarrays and lab on chip microsystems. Microelectron. Eng. 2015, 132, 135–155. [Google Scholar] [CrossRef]
- Von der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, A.Y.; Ichkitidze, L.P.; Podgaetsky, V.M.; Selishchev, S.V. Biomedical applications of promising nanomaterials with carbon nanotubes. Biomed. Eng. 2015, 48, 310–314. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B. Potential Impact of Multi-Walled Carbon Nanotubes Exposure to the Seedling Stage of Selected Plant Species. Nanomaterials 2014, 4, 203–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, D.A.; Webster, T.J. Carbon nanotubes for stem cell control. Mater. Today 2012, 15, 312–318. [Google Scholar] [CrossRef]
- Khalid, P.; Suman, V.B. Carbon Nanotube-Hydroxyapatite Composite for Bone Tissue Engineering and Their Interaction with Mouse Fibroblast L929 In Vitro. J. Bionanosci. 2017, 11, 233–240. [Google Scholar] [CrossRef]
- Wojtek, T.; Manish, C.; Federico, S. The chemical and physical characteristics of single-walled carbon nanotube film impact on osteoblastic cell response. Nanotechnology 2010, 21, 315102. [Google Scholar]
- Patel, H.; Kwon, S. Multi-walled carbon nanotube-induced inflammatory response and oxidative stress in a dynamic cell growth environment. J. Biol. Eng. 2012, 6, 22. [Google Scholar] [CrossRef]
- Zhang, F.; Weidmann, A.; Nebe, J.B.; Burkel, E. Osteoblast cell response to surface-modified carbon nanotubes. Mater. Sci. Eng. C 2012, 32, 1057–1061. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Moztarzadeh, F.; Haghighipour, N.; Ghazizadeh, L.; Baghbani, F.; Shokrgozar, M.A.; Allahyari, Z. Preparation and characterization of novel functionalized multiwalled carbon nanotubes/chitosan/beta-Glycerophosphate scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 365–372. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, B.R.C.; Rodrigues, K.F.; da Silva Fonseca, B.C.; Ribas, R.G.; do Amaral Montanheiro, T.L.; Thim, G.P. Recent advances in the use of carbon nanotubes as smart biomaterials. J. Mater. Chem. B 2019, 7, 1343–1360. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z. Carbon nanotubes in biology and medicine: An overview. Chinese Sci. Bull. 2011, 57, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Nayak, T.R.; Jian, L.; Phua, L.C.; Ho, H.K.; Ren, Y.; Pastorin, G. Thin films of functionalized multiwalled carbon nanotubes as suitable scaffold materials for stem cells proliferation and bone formation. ACS Nano 2010, 4, 7717–7725. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, Y.; Haniu, H.; Nomura, H.; Kobayashi, S.; Takanashi, S.; Okamoto, M.; Takizawa, T.; Aoki, K.; Usui, Y. A three-dimensional block structure consisting exclusively of carbon nanotubes serving as bone regeneration scaffold and as bone defect filler. PLoS ONE 2017, 12, e0172601. [Google Scholar] [CrossRef]
- Nam, K.; Eom, K.; Yang, J.; Park, J.; Lee, G.; Jang, K.; Lee, H.; Sang, W.L.; Yoon, D.S.; Chang, Y.L. Aptamer-functionalized nano-pattern based on carbon nanotube for sensitive, selective protein detection. J. Mater. Chem. 2012, 22, 23348–23356. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Paul, A.; Zhao, B.; Lee, C.; Rodes, L.; Prakash, S. Carbon nanotube lipid drug approach for targeted delivery of a chemotherapy drug in a human breast cancer xenograft animal model. Biomaterials 2013, 34, 10109–10119. [Google Scholar] [CrossRef]
- Shin, U.S.; Yoon, I.K.; Lee, G.S.; Jang, W.C.; Knowles, J.C.; Kim, H.W. Carbon nanotubes in nanocomposites and hybrids with hydroxyapatite for bone replacements. J. Tissue Eng. 2011, 2011, 674287. [Google Scholar] [CrossRef]
- Ju, S. Interactions between Carbon Nanotubes and Biomolecules. Prog. Chem. 2010, 22, 1767–1775. [Google Scholar]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Obregón, R.; Ramón-Azcón, J.; Salazar, G.; Shiku, H.; Ramalingam, M.; Matsue, T. Carbon Nanotubes and Graphene-Based Nanomaterials for Stem Cell Differentiation and Tissue Regeneration. J. Nanosci. Nanotechnol. 2016, 16, 8862–8880. [Google Scholar] [CrossRef]
- Anthony, D.B.; Sui, X.; Kellersztein, I.; De Luca, H.G.; White, E.R.; Wagner, H.D.; Greenhalgh, E.S.; Bismarck, A.; Shaffer, M.S.P. Continuous carbon nanotube synthesis on charged carbon fibers. Compos. Part A Appl. Sci. Manuf. 2018, 112, 525–538. [Google Scholar] [CrossRef]
- Namgung, S.; Baik, K.Y.; Park, J.; Hong, S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano 2011, 5, 7383–7390. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Yan, B.; Ma, H.; Li, N.; Hu, Y.; Ye, M. Covalent attaching protein to graphene oxide via diimide-activated amidation. Colloids Surf. B Biointerfaces 2010, 81, 434–438. [Google Scholar] [CrossRef]
- Kaiser, J.P.; Buerki-Thurnherr, T.; Wick, P. Influence of single walled carbon nanotubes at subtoxical concentrations on cell adhesion and other cell parameters of human epithelial cells. J. King Saud Univ. Sci. 2013, 25, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, H.; Niu, X.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012, 33, 4818–4827. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Ryu, J.; Budhathoki, M.; Gang, L.; Guo, Z. In situ stabilized carbon nanofiber (CNF) reinforced epoxy nanocomposites. J. Mater. Chem. 2010, 20, 4937–4948. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Jantou-Morris, V.; Horton, M.A.; Mccomb, D.W. The nano-morphological relationships between apatite crystals and collagen fibrils in ivory dentine. Biomaterials 2010, 31, 5275–5286. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Landis, W.J. Deposition of apatite in mineralizing vertebrate extracellular matrices: A model of possible nucleation sites on type I collagen. Connect. Tissue Res. 2011, 52, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Mcnally, E.A.; Schwarcz, H.P.; Botton, G.A.; Arsenault, A.L. A model for the ultrastructure of bone based on electron microscopy of ion-milled sections. PLoS ONE 2015, 46, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hui, H.; Mandal, S.K.; Haddon, R.C. A Bone Mimic Based on the Self-Assembly of Hydroxyapatite on Chemically Functionalized Single-Walled Carbon Nanotubes. Chem. Mater. 2005, 17, 3235–3241. [Google Scholar] [CrossRef]
- Lobo, A.O.; Marciano, F.R.; Regiani, I.; Ramos, S.C.; Matsushima, J.T.; Corat, E.J. Proposed model for growth preference of plate-like nanohydroxyapatite crystals on superhydrophilic vertically aligned carbon nanotubes by electrodeposition. Theor. Chem. Acc. 2011, 130, 1071–1082. [Google Scholar] [CrossRef]
- Sciortino, N.; Fedeli, S.; Paoli, P.; Brandi, A.; Chiarugi, P.; Severi, M.; Cicchi, S. Multiwalled carbon nanotubes for drug delivery: Efficiency related to length and incubation time. Int. J. Pharm. 2017, 521, 69–72. [Google Scholar] [CrossRef]
- Munir, K.S.; Wen, C.; Li, Y. Carbon Nanotubes and Graphene as Nanoreinforcements in Metallic Biomaterials: A Review. Adv. Biosyst. 2019, 3, 2366–7478. [Google Scholar] [CrossRef]
- Kim, H. Biomedical nanocomposites of hydroxyapatite/polycaprolactone obtained by surfactant mediation. J. Biomed. Mater. Res. Part A 2010, 83A, 169–177. [Google Scholar] [CrossRef]
- Neubauer, E.; Kitzmantel, M.; Hulman, M.; Angerer, P. Potential and challenges of metal-matrix-composites reinforced with carbon nanofibers and carbon nanotubes. Compos. Sci. Technol. 2010, 70, 2228–2236. [Google Scholar] [CrossRef] [Green Version]
- Ashkan, A.; Jayatissa, A.H.; Jayasuriya, A.C. Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. Part A 2014, 102, 2704–2712. [Google Scholar]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Liao, S.; Li, J. Carbon nanotubes reinforced composites for biomedical applications. Biomed. Res. Int. 2014, 2014, 518609. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Pallela, R.; Kim, S.K. Applications of carbon nanomaterials in bone tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 3105–3123. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, W.; Lin, D.; Yang, K. Influence of surface oxidation of multiwalled carbon nanotubes on the adsorption affinity and capacity of polar and nonpolar organic compounds in aqueous phase. Environ. Sci. Technol. 2012, 46, 5446–5454. [Google Scholar] [CrossRef] [PubMed]
- Gautam, V.; Singh, K.P.; Yadav, V.L. Polyaniline/multiwall carbon nanotubes/starch nanocomposite material and hemoglobin modified carbon paste electrode for hydrogen peroxide and glucose biosensing. Int. J. Biol. Macromol. 2018, 111, 1124–1132. [Google Scholar] [CrossRef]
- Peyvandi, A.; Soroushian, P.; Abdol, N.; Balachandra, A.M. Surface-modified graphite nanomaterials for improved reinforcement efficiency in cementitious paste. Carbon 2013, 63, 175–186. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface Chemical Functional Groups Modification of Porous Carbon. Recent Pat. Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T., Jr.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2016, 67, 1–47. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Ma, Q.; Luo, J.; Wei, W.; Liu, X. Noncovalent functionalization of carbon nanotube using poly(vinylcarbazole)-based compatibilizer for reinforcement and conductivity improvement in epoxy composite. J. Appl. Polym. Sci. 2017, 134, 45022. [Google Scholar] [CrossRef]
- Rostamizadeh, K.; Habibizadeh, M.; Dalali, N.; Ramazani, A. Preparation and characterization of PEGylated multiwall carbon nanotubes as covalently conjugated and non-covalent drug carrier: A comparative study. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 1–9. [Google Scholar] [PubMed]
- Stevens, J.L.; Huang, A.Y.; Peng, H.; Chiang, I.W.; Khabashesku, V.N.; Margrave, J.L. Sidewall Amino-Functionalization of Single-Walled Carbon Nanotubes through Fluorination and Subsequent Reactions with Terminal Diamines. Nano Lett. 2003, 3, 331–336. [Google Scholar] [CrossRef]
- Kang, E.S.; Kim, D.S.; Suhito, I.R.; Choo, S.S.; Kim, S.J.; Song, I.; Kim, T.H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Woods, M.D.; Illingworth, K.D.; Niemeier, R.; Schafer, I.; Cady, C.; Filip, P.; El-Amin, S.F., III. Single walled carbon nanotube composites for bone tissue engineering. J. Orthop. Res. 2013, 31, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Valverde, T.M.; Castro, E.G.; Cardoso, M.H.; Martins-Junior, P.A.; Souza, L.M.; Silva, P.P.; Ladeira, L.O.; Kitten, G.T. A novel 3D bone-mimetic scaffold composed of collagen/MTA/MWCNT modulates cell migration and osteogenesis. Life Sci. 2016, 162, 115–124. [Google Scholar] [CrossRef]
- Kiran, S.; Nune, K.C.; Misra, R.D. The significance of grafting collagen on polycaprolactone composite scaffolds: Processing-structure-functional property relationship. J. Biomed. Mater. Res. A 2015, 103, 2919–2931. [Google Scholar] [CrossRef]
- Cirillo, G.; Hampel, S.; Spizzirri, U.G.; Parisi, O.I.; Picci, N.; Iemma, F. Carbon nanotubes hybrid hydrogels in drug delivery: A perspective review. Biomed. Res. Int. 2014, 2014, 825017. [Google Scholar] [CrossRef]
- Usui, Y.; Aoki, K.; Narita, N.; Murakami, N.; Nakamura, I.; Nakamura, K.; Ishigaki, N.; Yamazaki, H.; Horiuchi, H.; Kato, H.; et al. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small 2008, 4, 240–246. [Google Scholar] [CrossRef]
- La, W.G.; Jin, M.; Park, S.; Yoon, H.H.; Jeong, G.J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 2014, 107–116. [Google Scholar]
- Murugan, E.; Arumugam, S. New dendrimer functionalized multi-walled carbon nanotube hybrids for bone tissue engineering. RSC Adv. 2014, 4, 35428–35441. [Google Scholar] [CrossRef]
- Li, Z.; de Barros, A.L.B.; Soares, D.C.F.; Moss, S.N.; Alisaraie, L. Functionalized single-walled carbon nanotubes: Cellular uptake, biodistribution and applications in drug delivery. Int. J. Pharm. 2017, 524, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.; Minett, A.; Ellis-Behnke, R.; Zreiqat, H. Carbon nanotubes: Their potential and pitfalls for bone tissue regeneration and engineering. Nanomedicine 2013, 9, 1139–1158. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2018, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lim, C.H.; Kenry; Su, C.; Loh, K.P.; Lim, C.T. Cell-assembled graphene biocomposite for enhanced chondrogenic differentiation. Small 2015, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Tu, X.; Liu, B.; Chen, L.; Zheng, M.; Zhou, C. Chirality-controlled synthesis of single-wall carbon nanotubes using vapour-phase epitaxy. Nat. Commun. 2012, 3, 1199. [Google Scholar] [CrossRef]
- Md Azahar, A.; Solanki, P.R.; Saurabh, S.; Samer, S.; Agrawal, V.V.; Renu, J.; Malhotra, B.D. Protein Functionalized Carbon Nanotubes-based Smart Lab-on-a-Chip. ACS Appl. Mater. Interfaces 2015, 7, 5837–5846. [Google Scholar]
- Ferrer-Anglada, N.; Gomis, V.; El-Hachemi, Z.; Weglikovska, U.D.; Kaempgen, M.; Roth, S.J.P.S.S. Carbon nanotube based composites for electronic applications: CNT–conducting polymers, CNT–Cu. Phys. Status Solidi 2010, 203, 1082–1087. [Google Scholar] [CrossRef]
- Saha, A.; Jiang, C.; Martí, A.A. Carbon Nanotube Networks on Different Platforms. Carbon 2014, 79, 1–18. [Google Scholar] [CrossRef]
- Shanta, A.S.; Mamun, K.A.A.; Islam, S.K.; Mcfarlane, N.; Hensley, D.K. Carbon Nanotubes, Nanofibers and Nanospikes for Electrochemical Sensing: A Review. Int. J. High Speed Electron. Syst. 2017, 26, 25–36. [Google Scholar] [CrossRef]
- Qamar, Z.; Zakria, M.; Shakoor, R.I.; Raffi, M.; Mehmood, M.; Mahmood, A. Reinforcement of electroactive characteristics in polyvinylidene fluoride electrospun nanofibers by intercalation of multi-walled carbon nanotubes. J. Polym. Res. 2017, 24, 39. [Google Scholar] [CrossRef]
- Mercante, L.A.; Pavinatto, A.; Iwaki, L.E.; Scagion, V.P.; Zucolotto, V.; Oliveira, O.N., Jr.; Mattoso, L.H.; Correa, D.S. Electrospun polyamide 6/poly(allylamine hydrochloride) nanofibers functionalized with carbon nanotubes for electrochemical detection of dopamine. ACS Appl. Mater. Interfaces 2015, 7, 4784–4790. [Google Scholar] [CrossRef] [PubMed]
- Mackle, J.N.; Blond, D.J.; Mooney, E.; McDonnell, C.; Blau, W.J.; Shaw, G.; Barry, F.P.; Murphy, J.M.; Barron, V. In vitro characterization of an electroactive carbon-nanotube-based nanofiber scaffold for tissue engineering. Macromol. Biosci. 2011, 11, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Iannazzo, D.; Piperno, A.; Pistone, A.; Fazio, M.; Russo, A.; Marcacci, M.; Galvagno, S. Hybrid composites made of multiwalled carbon nanotubes functionalized with Fe3O4 nanoparticles for tissue engineering applications. J. Nanotechnol. 2012, 23, 465102. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Kulanthaivel, S.; Arunachalam, T.; Banerjee, I.; Pramanik, K. Biological and mechanical evaluation of poly(lactic-co-glycolic acid) based composites reinforced with one, two and three dimensional carbon biomaterials for bone tissue regeneration. J. Biomed. Mater. 2017, 12, 025012. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Puerto, I.; Sandonis, I.; Ribeiro, S.; Lanceros-Mendez, S. Hydrolytic degradation and cytotoxicity of poly(lactic-co-glycolic acid)/multiwalled carbon nanotubes for bone regeneration. J. Appl. Polym. Sci. 2019, 136, 48439. [Google Scholar] [CrossRef]
- Park, S.J.; Khang, D. Conformational changes of fibrinogen in dispersed carbon nanotubes. Int. J. Nanomed. 2012, 7, 4325–4333. [Google Scholar] [Green Version]
- Hilder, T.A.; Hill, J.M. Maximum velocity for a single water molecule entering a carbon nanotube. Nanosci. Nanotechnol. 2009, 9, 1403–1407. [Google Scholar] [CrossRef]
- Mahmood, N.; Islam, M.; Hameed, A.; Saeed, S.; Khan, A.N. Polyamide-6-based composites reinforced with pristine or functionalized multi-walled carbon nanotubes produced using melt extrusion technique. J. Compos. Mater. 2014, 48, 1197–1207. [Google Scholar] [CrossRef]
- Mwangi, J.N.; Wang, N.; Ingersoll, C.G.; Hardesty, D.K.; Brunson, E.L.; Li, H.; Deng, B. Toxicity of carbon nanotubes to freshwater aquatic invertebrates. Environ. Toxicol. Chem. 2012, 31, 1823–1830. [Google Scholar] [CrossRef]
- Sinar, A.A.; Nur Azni, M.A.; Zainuddin, F.; Hazizan, M.A.; Siti Shuhadah, M.S.; Sahrim, H.A. Treatment Method for Dispersion of Carbon Nanotubes: A Review. Mater. Sci. Forum 2014, 803, 299–304. [Google Scholar] [CrossRef]
- Ko, H.; Peleshanko, S.; Tsukruk, V.V. Combing and Bending of Carbon Nanotube Arrays with Confined Microfluidic Flow on Patterned Surfaces. J. Phys. Chem. B 2014, 108, 4385–4393. [Google Scholar] [CrossRef]
- Homma, Y.; Chiashi, S.; Yamamoto, T.; Kono, K.; Matsumoto, D.; Shitaba, J.; Sato, S. Photoluminescence measurements and molecular dynamics simulations of water adsorption on the hydrophobic surface of a carbon nanotube in water vapor. Phys. Rev. Lett. 2013, 110, 157402. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2010, 14, 1857–1860. [Google Scholar] [CrossRef]
- Saleh, N.B.; Pfefferle, L.D.; Elimelech, M. Aggregation Kinetics of Multiwalled Carbon Nanotubes in Aquatic Systems: Measurements and Environmental Implications. Environ. Chem. 2015, 42, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Saltiel, C.; Manickavasagam, S.; Schadler, L.S.; Siegel, R.W.; Yang, H. Aggregation behavior of single-walled carbon nanotubes in dilute aqueous suspension. J. Colloid Interface Sci. 2004, 280, 91–97. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Yao, J.-M.; Qin, Z.-Y.; Liu, L.; Yang, X.-G. Comparison of covalent and noncovalent interactions of carbon nanotubes on the crystallization behavior and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci. 2013, 130, 4299–4307. [Google Scholar] [CrossRef]
- Niroula, J.; Premaratne, G.; Ali Shojaee, S.; Lucca, D.A.; Krishnan, S. Combined covalent and noncovalent carboxylation of carbon nanotubes for sensitivity enhancement of clinical immunosensors. Chem. Commun. 2016, 52, 13039–13042. [Google Scholar] [CrossRef] [Green Version]
- Usrey, M.L.; Strano, M.S. Controlling Single-Walled Carbon Nanotube Surface Adsorption with Covalent and Noncovalent Functionalization. J. Phys. Chem. C 2009, 113, 12443–12453. [Google Scholar] [CrossRef]
- Clavé, G.; Delport, G.; Roquelet, C.; Lauret, J.-S.; Deleporte, E.; Vialla, F.; Langlois, B.; Parret, R.; Voisin, C.; Roussignol, P.; et al. Functionalization of Carbon Nanotubes through Polymerization in Micelles: A Bridge between the Covalent and Noncovalent Methods. Chem. Mater. 2013, 25, 2700–2707. [Google Scholar] [CrossRef]
- Liu, J.Q.; Xiao, T.; Liao, K.; Wu, P. Interfacial design of carbon nanotube polymer composites: A hybrid system of noncovalent and covalent functionalizations. Nanotechnology 2007, 18, 165701. [Google Scholar] [CrossRef]
- Tu, W.; Lei, J.; Ju, H. Noncovalent nanoassembly of porphyrin on single-walled carbon nanotubes for electrocatalytic reduction of nitric oxide and oxygen. Electrochem. Commun. 2008, 10, 766–769. [Google Scholar] [CrossRef]
- Rajarajeswari, M.; Iyakutti, K.; Kawazoe, Y. Noncovalent and covalent functionalization of a (5, 0) single-walled carbon nanotube with alanine and alanine radicals. J. Mol. Model. 2012, 18, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Marques, P.A.A.P.; Sousa, A.C.M.; Gracio, J.; Silva, V.S.; Goncalves, P.P.; Ferreira, J.M.F.; Olhero, S. Biotoxicity study of bone cement based on a functionalised multi-walled carbon nanotube-reinforced PMMA/HAp nanocomposite. Int. J. Nano Biomater. 2009, 2, 442–453. [Google Scholar] [CrossRef]
- Liu, B.; Campo, E.M.; Bossing, T. Drosophila embryos as model to assess cellular and developmental toxicity of multi-walled carbon nanotubes (MWCNT) in living organisms. PLoS ONE 2014, 9, e88681. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, J.; Huang, Z.; Qu, L.; Lin, N.; Liang, C.; Dai, R.; Tang, L.; Tian, F. Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs. Int. J. Nanomed. 2017, 12, 3109–3120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Naskar, S.; Kuotsu, K. A review on carbon nanotubes: Influencing toxicity and emerging carrier for platinum based cytotoxic drug application. J. Drug Deliv. Sci. Technolo. 2019, 51, 708–720. [Google Scholar] [CrossRef]
- Liang, X.; Cheng, Q. Synergistic reinforcing effect from graphene and carbon nanotubes. Compos. Commun. 2018, 10, 122–128. [Google Scholar] [CrossRef]
- Che, A.C.; Azad, C.L.; Ovalle-Robles, R.; Fang, S.; Lima, M.D.; Lepró, X.; Collins, S.; Baughman, R.H.; Dalton, A.B.; Plant, N.J. Primary liver cells cultured on carbon nanotube substrates for liver tissue engineering and drug discovery applications. ACS Appl. Mater. Interfaces 2014, 6, 10373–10380. [Google Scholar]
- Song, G.; Guo, X.; Zong, X.; Du, L.; Zhao, J.; Lai, C.; Jin, X. Toxicity of functionalized multi-walled carbon nanotubes on bone mesenchymal stem cell in rats. Dent. Mater. J. 2019, 38, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Ema, M. Biological response and morphological assessment of individually dispersed multi-wall carbon nanotubes in the lung after intratracheal instillation in rats. Toxicology 2010, 276, 143–153. [Google Scholar] [Green Version]
- Donaldson, K.; Poland, C.A.; Murphy, F.A.; MacFarlane, M.; Chernova, T.; Schinwald, A. Pulmonary toxicity of carbon nanotubes and asbestos-similarities and differences. Adv. Drug Deliv. Rev. 2013, 65, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Gernand, J.M.; Casman, E.A. A meta-analysis of carbon nanotube pulmonary toxicity studies—How physical dimensions and impurities affect the toxicity of carbon nanotubes. Risk Anal. 2014, 34, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Figarol, A.; Pourchez, J.; Boudard, D.; Forest, V.; Berhanu, S.; Tulliani, J.M.; Lecompte, J.P.; Cottier, M.; Bernache-Assollant, D.; Grosseau, P. Thermal annealing of carbon nanotubes reveals a toxicological impact of the structural defects. J. Nanoparticle Res. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- Lanone, S.; Andujar, P.; Kermanizadeh, A.; Boczkowski, J. Determinants of carbon nanotube toxicity. Adv. Drug Deliv. Rev. 2013, 65, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, R. Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels. Environ. Int. 2012, 40, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Orecna, M.; Paoli, S.H.D.; Janouskova, O.; Tegegn, T.Z.; Filipova, M.; Bonevich, J.E.; Holada, K.; Simak, J. Toxicity of carboxylated carbon nanotubes in endothelial cells is attenuated by stimulation of the autophagic flux with the release of nanomaterial in autophagic vesicles. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 939–948. [Google Scholar] [CrossRef]
- Edwards, S.L.; Werkmeister, J.A.; Ramshaw, J.A. Carbon nanotubes in scaffolds for tissue engineering. Expert Rev. Med. Devices 2009, 6, 499–505. [Google Scholar] [CrossRef]
- Rafeeqi, T.; Kaul, G. Elucidation of Interaction Between Multi-Walled Carbon Nanotubes and Cell Culture Medium by Spectroscopy Supports Biocompatibility of These Nanotubes. J. Comput. Theor. Nanosci. 2011, 4, 536–540. [Google Scholar] [CrossRef]
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: A Perspective View. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Al-Jamal, K.T.; Kostarelos, K. Cytotoxic assessment of carbon nanotube interaction with cell cultures. Methods Mol. Biol. 2011, 726, 299–312. [Google Scholar]
- Ali-Boucetta, H.; Al-Jamal, K.T.; Müller, K.H.; Li, S.; Porter, A.E.; Eddaoudi, A.; Prato, M.; Bianco, A.; Kostarelos, K. Cellular Uptake and Cytotoxic Impact of Chemically Functionalized and Polymer-Coated Carbon Nanotubes. Small 2011, 7, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Myer, M.H.; Black, M.C. Multi-walled Carbon Nanotubes Reduce Toxicity of Diphenhydramine to Ceriodaphnia dubia in Water and Sediment Exposures. Bull. Environ. Contam. Toxicol. 2017, 99, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Jośko, I.; Oleszczuk, P.; Pranagal, J.; Lehmann, J.; Xing, B.; Cornelissen, G. Effect of biochars, activated carbon and multiwalled carbon nanotubes on phytotoxicity of sediment contaminated by inorganic and organic pollutants. Ecol. Eng. 2013, 60, 50–59. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Chen, C.; Wu, W.; Yao, K.; Jian, Z. Ozone-Mediated Functionalization of Multi-Walled Carbon Nanotubes and Their Activities for Oxygen Reduction Reaction. J. Mater. Sci. Technol. 2016, 32, 533–538. [Google Scholar] [CrossRef]
- Zhao, W.; Mi, J.L.; Kim, H.T.; Kim, I.J. The synthesis of carbon nanotubes (CNTs) by catalytic CVD using a Fe/Co-supported zeolite template. Electron. Mater. Lett. 2011, 7, 139–144. [Google Scholar] [CrossRef]
- Meng, D.; Shi, C.S.; Liu, E.Z.; He, C.N.; Zhao, N.Q. One-Step Synthesis of Thin Graphite Layers Supported CNTs in Porous Copper by CVD. Adv. Mater. Res. 2012, 588–589, 1677–1680. [Google Scholar] [CrossRef]
- Das, R.; Shahnavaz, Z.; Ali, M.E.; Islam, M.M.; Abd Hamid, S.B. Can We Optimize Arc Discharge and Laser Ablation for Well-Controlled Carbon Nanotube Synthesis? Nanoscale Res. Lett. 2016, 11, 510. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kundu, B.; Sen, S.; Chanda, A. Improved properties of hydroxyapatite–carbon nanotube biocomposite: Mechanical, in vitro bioactivity and biological studies. Ceram. Int. 2014, 40, 5635–5643. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Bai, Y.; Ma, W.; Jia, R.-L.; Zheng, X.-B. Improved Properties of Carbon Nanotube-Fluorhydroxyapatite Biocomposite: Mechanical, Chemical Stability, and Antibacterial Activity. Adv. Eng. Mater. 2016, 18, 1921–1929. [Google Scholar] [CrossRef]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Lee, B.Y.; Padalhin, A.R.; Sarker, A.; Carpena, N.; Kim, B.; Paul, K.; Choi, H.J.; Bae, S.H.; Lee, B.T. Brushite-based calcium phosphate cement with multichannel hydroxyapatite granule loading for improved bone regeneration. J. Biomater. Appl. 2016, 30, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Jung, H.D.; Jang, T.S.; Kim, S.W.; Kang, M.H.; Kim, H.E.; Koh, Y.H. Synthesis and evaluation of bone morphogenetic protein (BMP)-loaded hydroxyapatite microspheres for enhanced bone regeneration. Ceram. Int. 2016, 42, 7748–7756. [Google Scholar] [CrossRef]
- Sariibrahimoglu, K.; Wolke, J.G.C.; Leeuwenburgh, S.C.G.; Jansen, J.A. Characterization of α/β-TCP Based Injectable Calcium Phosphate Cement as a Potential Bone Substitute. Key Eng. Mater. 2013, 529–530, 157–160. [Google Scholar] [CrossRef]
- Pripatnanont, P.; Praserttham, P.; Suttapreyasri, S.; Leepong, N.; Monmaturapoj, N. Bone Regeneration Potential of Biphasic Nanocalcium Phosphate with High Hydroxyapatite/Tricalcium Phosphate Ratios in Rabbit Calvarial Defects. Int. J. Oral Maxillofac. Implants 2016, 31, 294–303. [Google Scholar] [CrossRef]
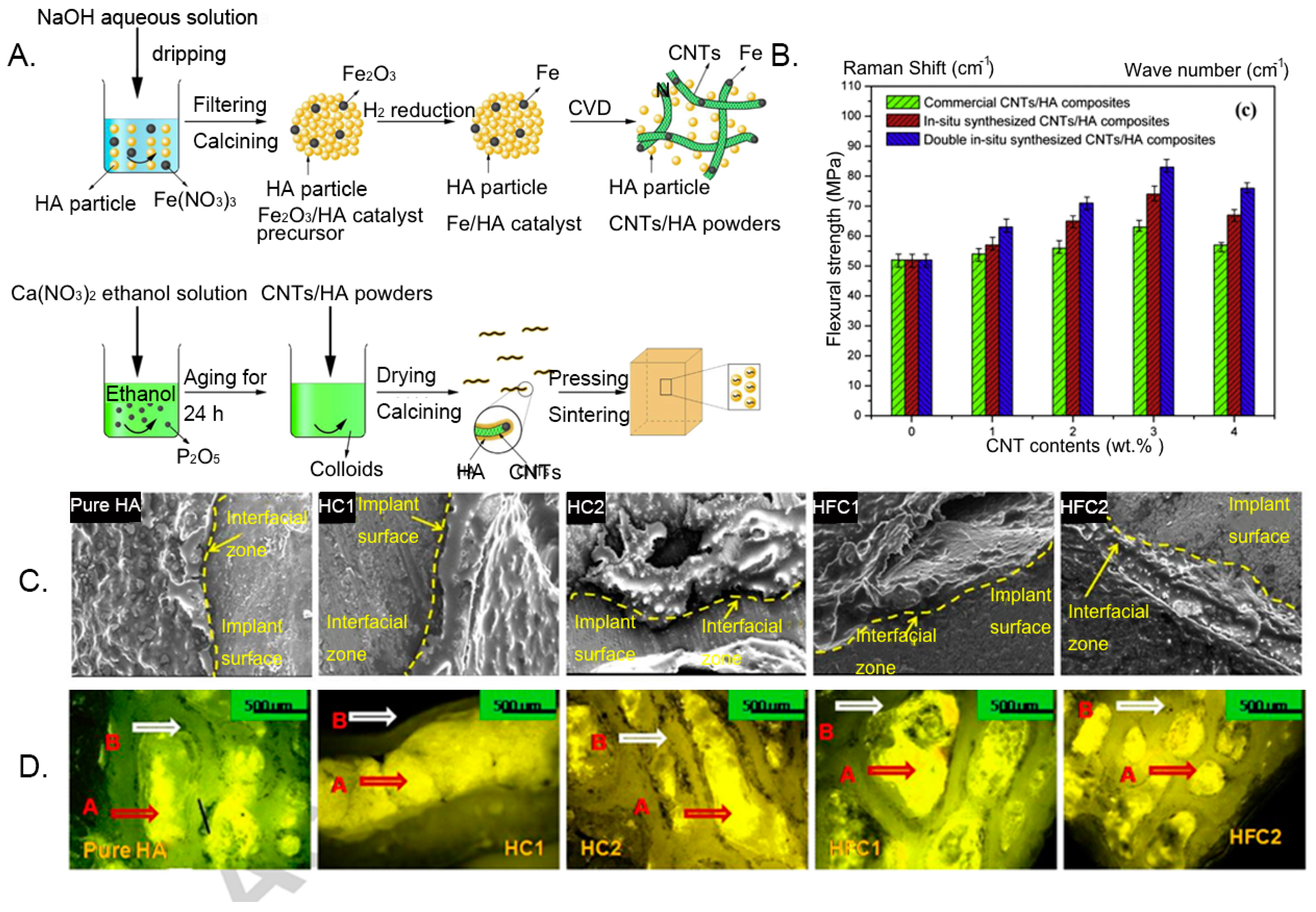
- Li, H.; Zhao, Q.; Li, B.; Kang, J.; Yu, Z.; Li, Y.; Song, X.; Liang, C.; Wang, H. Fabrication and properties of carbon nanotube-reinforced hydroxyapatite composites by a double in situ synthesis process. Carbon 2016, 101, 159–167. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nandi, S.K.; Kundu, B.; Chanda, A.; Sen, S.; Das, P.K. Enhanced bone regeneration with carbon nanotube reinforced hydroxyapatite in animal model. J. Mech. Behav. Biomed. Mater. 2016, 60, 243–255. [Google Scholar] [CrossRef]
- Park, J.E.; Jang, Y.S.; Bae, T.S.; Lee, M.H. Biocompatibility Characteristics of Titanium Coated with Multi Walled Carbon Nanotubes-Hydroxyapatite Nanocomposites. Materials 2019, 12, 224. [Google Scholar] [CrossRef]
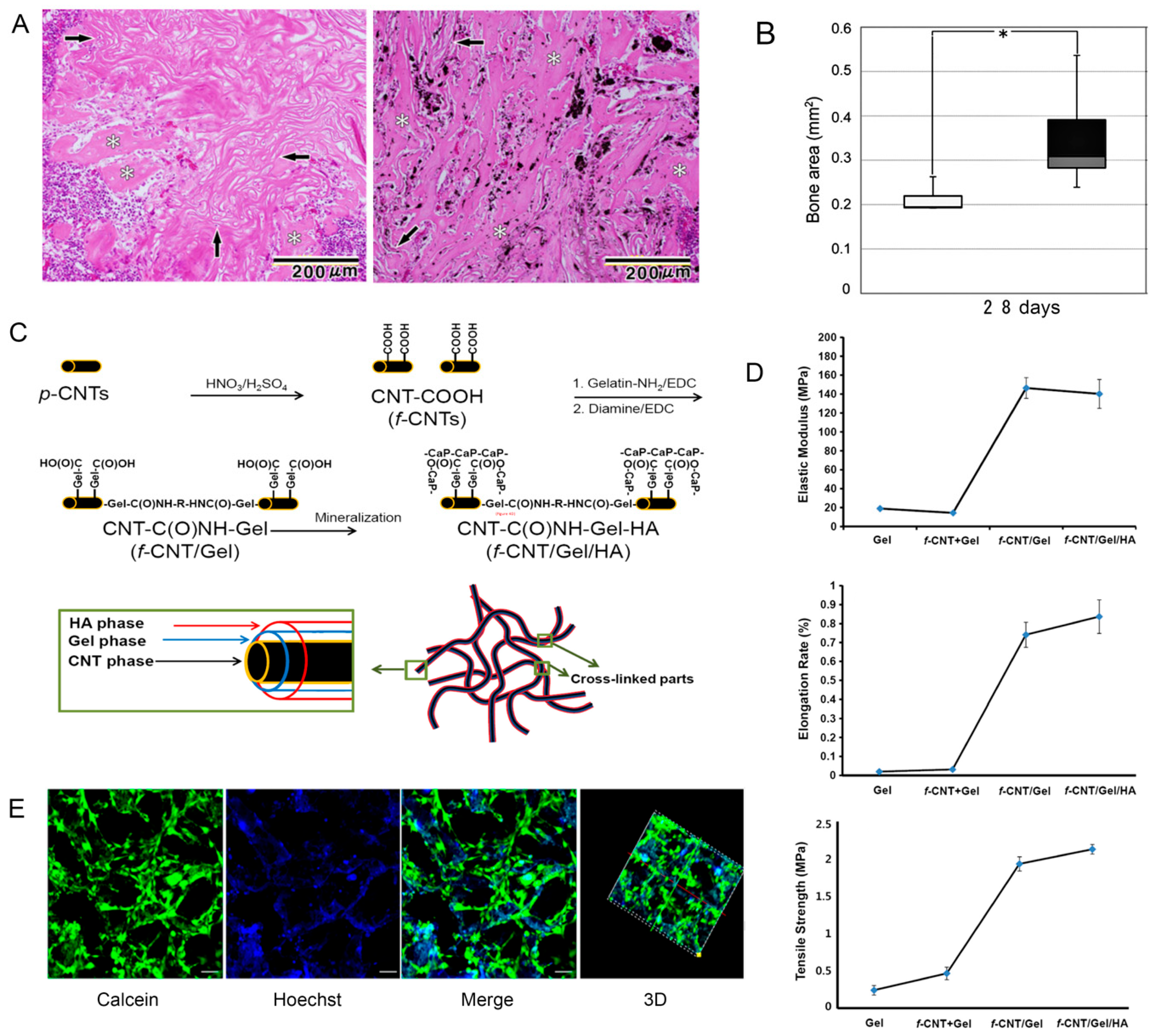
- Jing, Z.; Wu, Y.; Su, W.; Tian, M.; Jiang, W.; Cao, L.; Zhao, L.; Zhao, Z. Carbon Nanotube Reinforced Collagen/Hydroxyapatite Scaffolds Improve Bone Tissue Formation In Vitro and In Vivo. Ann. Biomed. Eng. 2017, 45, 2075–2087. [Google Scholar] [CrossRef]
- Naudi, K.B.; Ayoub, A.; Mcmahon, J.; Silvio, L.D.; Lappin, D.; Hunter, K.D.; Barbenel, J. Mandibular reconstruction in the rabbit using beta-tricalcium phosphate (β-TCP) scaffolding and recombinant bone morphogenetic protein 7 (rhBMP-7)–Histological, radiographic and mechanical evaluations. J. Cranio Maxillofac. Surg. 2012, 40, e461–e469. [Google Scholar] [CrossRef]
- Jensen, S.S.; Broggini, N.; Hjortinghansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2010, 17, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Lyne, D.V.; Barragan, M.; Berkland, C.J.; Detamore, M.S. Microsphere-based scaffolds encapsulating tricalcium phosphate and hydroxyapatite for bone regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 121. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, F.; Mohammadi, H.; Azimi, M.; Hafezi, M.; Abu Osman, N.A. Synthesis and characterization of β-TCP/CNT nanocomposite: Morphology, microstructure and in vitro bioactivity. Ceram. Int. 2017, 43, 7573–7580. [Google Scholar] [CrossRef]
- Tanahashi, M.; Matsuda, T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 2015, 34, 305–315. [Google Scholar] [CrossRef]
- Gholami, F.; Zein, S.H.S.; Gerhardt, L.C.; Low, K.L.; Tan, S.H.; Mcphail, D.S.; Grover, L.M.; Boccaccini, A.R. Cytocompatibility, bioactivity and mechanical strength of calcium phosphate cement reinforced with multi-walled carbon nanotubes and bovine serum albumin. Ceram. Int. 2013, 39, 4975–4983. [Google Scholar] [CrossRef]
- Wang, S.; Sun, X.; Wang, Y.; Sun, K.; Bi, J. Properties of reduced graphene/carbon nanotubes reinforced calcium phosphate bone cement in a microwave environment. J. Mater. Sci. Mater. Med. 2019, 30, 37. [Google Scholar] [CrossRef]
- Chew, K.K.; Low, K.L.; Sharif Zein, S.H.; McPhail, D.S.; Gerhardt, L.C.; Roether, J.A.; Boccaccini, A.R. Reinforcement of calcium phosphate cement with multi-walled carbon nanotubes and bovine serum albumin for injectable bone substitute applications. J. Mech. Behav. Biomed. Mater. 2011, 4, 331–339. [Google Scholar] [CrossRef]
- Venkatesan, J.; Ryu, B.; Sudha, P.N.; Kim, S.K. Preparation and characterization of chitosan-carbon nanotube scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 393–402. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252. [Google Scholar] [CrossRef]
- Di, M.A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar]
- Chen, L.; Hu, J.; Shen, X.; Tong, H. Synthesis and characterization of chitosan–multiwalled carbon nanotubes/hydroxyapatite nanocomposites for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; Gutierrez, M.C.; Moreno-Vicente, C.; Hortiguela, M.J.; Ramos, V.; Lopez-Lacomba, J.L.; Ferrer, M.L.; del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sato, Y.; Zhang, M.; Haniu, H.; Okamoto, M.; Aoki, K.; Takizawa, T.; Yoshida, K.; Sobajima, A.; Kamanaka, T.; et al. In Vitro and In Vivo Evaluation of a Three-Dimensional Porous Multi-Walled Carbon Nanotube Scaffold for Bone Regeneration. Nanomaterials 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Hu, R.; Yang, C.; Yoon, H.S.; Yong, K.-T. Pancreatic cancer gene therapy using an siRNA-functionalized single walled carbon nanotubes (SWNTs) nanoplex. Biomater. Sci. 2014, 2, 1244–1253. [Google Scholar] [CrossRef]
- Mikael, P.E.; Nukavarapu, S.P. Functionalized Carbon Nanotube Composite Scaffolds for Bone Tissue Engineering: Prospects and Progress. J. Biomater. Tissue Eng. 2011, 1, 76–85. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Chen, X.; Ismail, A.F.; Aziz, M.; Abdolahi, E.; Mahmoodiyan, F. Improved antibacterial properties of an Mg-Zn-Ca alloy coated with chitosan nanofibers incorporating silver sulfadiazine multiwall carbon nanotubes for bone implants. Polym. Adv. Technol. 2019, 30, 1333–1339. [Google Scholar] [CrossRef]
- Moreira, P.L.; An, Y.H.; Santos, A.R., Jr.; Genari, S.C. In vitro analysis of anionic collagen scaffolds for bone repair. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 229–237. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Gu, Y.; Zhu, Y.; Chen, L.; Chen, Y. Production of Composite Scaffold Containing Silk Fibroin, Chitosan, and Gelatin for 3D Cell Culture and Bone Tissue Regeneration. Med. Sci. Monit. 2017, 23, 5311–5320. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, G.S.; Gostynska, N.; Campodoni, E.; Dapporto, M.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Ribose mediated crosslinking of collagen-hydroxyapatite hybrid scaffolds for bone tissue regeneration using biomimetic strategies. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 594–605. [Google Scholar] [CrossRef]
- Castroceseña, A.B.; Camachovillegas, T.A.; Lugofabres, P.H.; Novitskaya, E.E.; Mckittrick, J.; Liceanavarro, A. Effect of starch on the mechanical and in vitro properties of collagen-hydroxyapatite sponges for applications in dentistry. Carbohydr. Polym. 2016, 148, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.; Twomey, J.; Guo, D.; Madhavan, K.; Li, M. Evaluation of nanostructural, mechanical, and biological properties of collagen-nanotube composites. IEEE Trans. Nanobiosci. 2010, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite–Carbon Nanotube Composites for Biomedical Applications: A Review. Int. J. Appl. Ceram. Technol. 2010, 4, 1–13. [Google Scholar] [CrossRef]
- Silva, E.E.D.; Colleta, H.H.M.D.; Ferlauto, A.S.; Moreira, R.L.; Resende, R.R.; Oliveira, S.; Kitten, G.T.; Lacerda, R.G.; Ladeira, L.O. Nanostructured 3-D collagen/nanotube biocomposites for future bone regeneration scaffolds. Nano Res. 2009, 2, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Hirata, E.; Uo, M.; Takita, H.; Akasaka, T.; Watari, F.; Yokoyama, A. Multiwalled carbon nanotube-coating of 3D collagen scaffolds for bone tissue engineering. Carbon 2011, 49, 3284–3291. [Google Scholar] [CrossRef]
- Yoon, I.K.; Hwang, J.Y.; Seo, J.W.; Jang, W.C.; Kim, H.W.; Shin, U.S. Carbon nanotube-gelatin-hydroxyapatite nanohybrids with multilayer core–shell structure for mimicking natural bone. Carbon 2014, 77, 379–389. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Jiang, S.; Wang, Y.; Parungao, R.; Zheng, S.; Nie, Y.; Liu, T.; Song, K. The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro. Polymers 2019, 11, 230. [Google Scholar] [CrossRef]
- Moßhammer, D.; Natanzon, I.; Manske, I.; Grutschkowski, P.; Rieger, M.A. Polymeric composites containing carbon nanotubes for bone tissue engineering. Int. J. Biol. Macromol. 2010, 46, 281–283. [Google Scholar]
- Gutierrez-Hernandez, J.M.; Escobar-Garcia, D.M.; Escalante, A.; Flores, H.; Gonzalez, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 445–453. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yun, Y.S.; Kim, E.S.; Kim, M.S.; Jin, H.J. Stem cell response to multiwalled carbon nanotube-incorporated regenerated silk fibroin films. J. Nanosci. Nanotechnol. 2011, 11, 801–805. [Google Scholar] [CrossRef]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N.J.N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials 2018, 8, 337. [Google Scholar] [CrossRef]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.M.; Choi, D.Y.; Jung, S.C.; Kim, B.H. Characteristics of Plasma Treated Electrospun Polycaprolactone (PCL) Nanofiber Scaffold for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2015, 15, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Bose, S. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: In vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl. Mater. Interfaces 2014, 6, 9955–9965. [Google Scholar] [CrossRef] [PubMed]
- Mattioli-Belmonte, M.; Vozzi, G.; Whulanza, Y.; Seggiani, M.; Fantauzzi, V.; Orsini, G.; Ahluwalia, A. Tuning polycaprolactone–carbon nanotube composites for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2012, 32, 152–159. [Google Scholar] [CrossRef]
- Flores-Cedillo, M.L.; Alvarado-Estrada, K.N.; Pozos-Guillen, A.J.; Murguia-Ibarra, J.S.; Vidal, M.A.; Cervantes-Uc, J.M.; Rosales-Ibanez, R.; Cauich-Rodriguez, J.V. Multiwall carbon nanotubes/polycaprolactone scaffolds seeded with human dental pulp stem cells for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 35. [Google Scholar] [CrossRef]
- Huang, B.; Vyas, C.; Roberts, I.; Poutrel, Q.A.; Chiang, W.H.; Blaker, J.J.; Huang, Z.; Bartolo, P. Fabrication and characterisation of 3D printed MWCNT composite porous scaffolds for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 266–278. [Google Scholar] [CrossRef]
- Shuilin, W.U.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R 2014, 80, 1–36. [Google Scholar]
- Pan, L.; Pei, X.; He, R.; Wan, Q.; Wang, J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surf. B Biointerfaces 2012, 93, 226–234. [Google Scholar] [CrossRef]
- Dorj, B.; Won, J.E.; Kim, J.H.; Choi, S.J.; Shin, U.S.; Kim, H.W. Robocasting nanocomposite scaffolds of poly(caprolactone)/hydroxyapatite incorporating modified carbon nanotubes for hard tissue reconstruction. J. Biomed. Mater. Res. Part. A 2013, 101A, 1670–1681. [Google Scholar] [CrossRef]
- Ormsby, R.; McNally, T.; O’Hare, P.; Burke, G.; Mitchell, C.; Dunne, N. Fatigue and biocompatibility properties of a poly(methyl methacrylate) bone cement with multi-walled carbon nanotubes. Acta Biomater. 2012, 8, 1201–1212. [Google Scholar] [CrossRef]
- Wang, C.; Yu, B.; Fan, Y.; Ormsby, R.W.; McCarthy, H.O.; Dunne, N.; Li, X. Incorporation of multi-walled carbon nanotubes to PMMA bone cement improves cytocompatibility and osseointegration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109823. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, R.; McNally, T.; Mitchell, C.; Halley, P.; Martin, D.; Nicholson, T.; Dunne, N. Effect of MWCNT addition on the thermal and rheological properties of polymethyl methacrylate bone cement. Carbon 2011, 49, 2893–2904. [Google Scholar] [CrossRef]
- Mikael, P.E.; Amini, A.R.; Basu, J.; Josefina Arellano-Jimenez, M.; Laurencin, C.T.; Sanders, M.M.; Barry Carter, C.; Nukavarapu, S.P. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: Fabrication, in vitro and in vivo evaluation. Biomed. Mater. 2014, 9, 035001. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Rutledge, K.; Jabbarzadeh, E. Carbon nanotube-poly(lactide-co-glycolide) composite scaffolds for bone tissue engineering applications. Ann. Biomed. Eng. 2013, 41, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, Y.; Lai, Y.; Yang, W.; Jiao, F.; Zhang, H.; Ye, S.; Zhang, Q. Incorporation of carboxylation multiwalled carbon nanotubes into biodegradable poly(lactic-co-glycolic acid) for bone tissue engineering. Colloids Surf. B Biointerfaces 2011, 83, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lei, L.; Yang, B.; Li, J.; Ren, J. Preparation and characterization of polylactic acid (PLA) carbon nanotube nanocomposites. Polym. Test. 2018, 68, 34–38. [Google Scholar] [CrossRef]
- Martinez de Arenaza, I.; Obarzanek-Fojt, M.; Sarasua, J.R.; Meaurio, E.; Meyer, F.; Raquez, J.M.; Dubois, P.; Bruinink, A. Pyrene-end-functionalized poly(L-lactide) as an efficient carbon nanotube dispersing agent in poly(L-lactide): Mechanical performance and biocompatibility study. Biomed. Mater. 2015, 10, 045003. [Google Scholar] [CrossRef]
- Shao, S.; Zhou, S.; Li, L.; Li, J.; Luo, C.; Wang, J.; Li, X.; Weng, J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef]
- Shao, D.; Sawyer, L.Q.S. Ultraviolet (UV) photodetectors fabricated from multi-walled carbon nanotubes (MWCNTs) and polyvinyl-alcohol (PVA) coated ZnO nanoparticles. MRS Online Proc. Libr. Arch. 2012, 1454, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Park, H.B.; Chang, H.L.; Sohn, J.Y.; Lee, Y.M.; Freeman, B.D.; Kim, H.J. Effect of crosslinked chain length in sulfonated polyimide membranes on water sorption, proton conduction, and methanol permeation properties. J. Membr. Sci. 2006, 285, 432–443. [Google Scholar] [CrossRef]
- Wang, K.; Luo, G.F.; Liu, Y.; Li, C.; Cheng, S.X.; Zhuo, R.X.; Zhang, X.Z. Redox-sensitive shell cross-linked PEG–polypeptide hybrid micelles for controlled drug release. Polym. Chem. 2012, 3, 1084–1090. [Google Scholar] [CrossRef]
- Sitharaman, B.; Shi, X.; Tran, L.A.; Spicer, P.P.; Rusakova, I.; Wilson, L.J.; Mikos, A.G. Injectable in situ cross-linkable nanocomposites of biodegradable polymers and carbon nanostructures for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 655–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.Z.; Li, K.; Wong, H.M.; Tong, W.Y.; Yeung, K.W.; Tjong, S.C. Novel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacements. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Dahman, Y. A Novel Approach for the Utilization of Biocellulose Nanofibres in Polyurethane Nanocomposites for Potential Applications in Bone Tissue Implants. Des. Monomers Polym. 2012, 15, 1–29. [Google Scholar] [CrossRef]
- Liao, H.; Qi, R.; Shen, M.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Improved cellular response on multiwalled carbon nanotube-incorporated electrospun polyvinyl alcohol/chitosan nanofibrous scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 528–535. [Google Scholar] [CrossRef]
- Cao, J.; Lu, Y.; Chen, H.; Zhang, L.; Xiong, C. Bioactive poly(etheretherketone) composite containing calcium polyphosphate and multi-walled carbon nanotubes for bone repair: Mechanical property and in vitro biocompatibility. J. Bioact. Compat. Polym. 2018, 33, 543–557. [Google Scholar] [CrossRef]
- Hai, C.; Wei, B.; Dongsheng, M.A. Energy Storage and Management System With Carbon Nanotube Supercapacitor and Multidirectional Power Delivery Capability for Autonomous Wireless Sensor Nodes. IEEE Trans. Power Electron. 2010, 25, 2897–2909. [Google Scholar]
- Nivethaa, E.A.K.; Dhanavel, S.; Narayanan, V.; Stephen, A. Fabrication of chitosan/MWCNT nanocomposite as a carrier for 5-fluorouracil and a study of the cytotoxicity of 5-fluorouracil encapsulated nanocomposite towards MCF-7. Polym. Bull. 2016, 73, 1–16. [Google Scholar] [CrossRef]
- Riggio, C.; Ciofani, G.; Raffa, V.; Cuschieri, A.; Micera, S. Combination of Polymer Technology and Carbon Nanotube Array for the Development of an Effective Drug Delivery System at Cellular Level. Nanoscale Res. Lett. 2009, 4, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Nie, L.; Wang, T.; Qin, Y.; Guo, Z.; Yang, D.; Yan, X. Carbon nanotube delivery of the GFP gene into mammalian cells. Chembiochem 2010, 7, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Bhirde, A.A.; Patel, S.; Sousa, A.A.; Patel, V.; Molinolo, A.A.; Ji, Y.; Leapman, R.D.; Gutkind, J.S.; Rusling, J.F. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine 2010, 5, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.M.; Karthivashan, G.; Arulselvan, P.; Fakurazi, S.; Hussein, M.Z. Characterization andIn VitroSustained Release of Silibinin from pH Responsive Carbon Nanotube-Based Drug Delivery System. J. Nanomater. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Siu, K.S.; Chen, D.; Zheng, X.; Zhang, X.; Johnston, N.; Liu, Y.; Yuan, K.; Koropatnick, J.; Gillies, E.R.; Min, W.P. Non-covalently functionalized single-walled carbon nanotube for topical siRNA delivery into melanoma. Biomaterials 2014, 35, 3435–3442. [Google Scholar] [CrossRef]
- Kamalha, E.; Mwasiagi, J.I.; Zeng, Y. Nanotechnology and carbon nanotubes; A review of potential in drug delivery. Macromol. Res. 2012, 20, 891–898. [Google Scholar] [CrossRef]
- Murakami, T.; Ajima, K.; Miyawaki, J.; Yudasaka, M.; Iijima, S.; Shiba, K. Drug-Loaded Carbon Nanohorns: Adsorption and Release of Dexamethasone in Vitro. Mol. Pharm. 2004, 1, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.Z.; Huang-Fu, M.Y.; Liu, H.N.; Wang, X.R.; Sheng, X.; Gao, J.Q. Fabrication and characterization of drug-loaded nano-hydroxyapatite/polyamide 66 scaffolds modified with carbon nanotubes and silk fibroin. Int. J. Nanomed. 2016, 11, 6181–6194. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, Y.; Yu, D.; Li, R.; Luo, W.; Ma, G.; Zhang, C. Isoniazid-loaded chitosan/carbon nanotubes microspheres promote secondary wound healing of bone tuberculosis. J. Biomater. Appl. 2019, 33, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, D.G.; Power, K.; Kadir, O.; Dekany, I.; Yannopoulos, S.N.; Bouropoulos, N.; Bakandritsos, A.; Antonijevic, M.D.; Zouganelis, G.D.; Roldo, M. Stabilisation of SWNTs by alkyl-sulfate chitosan derivatives of different molecular weight: Towards the preparation of hybrids with anticoagulant properties. Nanoscale 2011, 3, 1218–1224. [Google Scholar] [CrossRef]
- Sukhodub, L.B.; Sukhodub, L.F.; Prylutskyy, Y.I.; Strutynska, N.Y.; Vovchenko, L.L.; Soroca, V.M.; Slobodyanik, N.S.; Tsierkezos, N.G.; Ritter, U. Composite material based on hydroxyapatite and multi-walled carbon nanotubes filled by iron: Preparation, properties and drug release ability. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 606–614. [Google Scholar] [CrossRef]
- Costantini, L.; Bouropoulos, N.; Fatouros, D.G.; Kontopoulou, I.; Roldo, M. Synthesis of carbon nanotubes loaded hydroxyapatite: Potential for controlled drug release from bone implants. J. Adv. Ceram. 2016, 5, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.Y.; Qiu, T.; Wang, X.F.; Zhang, M.; Gao, X.L.; Li, R.X.; Lu, X.; Weng, J. Preparation of foam-like carbon nanotubes/hydroxyapatite composite scaffolds with superparamagnetic properties. Appl. Surf. Sci. 2012, 262, 227–230. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Panseri, S.; Montesi, M.; Tampieri, A.; Galvagno, S. Hydroxyapatite-magnetite-MWCNT nanocomposite as a biocompatible multifunctional drug delivery system for bone tissue engineering. Nanotechnology 2014, 25, 425701. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Cicuendez, M.; Sanchez-Marcos, J.; Fal-Miyar, V.; Manzano, M.; Prieto, C.; Vallet-Regi, M. Electrical stimuli to increase cell proliferation on carbon nanotubes/mesoporous silica composites for drug delivery. J. Biomed. Mater. Res. A 2013, 101, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, X.; Liu, Y.; Gao, X.; Zhu, T.; Lu, L. Acceleration of Bone Regeneration in Critical-Size Defect Using BMP-9-Loaded nHA/ColI/MWCNTs Scaffolds Seeded with Bone Marrow Mesenchymal Stem Cells. Biomed. Res. Int. 2019, 2019, 7343957. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Ma, G.; Zhang, F.; Hu, R. Efficacy and safety of a live canine adenovirus-vectored rabies virus vaccine in swine. Vaccine 2008, 26, 5368–5372. [Google Scholar] [CrossRef]
- Han, Z.J.; Ostrikov, K.K.; Tan, C.M.; Tay, B.K.; Peel, S.A. Effect of hydrophilicity of carbon nanotube arrays on the release rate and activity of recombinant human bone morphogenetic protein-2. Nanotechnology 2011, 22, 295712. [Google Scholar] [CrossRef]
- Qian, S.; Yan, Z.; Xu, Y.; Tan, H.; Chen, Y.; Ling, Z.; Niu, X. Carbon nanotubes as electrophysiological building blocks for a bioactive cell scaffold through biological assembly to induce osteogenesis. RSC Adv. 2019, 9, 12001–12009. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Zhou, H.; Leaman, D.W.; Goel, V.K.; Agarwal, A.K.; Bhaduri, S.B. Sustained release of small molecules from carbon nanotube-reinforced monetite calcium phosphate cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 92–96. [Google Scholar] [CrossRef]
- Hedlund, J.; Lundgren, A.; Lundgren, B.; Elwing, H. A new compact electrochemical method for analyzing complex protein films adsorbed on the surface of modified interdigitated gold electrodes. Sens. Actuators B Chem. 2009, 142, 494–501. [Google Scholar] [CrossRef]
- Geyik, C.; Evran, S.; Timur, S.; Telefoncu, A. The covalent bioconjugate of multiwalled carbon nanotube and amino-modified linearized plasmid DNA for gene delivery. Biotechnol. Prog. 2014, 30, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Blais, M.O.; Harris, G.M.; Jabbarzadeh, E. PLGA-carbon nanotube conjugates for intercellular delivery of caspase-3 into osteosarcoma cells. PLoS ONE 2013, 8, e81947. [Google Scholar] [CrossRef] [PubMed]






| Substrate Materials | CNT Application | Consequences | References |
|---|---|---|---|
| Calcium Phosphate | |||
| hydroxyapatite (HA) | bone implant materials |
| [147,148] |
| hydroxyapatite (HA) | coating material for implants |
| [149] |
| beta-tricalcium phosphate (β-TCP) | bone repair biomaterials |
| [154] |
| calcium phosphate cements (CPC) | injectable bone substitutes |
| [157] |
| Natural Polymers | |||
| chitosan (CS) | nanocomposite films |
| [62] |
| chitosan (CS) | bone tissue scaffolds |
| [159,163] |
| chitosan(CS)–hydroxyapatite (HA) | bone tissue engineering |
| [162,163] |
| silver sulfadiazine (AgSD)–chitosan (CS) nanofiber | coating material for implants |
| [94] |
| collagen | bone repair biomaterials |
| [172] |
| collagen | 3D CNT-coated bone scaffolds |
| [175] |
| collagen–hydroxyapatite (HA) | bone tissue scaffolds |
| [150] |
| gelatin–hydroxyapatite (HA) | artificial bone grafts |
| [176] |
| gelatin–chitosan (CS) | bone scaffold materials |
| [177] |
| bacterial cellulose | bone tissue scaffolds |
| [179] |
| silk fibroin | nanocomposite films |
| [180] |
| Synthetic Polymers | |||
| polycaprolactone (PCL) | 3D bone scaffolds |
| [187,189] |
| polycaprolactone (PCL)–hydroxyapatite (HA) | 3D bone scaffolds |
| [190] |
| polymethyl-methacrylate (PMMA) | bone cements |
| [191] |
| polymethyl-methacrylate (PMMA) | bone cements |
| [192] |
| Poly(lactide-co-glycolide) (PLGA) | load-bearing bone tissue scaffolds |
| [194] |
| Poly(lactide-co-glycolide) (PLGA) | bone repair scaffolds |
| [195,196] |
| polylactic acid (PLA) | nanocomposite materials |
| [197] |
| polylactic acid (PLA) | bone tissue engineering |
| [199] |
| poly-L-lactic acid (PLLA) | bone tissue engineering |
| [198] |
| polyvinyl alcohol (PVA)–chitosan (CS) | bone tissue engineering |
| [207] |
| poly(etheretherketone)-calcium polyphosphate cements (CPPs) | load-bearing orthopedic application |
| [208] |
| Delivery System | Drugs/Molecular Type | Consequences | References |
|---|---|---|---|
| carbon nanotube (CNTs)/silk fibroin–hydroxyapatite (HA)/polyamide 66 (nHA/PA66) scaffolds | Dexamethasone (DEX) | Promoted the expression of osteoblast genes and induced the osteogenic differentiation | [217,218] |
| Chitosan (CS)–CNTs nanoparticles | isoniazid | Prolonged the release time, stabilized the release rate of isoniazid, retained the biological function, and reduced the cytotoxicity and inflammatory response of isoniazid | [219] |
| HA–alginate–MWCNT + Fe beads | chlorhexidine | Prolonged chlorhexidine release time and showed high a young’s modulus comparable to steel | [221] |
| CNT–chitosan (CS)–hydroxyapatite (HA) composite materials | ibuprofen (IBU) ibuprofen sodium (IBU-Na) fluorescein isothiocyanate-dextran (FITC-Dex) | Controlled the release of both low and high molecular weight hydrophilic drugs | [222] |
| HA–magnetite–MWCNT nanocomposite with magnetite nanoparticles (MWCNT/Fe3O4) | clodronate | Improved magnetic properties, induced bone biomineralization, and inhibited osteoclast activity in vitro | [223,224] |
| CNT–mesoporous silica composites | zoledronic acid (Zol) | Ensured the 3D conductive network to transmit the electrical stimuli, affected osteoblasts cultured over the surface, and increased the drug loading | [225] |
| CNT gel scaffold via specific pairing of functionalized nucleobases | human bone morphogenetic protein-2 (BMP-2) | Significantly increased the spontaneous osteogenesis on bio-electrical gel scaffolds and enhanced cell differentiation and organization via extra electrical stimulus. | [229] |
| CNT arrays | recombinant human bone morphogenetic protein-2 (rhBMP-2), poloxamer | Retained a larger amount of rhBMP-2, delayed protein release and inhibited the large initial burst | [228] |
| hydroxyapatite (HA)–collagen–MWCNT composite scaffolds | recombinant bone morphogenetic protein-9 (BMP-9) | Enhanced osteogenic differentiation in vitro and induced more new bone formation in vivo | [226] |
| carboxylic acid-functionalized MWCNT–monetite-based CPC | Z-Leu-Leu-Leu-al (MG132) | Exhibited a sustained drug release, and confirmed the therapeutic effect by the inhibition of cytokine-induced osteoclast differentiation | [222] |
| poly(lactic-co-glycolic) (PLGA)-functionalized CNTs materials | pro-apoptotic protein caspase-3 (CP3) | Promoted delivery of RNA and transcription factor to cells and demonstrated a pronounced ability of cell penetration | [233] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. https://doi.org/10.3390/nano9101501
Pei B, Wang W, Dunne N, Li X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials. 2019; 9(10):1501. https://doi.org/10.3390/nano9101501
Chicago/Turabian StylePei, Baoqing, Wei Wang, Nicholas Dunne, and Xiaoming Li. 2019. "Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects" Nanomaterials 9, no. 10: 1501. https://doi.org/10.3390/nano9101501
APA StylePei, B., Wang, W., Dunne, N., & Li, X. (2019). Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials, 9(10), 1501. https://doi.org/10.3390/nano9101501






