Mineral-Enriched Deep-Sea Water Modulates Lactate Metabolism via PGC-1α-Mediated Metabolic Reprogramming
Abstract
1. Introduction
2. Results
2.1. BDSW Cytotoxicity
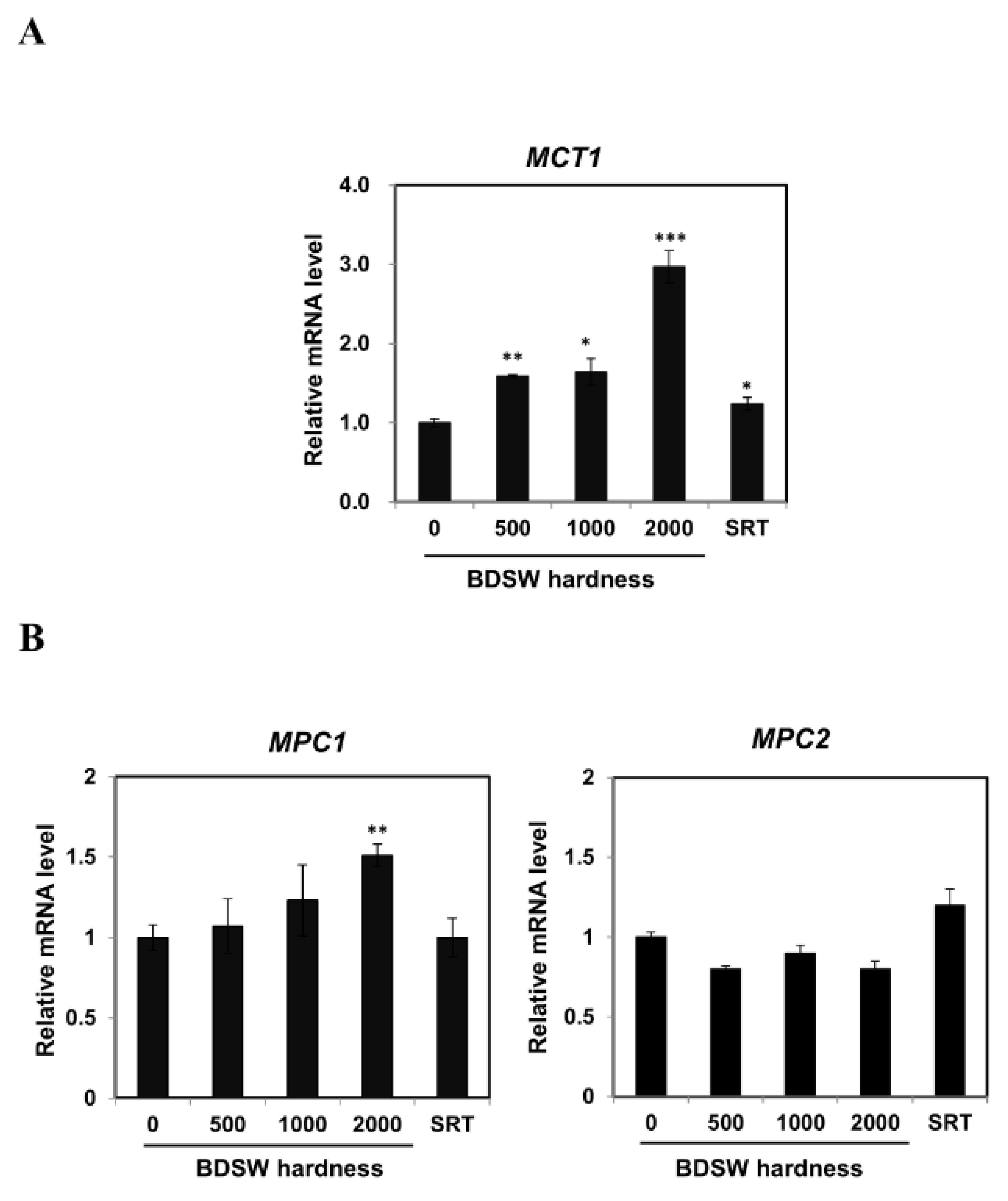
2.2. BDSW Modulated Expression of Lactate Production-Related Genes
2.3. BDSW Enhanced PGC-1α Expression and Activity in C2C12 Myotubes
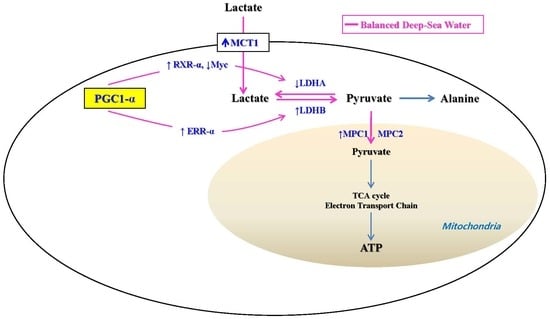
2.4. Potential Mechanisms of BDSW Effects on Lactate Metabolism
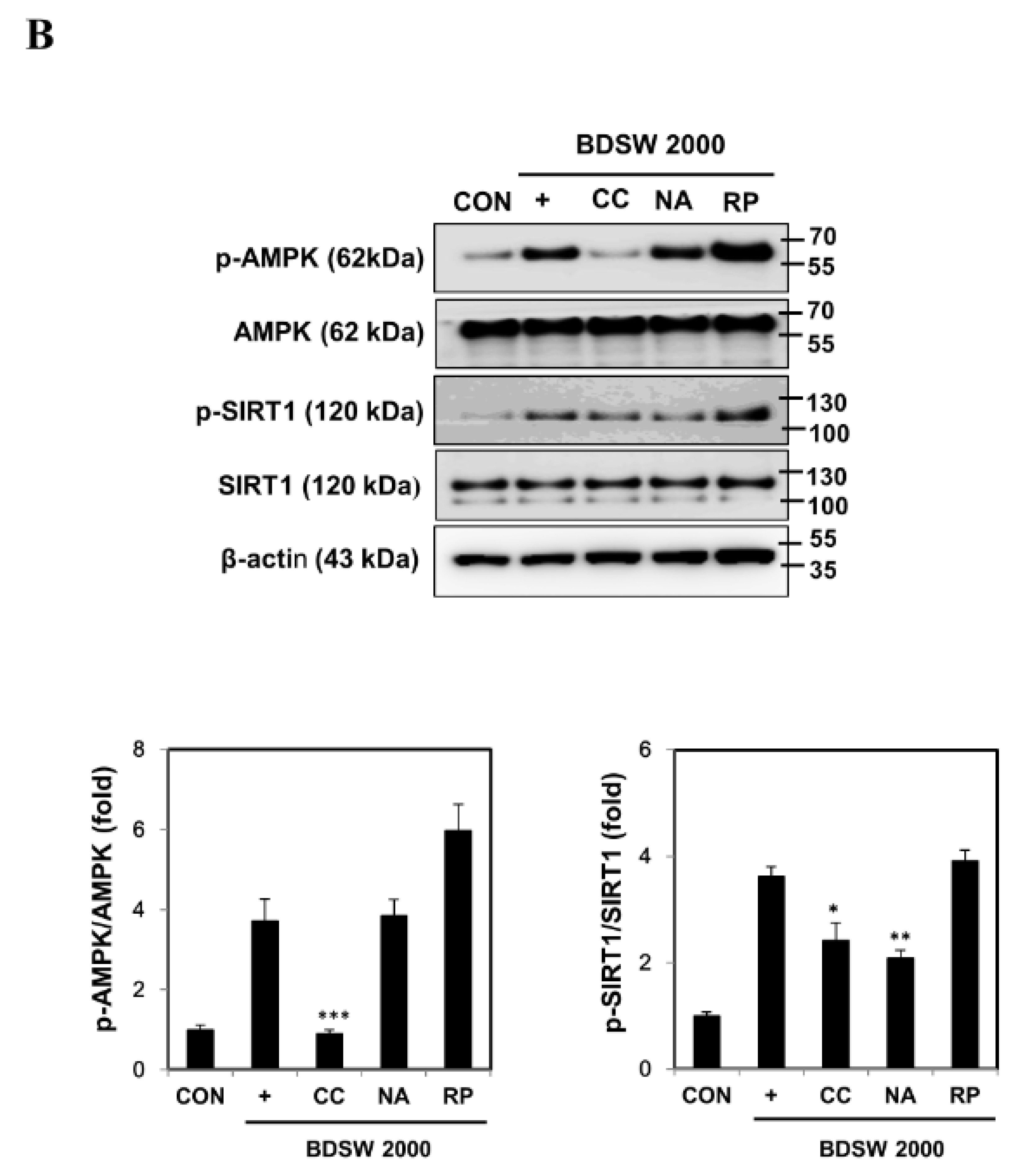
2.5. Signaling Molecules Involved in PGC-1α Activation
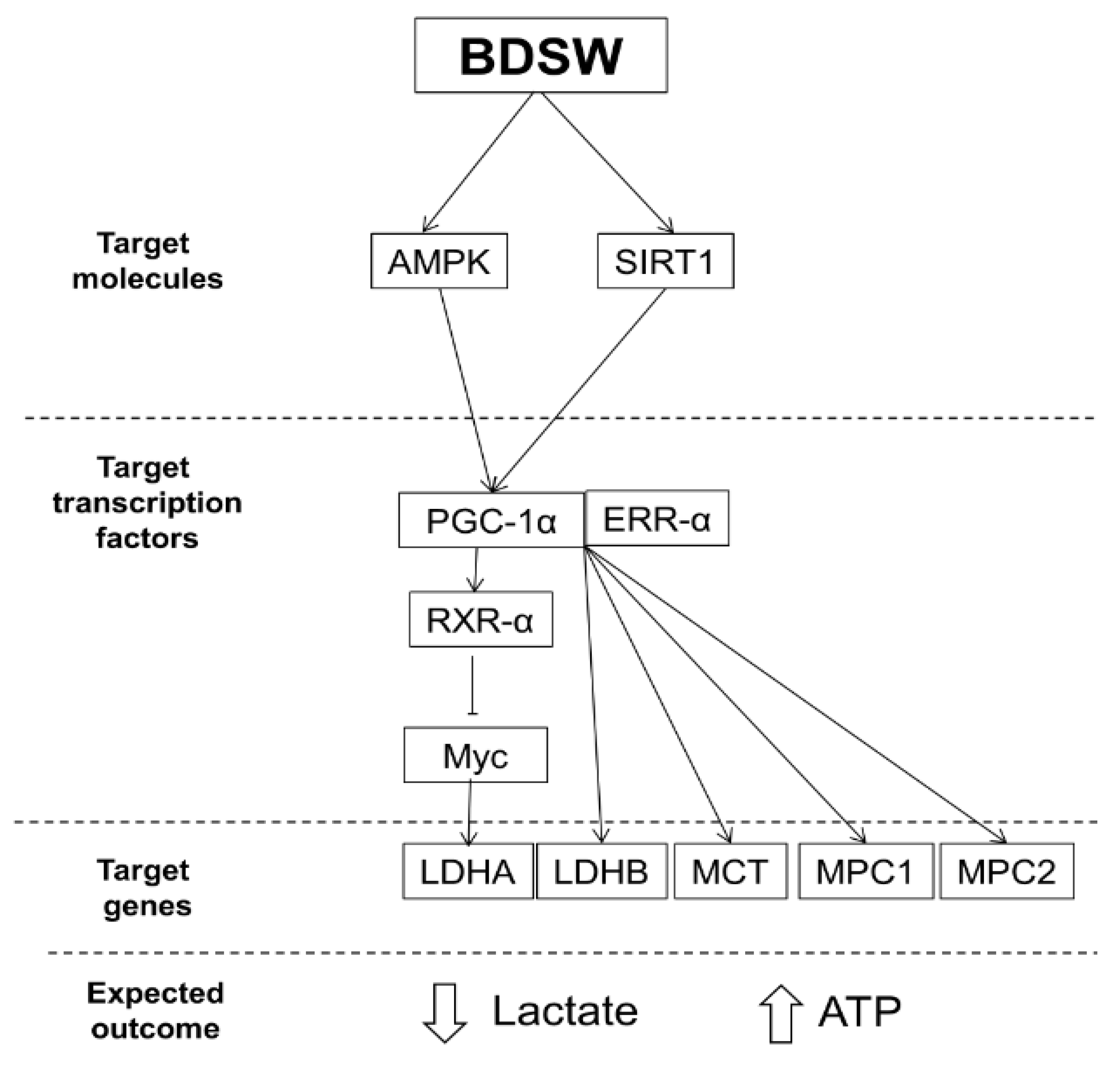
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. BDSW Preparation
4.3. C2C12 Cell Culture and Differentiation
4.4. BDSW Treatment
4.5. Inhibitor Treatments
4.6. Cell Toxicity
4.7. qRT-PCR
4.8. Western Blot Analysis
4.9. Immunoprecipitation
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Barros, L.F. Metabolic signaling by lactate in the brain. Trends Neurosci. 2013, 36, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 2013, 123, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Santos, C.R.; Spencer-Dene, B.; Martinez, P.; Endesfelder, D.; Burrell, R.A.; Vetter, M.; Jiang, M.; Saunders, R.E.; Kelly, G.; et al. Genome-wide RNA interference analysis of renal carcinoma survival regulators identifies MCT4 as a Warburg effect metabolic target. J. Pathol. 2012, 227, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.C.; Cox, J.E.; Cardon, C.M.; Van Vranken, J.G.; Dephoure, N.; et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef]
- Handschin, C. Regulation of skeletal muscle cell plasticity by the peroxisome proliferator-activated receptor γ coactivator. J. Recept. Signal Transduct. Res. 2010, 30, 376–384. [Google Scholar] [CrossRef]
- Summermatter, S.; Santos, G.; Pérez-Schindler, J.; Handschin, C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc. Natl. Acad. Sci. USA 2013, 110, 8738–8743. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Fryer, L.G.; Parbu-Patel, A.; Carling, D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef]
- Barnes, B.R.; Long, Y.C.; Steiler, T.L.; Leng, Y.; Galuska, D.; Wojtaszewski, J.F.; Andersson, L.; Zierath, J.R. Changes in exercise-induced gene expression in 5′-AMP-activated protein kinase gamma3-null and gamma3 R225Q transgenic mice. Diabetes 2005, 54, 3484–3489. [Google Scholar] [CrossRef]
- Zong, H.; Ren, J.M.; Young, L.H.; Pypaert, M.; Mu, J.; Birnbaum, M.J.; Shulman, G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15983–15987. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.J.; Chou, P.Y.; Lin, W.H.; Pan, C.H.; Chien, Y.C.; Chung, Y.L.; Liu, F.C.; Wu, C.H. Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: an in vivo study. Mar. Drugs 2013, 11, 2183–2202. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.P.; Kim, Y.M.; Son, J.; Kim, C.J.; Kim, E.H. Application of concentrated deep sea water inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. BMC Complement. Altern. Med. 2012, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, S.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar] [CrossRef]
- Ha, B.G.; Park, J.E.; Shin, E.J.; Shon, Y.H. Modulation of glucose metabolism by balanced deep-sea water ameliorates hyperglycemia and pancreatic function in streptozotocin-induced diabetic mice. PLoS One 2014, 9, e102095. [Google Scholar] [CrossRef]
- Ha, B.G.; Shin, E.J.; Park, J.E.; Shon, Y.H. Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice. Mar. Drugs 2013, 11, 4193–4212. [Google Scholar] [CrossRef]
- Ha, B.G.; Park, J.E.; Shin, E.J.; Shon, Y.H. Effects of balanced deep-sea water on adipocyte hypertrophy and liver steatosis in high-fat, diet-induced obese mice. Obesity 2014, 22, 1669–1678. [Google Scholar] [CrossRef]
- Ha, B.G.; Park, J.E.; Cho, H.J.; Shon, Y.H. Stimulatory effects of balanced deep sea water on mitochondrial biogenesis and function. PLoS One 2015, 10, e0129972. [Google Scholar] [CrossRef]
- Ha, B.G.; Moon, D.S.; Kim, H.J.; Shon, Y.H. Magnesium and calcium-enriched deep-sea water promotes mitochondrial biogenesis by AMPK-activated signals pathway in 3T3-L1 preadipocytes. Biomed. Pharmacother. 2016, 83, 477–484. [Google Scholar] [CrossRef]
- Crawford, S.O.; Ambrose, M.S.; Hoogeveen, R.C.; Brancati, F.L.; Ballantyne, C.M.; Young, J.H. Association of lactate with blood pressure before and after rapid weight loss. Am. J. Hypertens. 2008, 21, 1337–1342. [Google Scholar] [CrossRef]
- van der Merwe, M.T.; Schlaphoff, G.P.; Crowther, N.J.; Boyd, I.H.; Gray, I.P.; Joffe, B.I.; Lonnroth, P.N. Lactate and glycerol release from adipose tissue in lean, obese, and diabetic women from South Africa. J. Clin. Endocrinol. Metab. 2001, 86, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, L.O.; Larsson, B.; Bjorntorp, P.; Eriksson, H.; Svärdsudd, K.; Welin, L.; Tibblin, G.; Wilhelmsen, L. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus. Thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia 1988, 31, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.E.; Caro, J.F.; Pories, W.J.; Azevedo, J.L.; Dohm, G.L. Glucose metabolism in incubated human muscle: effect of obesity and non-insulin-dependent diabetes mellitus. Metabolism 1994, 43, 1047–1054. [Google Scholar] [CrossRef]





| Mineral | BDSW (mg/L) | Mass Concentration (mg/L) | Ionic Concentration (mM) | Ionic Charge | Ionic Concentration × Ionic Charge |
|---|---|---|---|---|---|
| Ca2+ | 134 | 40 | 3.35 | +2 | 13.40 |
| Mg2+ | 405 | 24 | 16.88 | +2 | 67.50 |
| K+ | 0.8 | 39 | 0.02 | +1 | 0.02 |
| Na+ | 106 | 23 | 4.61 | +1 | 4.61 |
| Cl− | 5844.7 | 35 | 166.99 | −1 | 166.99 |
| SO42− | 1525.7 | 96 | 15.89 | −2 | 63.57 |
| SeO32− | 1.3 | 128 | 0.01 | −2 | 0.04 |
| H2VO4− | 0.7 | 117 | 0.01 | −1 | 0.01 |
| Zn2+ | 2.8 | 64 | 0.04 | +2 | 0.18 |
| Total (mM) | 316 | ||||
| Ionic strength of solution (M) | 0.16 | ||||
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| LDH A | GGTTACACATCCTGGGCCAT | CAGCTCAGACGAGAAGGGTG |
| LDH B | GTGTGATTGGAAGCGGATGC | TGCCGTACATTCCCTTCACC |
| PGC-1α | GGAACTGCAGGCCTAACTCC | TTGGAGCTGTTTTCTGGTGC |
| ERR-α | AGCCAGTCCTGACAGTCCAA | CCGGACAGCTGTACTCGATG |
| RXR-α | TGCGTCACTAGAAGCGTACT | GAGTAAAGATGGCGAGAGTGG |
| Myc | GCCCAGTGAGGATATCTGGA | ATCGCAGATGAAGCTCTGGT |
| MCT1 | TTCAGTGCAACGACCAGTGA | AGTGGAGCCAGGGTAGAGAG |
| MPC1 | CACAGCGGTGTCATCTGTCT | ATGGCCGCTTACTCATCTCG |
| MPC2 | GCCTCTCAGCTGTTTCGGAT | CATCCACAAGCAAGTCCCCT |
| β-actin | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, B.G.; Jung, S.S.; Jang, Y.K.; Jeon, B.Y.; Shon, Y.H. Mineral-Enriched Deep-Sea Water Modulates Lactate Metabolism via PGC-1α-Mediated Metabolic Reprogramming. Mar. Drugs 2019, 17, 611. https://doi.org/10.3390/md17110611
Ha BG, Jung SS, Jang YK, Jeon BY, Shon YH. Mineral-Enriched Deep-Sea Water Modulates Lactate Metabolism via PGC-1α-Mediated Metabolic Reprogramming. Marine Drugs. 2019; 17(11):611. https://doi.org/10.3390/md17110611
Chicago/Turabian StyleHa, Byung Geun, Sung Suk Jung, You Kyung Jang, Byong Yeob Jeon, and Yun Hee Shon. 2019. "Mineral-Enriched Deep-Sea Water Modulates Lactate Metabolism via PGC-1α-Mediated Metabolic Reprogramming" Marine Drugs 17, no. 11: 611. https://doi.org/10.3390/md17110611
APA StyleHa, B. G., Jung, S. S., Jang, Y. K., Jeon, B. Y., & Shon, Y. H. (2019). Mineral-Enriched Deep-Sea Water Modulates Lactate Metabolism via PGC-1α-Mediated Metabolic Reprogramming. Marine Drugs, 17(11), 611. https://doi.org/10.3390/md17110611