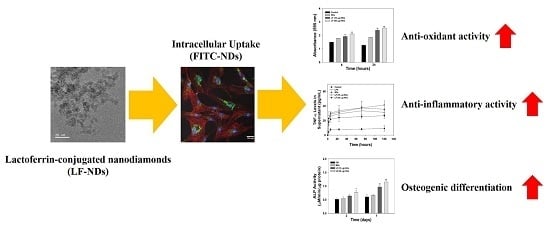
Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lactoferrin (LF) Conjugated Carboxylated-Nanodiamonds (cNDs)
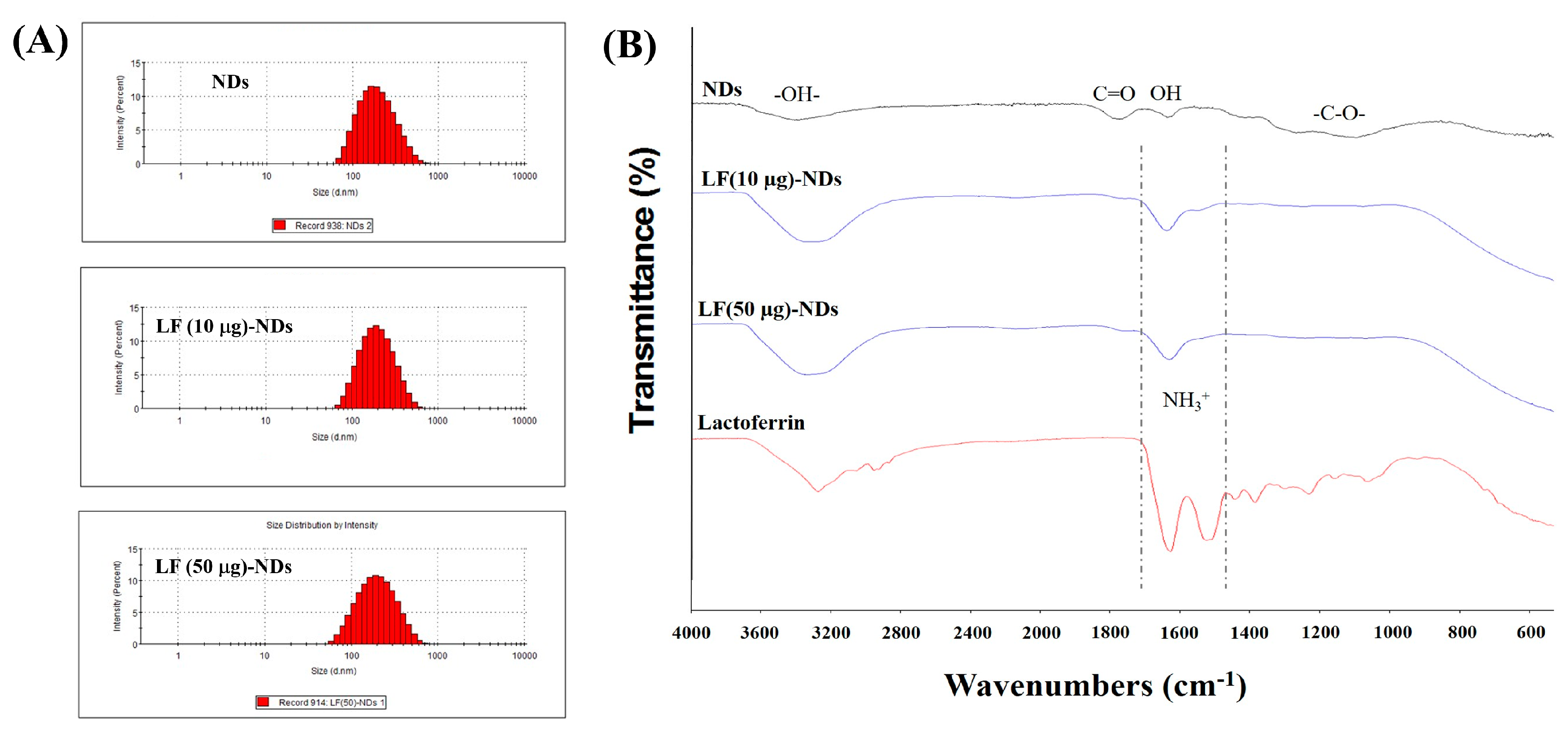
2.2. Characterization
2.3. In Vitro LF Release
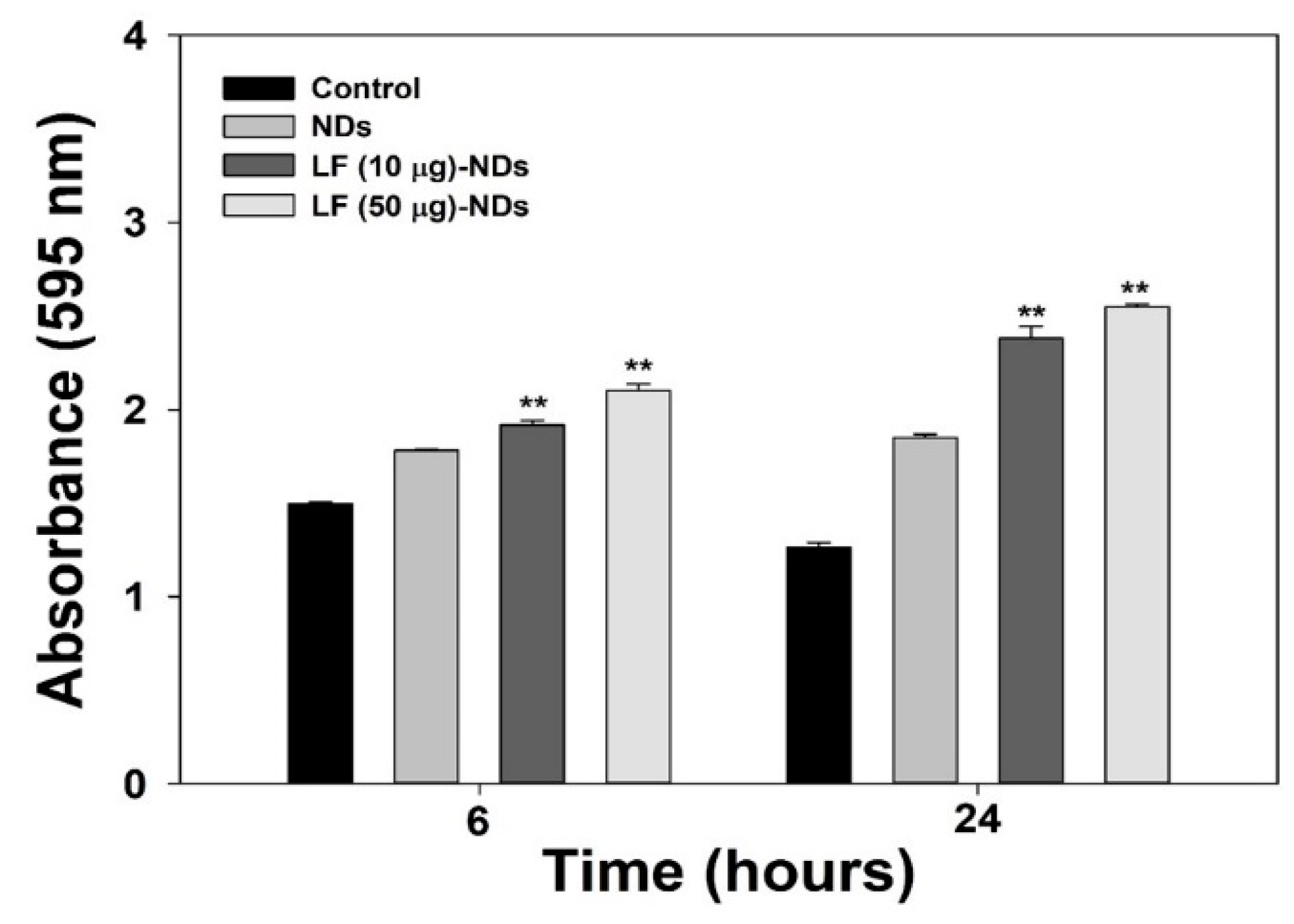
2.4. Cytotoxicity
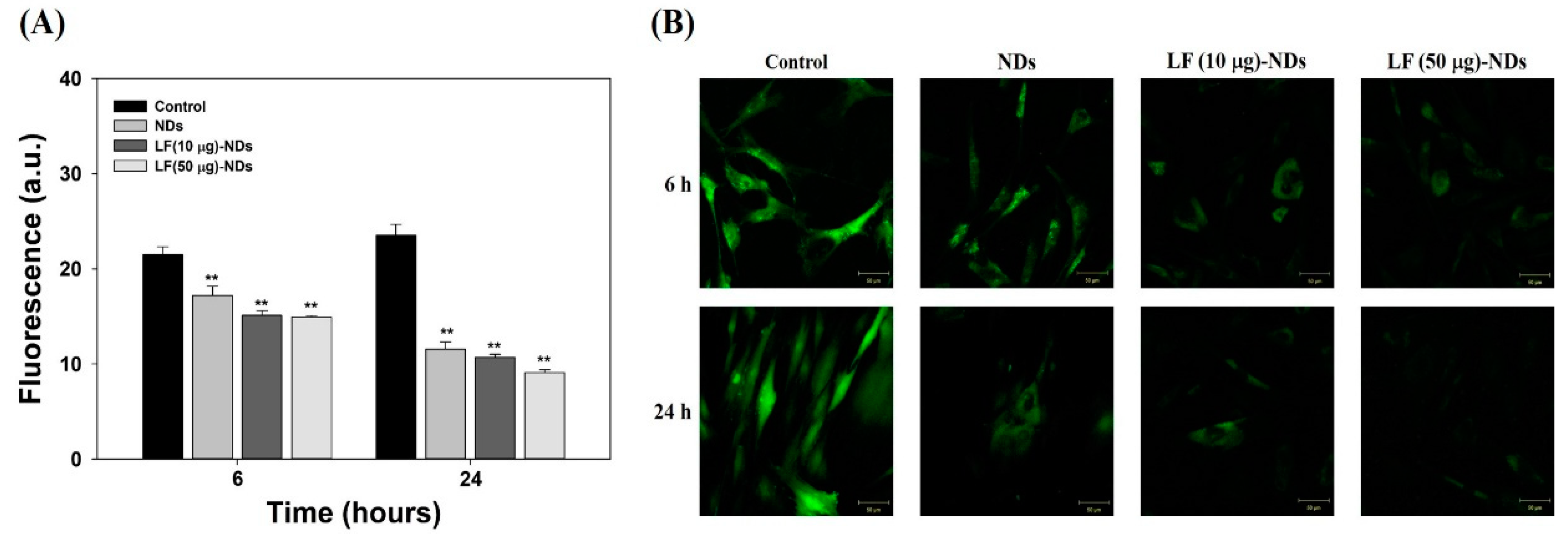
2.5. Cellular Uptake Anaalysis
2.6. Evaluation of ROS Scavenging Activity
2.6.1. Suppression of ROS at the Cell Level
2.6.2. Protection of Cell Suppression in the ROS Condition
2.7. Interleukin-1β (IL-1β) and Tumor Necrosis Factor Alpha (TNF-α) Content
2.8. Alkaline Phosphatase (ALP) Activity
2.9. Calcium Deposition
2.10. Statistical Analysis
3. Results
3.1. Characterization of NDs with and without LF
3.2. In Vitro LF Release
3.3. Cytotoxicity and Cellular Internalization
3.4. ROS Scavenging Effects of LF-NDs in Cells
3.5. Cellular Protection Against ROS
3.6. Levels of Pro-Inflammatory Cytokines in Cell Supernatants of LPS-Induced MC3T3-E1 Cells Treated with LF-NDs
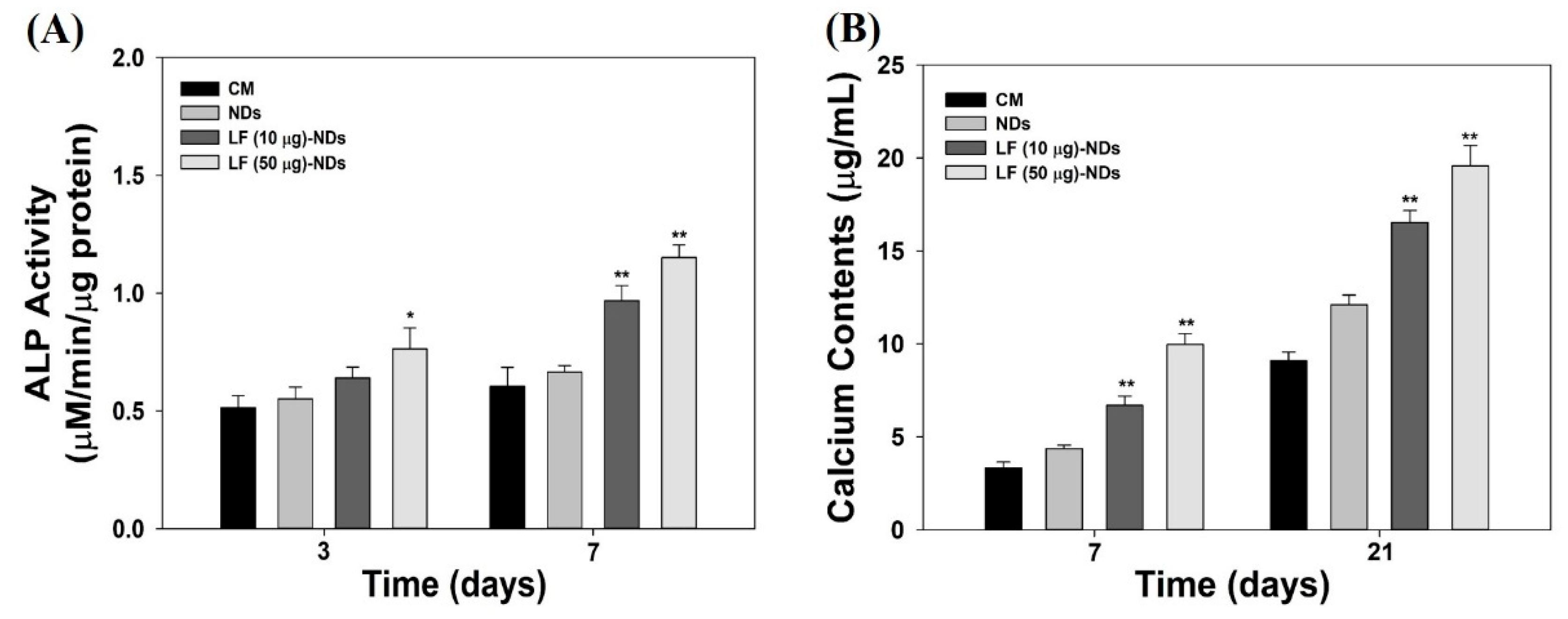
3.7. Alkaline Phosphatase (ALP) Activity and Calcium Deposition
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zimmermann, C.E.; Borner, B.I.; Hasse, A.; Sieg, P. Donor site morbidity after microvascular fibula transfer. Clin. Oral Investig. 2001, 5, 214–219. [Google Scholar] [CrossRef]
- Silber, J.S.; Anderson, D.G.; Daffner, S.D.; Brislin, B.T.; Leland, J.M.; Hilibrand, A.S.; Vaccaro, A.R.; Albert, T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2003, 28, 134–139. [Google Scholar] [CrossRef]
- Moreau, M.F.; Gallois, Y.; Basle, M.F.; Chappard, D. Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells. Biomaterials 2000, 21, 369–376. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Rebmann, V.; Passler, M.; Schollmeier, G.; Ekkernkamp, A.; Grosse-Wilde, H.; Tomford, W.W. Immune response to perforated and partially demineralized bone allografts. J. Orthop. Sci. 2001, 6, 545–555. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative stress antagonizes wnt signaling in osteoblast precursors by diverting beta-catenin from t cell factor-to forkhead box o-mediated transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.; Ambrogini, E.; Han, L.; Manolagas, S.C.; Jilka, R.L. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic wnt signaling in the skeleton. J. Biol. Chem. 2009, 284, 27438–27448. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.; Martin-Millan, M.; Ambrogini, E.; Bradsher, R., 3rd; Han, L.; Chen, X.D.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.A.; Jilka, R.L.; et al. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the eralpha. J. Bone Miner. Res. 2010, 25, 769–781. [Google Scholar]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by erk and nf-kappab. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [Green Version]
- Brock, J.H. The physiology of lactoferrin. Biochem. Cell Biol. 2002, 80, 1–6. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, D.; Zhao, F.; Wang, J.; Zhu, W. The effects of the combination of oral lactoferrin and iron injection on iron homestasis, antioxidative abilities and cytokines activities of suckling piglets. Animals (Basel) 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Maneva, A.; Taleva, B.; Maneva, L. Lactoferrin-protector against oxidative stress and regulator of glycolysis in human erythrocytes. Z. Naturforschung C J. Biosci. 2003, 58, 256–262. [Google Scholar] [CrossRef]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef]
- Cohen, M.S.; Mao, J.; Rasmussen, G.T.; Serody, J.S.; Britigan, B.E. Interaction of lactoferrin and lipopolysaccharide (lps): Effects on the antioxidant property of lactoferrin and the ability of lps to prime human neutrophils for enhanced superoxide formation. J. Infect. Dis. 1992, 166, 1375–1378. [Google Scholar] [CrossRef]
- Spadaro, M.; Caorsi, C.; Ceruti, P.; Varadhachary, A.; Forni, G.; Pericle, F.; Giovarelli, M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008, 22, 2747–2757. [Google Scholar] [CrossRef] [Green Version]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, S.A.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Effect of hydroxyapatite nanocrystals functionalized with lactoferrin in osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 224–234. [Google Scholar] [CrossRef]
- Kim, S.E.; Yun, Y.P.; Lee, J.Y.; Park, K.; Suh, D.H. Osteoblast activity of mg-63 cells is enhanced by growth on a lactoferrin-immobilized titanium substrate. Colloids Surf. B Biointerfaces 2014, 123, 191–198. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, H.Y.; Jing, H.; Li, Y.X.; Wang, X.Y.; Zhang, H.; Jiang, L.; Ren, F.Z. Lactoferrin stimulates osteoblast differentiation through pka and p38 pathways independent of lactoferrin’s receptor lrp1. J. Bone Miner. Res. 2014, 29, 1232–1243. [Google Scholar] [CrossRef] [Green Version]
- Bolanos, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387–6406. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Javed, M.N.; Pottoo, F.H.; Rabbani, S.A.; Barkat, M.A.; Sarafroz, M.; Amir, M. Bioresponse inspired nanomaterials for targeted drug and gene delivery. Pharm. Nanotechnol. 2019, 7, 220–233. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar] [CrossRef]
- Neugart, F.; Zappe, A.; Jelezko, F.; Tietz, C.; Boudou, J.P.; Krueger, A.; Wrachtrup, J. Dynamics of diamond nanoparticles in solution and cells. Nano Lett. 2007, 7, 3588–3591. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Pentecost, A.; Li, X.M.; Neitzel, I.; Nelson, M.; Wei, C.; He, T.; Guo, F.; Gogotsi, Y. Adsorption of drugs on nanodiamond: Toward development of a drug delivery platform. Mol. Pharm. 2013, 10, 3728–3735. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent plla-nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Hazeli, K.; Niu, J.; Kontsos, A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Mechanical properties and biomineralization of multifunctional nanodiamond-plla composites for bone tissue engineering. Biomaterials 2012, 33, 5067–5075. [Google Scholar] [CrossRef]
- Parizek, M.; Douglas, T.E.L.; Novotna, K.; Kromka, A.; Brady, M.A.; Renzing, A.; Voss, E.; Jarosova, M.; Palatinus, L.; Tesarek, P.; et al. Nanofibrous poly (lactide-co-glycolide) membranes loaded with diamond nanoparticles as promising substrates for bone tissue engineering. Int. J. Nanomed. 2012, 7, 5873. [Google Scholar]
- Ahn, G.Y.; Ryu, T.K.; Choi, Y.R.; Park, J.R.; Lee, M.J.; Choi, S.W. Fabrication and optimization of nanodiamonds-composited poly(epsilon-caprolactone) fibrous matrices for potential regeneration of hard tissues. Biomater. Res. 2018, 22, 16. [Google Scholar] [CrossRef]
- Kratochvilova, I.; Sebera, J.; Ashcheulov, P.; Golan, M.; Ledvina, M.; Micova, J.; Mravec, F.; Kovalenko, A.; Zverev, D.; Yavkin, B.; et al. Magnetical and optical properties of nanodiamonds can be tuned by particles surface chemistry: Theoretical and experimental study. J. Phys. Chem. C 2014, 118, 25245–25252. [Google Scholar] [CrossRef]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Park, K.; Choi, S.W.; Suh, D.H. Effect of lactoferrin-impregnated porous poly(lactide-co-glycolide) (plga) microspheres on osteogenic differentiation of rabbit adipose-derived stem cells (radscs). Colloids Surf. B Biointerfaces 2014, 122, 457–464. [Google Scholar] [CrossRef]
- Vacek, T.P.; Kalani, A.; Voor, M.J.; Tyagi, S.C.; Tyagi, N. The role of homocysteine in bone remodeling. Clin. Chem. Lab. Med. 2013, 51, 579–590. [Google Scholar] [CrossRef] [Green Version]
- Agidigbi, T.S.; Kim, C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ros-mediated osteoclast diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [Green Version]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J.K. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef]
- Collins, J.A.; Diekman, B.O.; Loeser, R.F. Targeting aging for disease modification in osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 101–107. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Ambrogini, E.; Bartell, S.M.; Manolagas, S.C. Oxidative stress stimulates apoptosis and activates nf-kappab in osteoblastic cells via a pkcbeta/p66shc signaling cascade: Counter regulation by estrogens or androgens. Mol. Endocrinol. 2010, 24, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
- Valentine, J.S.; Wertz, D.L.; Lyons, T.J.; Liou, L.L.; Goto, J.J.; Gralla, E.B. The dark side of dioxygen biochemistry. Curr. Opin. Chem. Biol. 1998, 2, 253–262. [Google Scholar] [CrossRef]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of cerium oxide nanoparticle functionalised pcl-gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef]
- Lacey, D.C.; Simmons, P.J.; Graves, S.E.; Hamilton, J.A. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: Implications for bone repair during inflammation. Osteoarthr. Cartil. 2009, 17, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Bastidas-Coral, A.P.; Bakker, A.D.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Bravenboer, N.; Forouzanfar, T.; Klein-Nulend, J. Cytokines tnf-alpha, il-6, il-17f, and il-4 differentially affect osteogenic differentiation of human adipose stem cells. Stem Cells Int. 2016, 2016, 1318256. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Jeon, D.I.; Park, K.; Kim, H.J. In vitro and in vivo anti-inflammatory and tendon-healing effects in achilles tendinopathy of long-term curcumin delivery using porous microspheres. J. Ind. Eng. Chem. 2018, 58, 123–130. [Google Scholar] [CrossRef]
- Park, J.W.; Yun, Y.P.; Park, K.; Lee, J.Y.; Kim, H.J.; Kim, S.E.; Song, H.R. Ibuprofen-loaded porous microspheres suppressed the progression of monosodium iodoacetate-induced osteoarthritis in a rat model. Colloids Surf. B Biointerfaces 2016, 147, 265–273. [Google Scholar] [CrossRef]
- Mattsby-Baltzer, I.; Roseanu, A.; Motas, C.; Elverfors, J.; Engberg, I.; Hanson, L.A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr. Res. 1996, 40, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouch, S.P.; Slater, K.J.; Fletcher, J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood 1992, 80, 235–240. [Google Scholar] [CrossRef]
- Rasheed, N.; Alghasham, A.; Rasheed, Z. Lactoferrin from camelus dromedarius inhibits nuclear transcription factor-kappa b activation, cyclooxygenase-2 expression and prostaglandin e2 production in stimulated human chondrocytes. Pharmacogn. Res. 2016, 8, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Cheng, S.; Wang, W.; Lin, Z.; Chen, Q.; Zhang, W.; Kou, D.; Shen, Y.; Cheng, X.; Peng, L.; et al. Effect of lactoferrin on osteogenic differentiation of human adipose stem cells. Int. Orthop. 2012, 36, 647–653. [Google Scholar] [CrossRef] [Green Version]




| Elements | C1s (%) | N1s (%) | O1s (%) | Total (%) | |
|---|---|---|---|---|---|
| Sample | |||||
| NDs | 87.32 | 1.83 | 10.85 | 100 | |
| LF (10 μg)-NDs | 85.99 | 3.09 | 10.92 | 100 | |
| LF (10 μg)-NDs | 84.45 | 4.74 | 10.81 | 100 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.E.; Choi, S.; Hong, J.-Y.; Shim, K.-S.; Kim, T.-H.; Park, K.; Lee, S.-H. Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects. Nanomaterials 2020, 10, 50. https://doi.org/10.3390/nano10010050
Kim SE, Choi S, Hong J-Y, Shim K-S, Kim T-H, Park K, Lee S-H. Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects. Nanomaterials. 2020; 10(1):50. https://doi.org/10.3390/nano10010050
Chicago/Turabian StyleKim, Sung Eun, Somang Choi, Jae-Young Hong, Kyu-Sik Shim, Tae-Hoon Kim, Kyeongsoon Park, and Suk-Ha Lee. 2020. "Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects" Nanomaterials 10, no. 1: 50. https://doi.org/10.3390/nano10010050
APA StyleKim, S. E., Choi, S., Hong, J.-Y., Shim, K.-S., Kim, T.-H., Park, K., & Lee, S.-H. (2020). Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects. Nanomaterials, 10(1), 50. https://doi.org/10.3390/nano10010050