Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Information Processing
2.2. Selection of Relevant Studies and Data Analysis
3. Results and Discussion
3.1. Compilation of Relevant Bibliographic Sources
3.2. Bibliographical Analysis
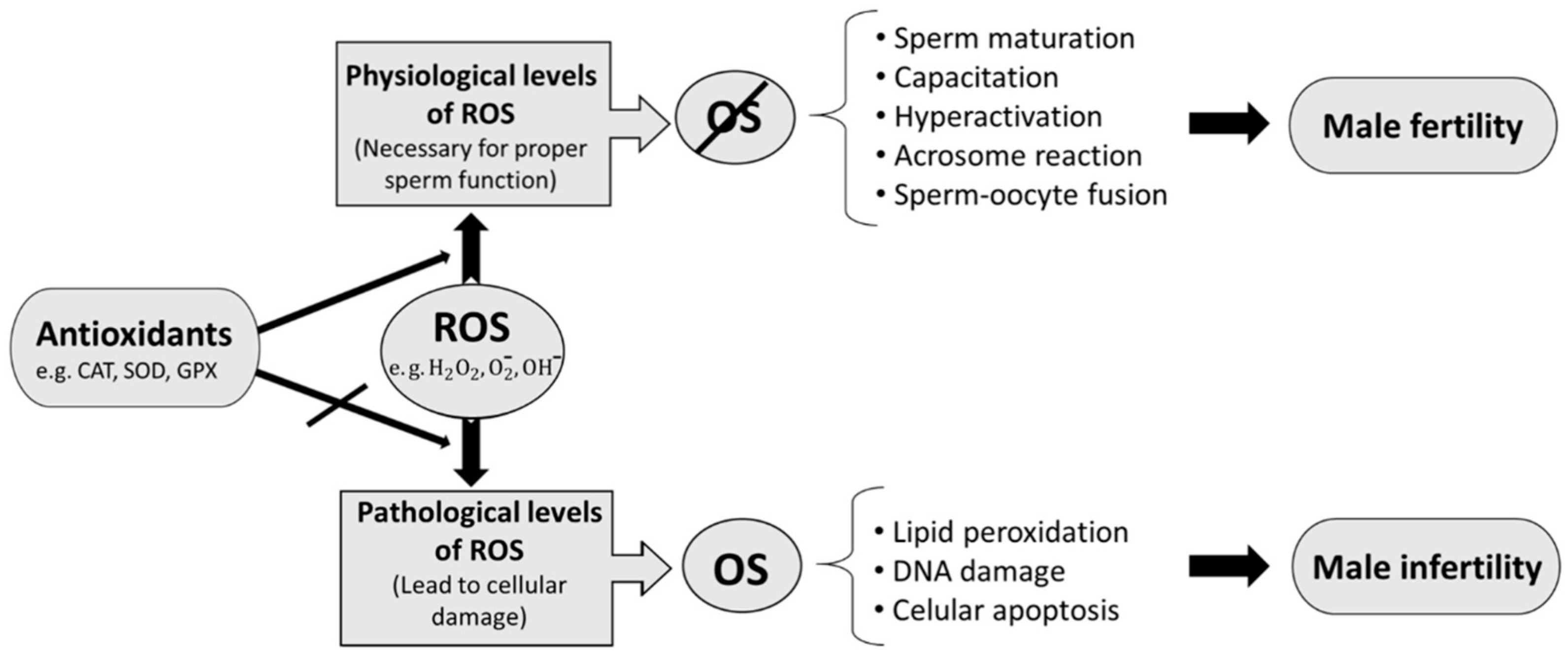
3.2.1. ROS and Male Fertility
3.2.2. Catalase and Male Fertility
3.3. Current Evidence Sustaining the Relationship between Catalase and Male Fertility
3.3.1. Antioxidant Therapies
3.3.2. Pathology of the Male Genital Tract and Genetics Related to CAT and Male Fertility
3.3.3. Lifestyle Factors: Harmful vs. Beneficial Factors
3.4. Main Findings Obtained after Reviewing the Clinical Trials Which Study the Relationship between Catalase Levels and Male Fertility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Bach, P.V.; Schlegel, P.N. Male Infertility. In Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, 8th ed.; Strauss, J.F., Barbieri, R.L., III, Gargiulo, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 582–593.e2. [Google Scholar] [CrossRef]
- Honig, S.C.; Lipshultz, L.I.; Jarow, J. Significant medical pathology uncovered by a comprehensive male infertility evaluation. Fertil. Steril. 1994, 62, 1028–1034. [Google Scholar] [CrossRef]
- Walsh, T.J.; Schembri, M.; Turek, P.J.; Chan, J.M.; Carroll, P.R.; Smith, J.F.; Eisenberg, M.L.; Van Den Eeden, S.K.; Croughan, M.S. Increased risk of high-grade prostate cancer among infertile men. Cancer 2010, 116, 2140–2147. [Google Scholar] [CrossRef] [Green Version]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Network, Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Sundaram, R.; Schisterman, E.F.; Sweeney, A.; Lynch, C.D.; Kim, S.; Maisog, J.M.; Gore-Langton, R.; Eisenberg, M.L.; Chen, Z. Semen quality and time to pregnancy: The Longitudinal Investigation of Fertility and the Environment Study. Fertil. Steril. 2014, 101, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, M.; Lewis, S.; Morroll, D. Sperm quality and its relationship to natural and assisted conception: British Fertility Society Guidelines for practice. Hum. Fertil. 2013, 16, 175–193. [Google Scholar] [CrossRef]
- Ghuman, N.; Ramalingam, M. Male infertility. Obstet. Gynaecol. Reprod. Med. 2018, 28, 7–14. [Google Scholar] [CrossRef]
- Wald, M. Male infertility: Causes and cures. Sex. Reprod. Menopause 2005, 3, 83–87. [Google Scholar] [CrossRef]
- Kenkel, S.; Rolf, C.; Nieschlag, E. Occupational risks for male fertility: An analysis of patients attending a tertiary referral centre. Int. J. Androl. 2001, 24, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef]
- Agarwal, A.; Arafa, M.M.; Elbardisi, H.; Majzoub, A.; Alsaid, S.S. Relationship between seminal oxidation reduction potential and sperm DNA fragmentation in infertile men. Fertil. Steril. 2017, 108, e316. [Google Scholar] [CrossRef]
- Tremblay, A.R.; Delbes, G. In vitro study of doxorubicin-induced oxidative stress in spermatogonia and immature Sertoli cells. Toxicol. Appl. Pharmacol. 2018, 348, 32–42. [Google Scholar] [CrossRef]
- Vilanova, J.C. Revisión bibliográfica del tema de estudio de un proyecto de investigación. Radiologia 2012, 54, 108–114. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Garrido, N.; Meseguer, M.; Simon, C.; Pellicer, A.; Remohi, J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J. Androl. 2004, 6, 59–65. [Google Scholar]
- Ochsendorf, F.R. Infections in the male genital tract and reactive oxygen species. Hum. Reprod. Update 1999, 5, 399–420. [Google Scholar] [CrossRef] [Green Version]
- Lavranos, G.; Balla, M.; Tzortzopoulou, A.; Syriou, V.; Angelopoulou, R. Investigating ROS sources in male infertility: A common end for numerous pathways. Reprod. Toxicol. 2012, 34, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Allamaneni, S.S.R.; Said, T.M. Chemiluminescence technique for measuring reactive oxygen species. Reprod. Biomed. Online 2004, 9, 466–468. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drevet, J.R. The antioxidant glutathione peroxidase family and spermatozoa: A complex story. Mol. Cell. Endocrinol. 2006, 250, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species in non-phagocytic cells. J. Leukoc. Biol. 1999, 65, 337–340. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.M.; Beorlegui, N.B.; Beconi, M.T. Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction. Theriogenology 1999, 52, 289–301. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Iwasaki, A.; Gagnon, C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil. Steril. 1992, 57, 409–416. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.; Allamaneni, S. What an andrologist/urologist should know about free radicals and why. Urology 2006, 67, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C.; Rajasekaran, M.; Hellstrom, W.J.G. Role of oxidative stress and antioxidants in male infertility. J. Androl. 1995, 16, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. 2009, 16, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Lenzi, A.; Gandini, L.; Picardo, M.; Tramer, F.; Sandri, G.; Panfili, E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): Scavenger mechanisms and possible scavenger therapies. Front. Biosci. 2000, 5, E1–E15. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Harkiss, D.; Buckingham, D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J. Reprod. Fertil. 1993, 98, 257–265. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous Lipid Peroxidation and Production of Hydrogen Peroxide and Superoxide in Human Spermatozoa Superoxide Dismutase as Major Enzyme Protectant Against Oxygen Toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Aisen, E.; Fernández-Santos, M.R.; Esteso, M.C.; Maroto-Morales, A.; García-Álvarez, O.; Garde, J.J. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in red deer spermatozoa. Reproduction 2009, 137, 225–235. [Google Scholar] [CrossRef]
- Awda, B.J.; Mackenzie-Bell, M.; Buhr, M.M. Reactive oxygen species and boar sperm function. Biol. Reprod. 2009, 81, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J. Androl. 1992, 13, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Griveau, J.F.; Dumont, E.; Renard, P.; Callegari, J.P.; Le Lannou, D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J. Reprod. Fertil. 1995, 103, 17–26. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, J. The role of oxygen in the metabolism and motility of human spermatozoa. Am. J. Physiol. 1943, 138, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Tosic, J.; Walton, A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect on respiration. Nature 1946, 158, 485. [Google Scholar] [CrossRef]
- Dai, D.F.; Chiao, Y.A.; Martin, G.M.; Marcinek, D.J.; Basisty, N.; Quarles, E.K.; Rabinovitch, P.S. Mitochondrial-Targeted Catalase: Extended Longevity and the Roles in Various Disease Models. Prog. Mol. Biol. Transl. Sci. 2017, 146, 203–241. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes: Production, Biocatalysis and Industrial Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 267–298. [Google Scholar] [CrossRef]
- Jeulin, C.; Soufir, J.C.; Weber, P.; Laval-Martin, D.; Calvayrac, R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989, 24, 185–196. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Messner, D.J.; Murray, K.F.; Kowdley, K.V. Mechanisms of Hepatocyte Detoxification. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L.R., Kaunitz, J.D., Said, H.M., Ghishan, F.K., Merchant, J.L., Wood, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1507–1527. [Google Scholar] [CrossRef]
- Dhawan, V. Reactive Oxygen and Nitrogen Species: General Considerations. Oxidative Stress in Applied Basic Research and Clinical Practice. JPMER 2014, 27–47. [Google Scholar] [CrossRef]
- Dandekar, S.P.; Nadkarni, G.D.; Kulkarni, V.S.; Punekar, S. Lipid peroxidation and antioxidant enzymes in male infertility. J. Postgrad. Med. 2002, 48, 186–189. [Google Scholar] [PubMed]
- Nakamura, H.; Kimura, T.; Nakajima, A.; Shimoya, K.; Takemura, M.; Hashimoto, K.; Isaka, S.; Azuma, C.; Koyama, M.; Murata, Y. Detection of oxidative stress in seminal plasma and fractionated sperm from subfertile male patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 105, 155–160. [Google Scholar] [CrossRef]
- Siciliano, L.; Tarantino, P.; Longobardi, F.; Rago, V.; De Stefano, C.; Carpino, A. Impaired seminal antioxidant capacity in human semen with hyperviscosity or oligoasthenozoospermia. J. Androl. 2001, 22, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Tilmisany, A.M.; Al-Sawaf, H. Mechanisms of male infertility: Role of antioxidants. Curr. Drug Metab. 2005, 6, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Zini, A.; Gabriel, M.S.; Baazeem, A. Antioxidants and sperm DNA damage: A clinical perspective. J. Assist. Reprod. Genet. 2009, 26, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Safarinejad, M.R. Effect of pentoxifylline on semen parameters, reproductive hormones, and seminal plasma antioxidant capacity in men with idiopathic infertility: A randomized double-blind placebo-controlled study. Int. Urol. Nephrol. 2011, 43, 315–328. [Google Scholar] [CrossRef]
- Nadjarzadeh, A.; Shidfar, F.; Amirjannati, N.; Vafa, M.R.; Motevalian, S.A.; Gohari, M.R.; Kakhki, S.A.N.; Akhondi, M.M.; Sadeghi, M.R. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: A double-blind randomised clinical trial. Andrologia 2014, 46, 177–183. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H. The Effects of Honey Supplementation on Seminal Plasma Cytokines, Oxidative Stress Biomarkers, and Antioxidants During 8 Weeks of Intensive Cycling Training. J. Androl. 2012, 33, 449–461. [Google Scholar] [CrossRef]
- Sengupta, P.; Agarwal, A.; Pogrebetskaya, M.; Roychoudhury, S.; Durairajanayagam, D.; Henkel, R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod. Biomed. Online 2018, 36, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Huang, Z. Variations in Antioxidant Genes and Male Infertility. Biomed. Res. Int. 2015, 513196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshahrani, S.; Mcgill, J.; Agarwal, A. Prostatitis and male infertility. J. Reprod. Immunol. 2013, 100, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-F.; Xiao, W.-Q.; Zheng, Y.-C.; Dong, J.; Zhang, S.-M. Increased oxidative stress and oxidative damage associated with chronic bacterial prostatitis. Asian J. Androl. 2006, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zhou, Z.; Liu, S.; Li, Q.; Yao, J.; Li, W.; Yan, J. The Effect of Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) on Semen Parameters in Human Males: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e94991. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Moltó, J.; Ortuño, N.; Romero, A.; Bezos, C.; Aizpurua, J.; Gómez-Torres, M.J. Relationship between serum dioxin-like polychlorinated biphenyls and post-testicular maturation in human sperm. Reproductive Toxicol. 2017, 73, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilacqua, A.; Izzo, G.; Pietro Emerenziani, G.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef]
- Maneesh, M.; Dutta, S.; Chakrabarti, A.; Vasudevan, D.M. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J. Physiol. Pharmacol. 2006, 50, 291–296. [Google Scholar]
- Dai, J.B.; Wang, Z.X.; Qiao, Z.D. The hazardous effects of tobacco smoking on male fertility. Asian J. Androl. 2015, 17, 954–960. [Google Scholar] [CrossRef]
- Agarwal, A.; Singh, A.; Hamada, A.; Kesari, K. Cell Phones and Male Infertility: A Review of Recent Innovations in Technology and Consequences. Int. Braz. J. Urol. 2011, 37, 432–454. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.R.; Kesari, K.K.; Agarwal, A. Pathophysiology of cell phone radiation: Oxidative stress and carcinogenesis with focus on male reproductive system. Reprod. Biol. Endocrinol. 2009, 9, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Marí, M.; Wu, D.; Nieto, N.; Cederbaum, A.I. CYP2E 1-Dependent Toxicity and Up-Regulation of Antioxidant Genes. J. Biomed. Sci. 2001, 10029, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Maleki, B.H.; Tartibian, B.; Chehrazi, M. The effects of three different exercise modalities on markers of male reproduction in healthy subjects: A randomized controlled trial. Reproduction 2017, 153, 157–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, B.H.; Tartibian, B. Combined aerobic and resistance exercise training for improving reproductive function in infertile men: A randomized controlled trial. Appl. Physiol. Nutr. Metab. 2017, 42, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Maleki, B.H.; Tartibian, B. Resistance exercise modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: A RCT. Life Sci. 2018, 203, 150–160. [Google Scholar] [CrossRef]
- Rocha, S.; Martins, A.D.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Melatonin alters the glycolytic profile of Sertoli cells: Implications for male fertility. Mol. Hum. Reprod. 2014, 20, 1067–1076. [Google Scholar] [CrossRef] [Green Version]
- Rocha, C.S.; Rato, L.; Martins, A.D.; Alves, M.G.; Oliveira, P.F. Melatonin and Male Reproductive Health: Relevance of Darkness and Antioxidant Properties. Curr. Mol. Med. 2015, 15, 299–311. [Google Scholar] [CrossRef]
- Vellani, E.; Colasante, A.; Mamazza, L.; Minasi, M.G.; Greco, E.; Bevilacqua, A. Association of state and trait anxiety to semen quality of in vitro fertilization patients: A controlled study. Fertil. Steril. 2013, 99, 1565–1572. [Google Scholar] [CrossRef]
- Pook, M.; Tischen-Caffier, B.; Krause, W. Is infertility a risk factor for impaired male fertility? Hum. Reprod. 2004, 19, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Giblin, P.T.; Poland, M.L.; Moghissi, K.S.; Ager, J.W.; Olson, J.M. Effects of stress and characteristic adaptability on semen quality in healthy men. Fertil. Steril. 1988, 49, 127–132. [Google Scholar] [CrossRef]
- Zhou, J.F.; Wang, X.Y.; Shangguan, X.J.; Gao, Z.M.; Zhang, S.M.; Xiao, W.Q.; Chen, C.G. Increased oxidative stress in bodies of women with pregnancy-induced hypertension. Biomed. Environ. Sci. 2005, 18, 419–426. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Yeung, C.H.; De Geyter, C.; De Geyter, M.; Nieschlag, E. Production of reactive oxygen species by and hydrogen peroxide scavenging activity of spermatozoa in an IVF program. J. Assist. Reprod. Genet. 1996, 13, 495–500. [Google Scholar] [CrossRef] [PubMed]

| Keyword | PubMed | Web of Science | Scopus |
|---|---|---|---|
| Catalase | 1620 | 2708 | 2880 |
| Male fertility | 897 | 1447 | 1393 |
| Oxidative stress | 32,933 | 56,578 | 37,182 |
| Catalase & male fertility | 1 | 6 | 17 |
| Catalase & oxidative stress | 862 | 1,523 | 1,668 |
| Male fertility & oxidative stress | 54 | 138 | 136 |
| Keyword | Total Number of Publications | After IC * | IC and Reproductive Biology (%) ** |
|---|---|---|---|
| Catalase & male fertility | 82 | 6 | 5 (6.1%) |
| Catalase & oxidative stress | 39,712 | 1515 | 86 (0.2%) |
| Male fertility & oxidative stress | 667 | 136 | 106 (15.9%) |
| Publication Main Topic | Number of Publications |
|---|---|
| Publications including non-human study models | 36 |
| Publications about female fertility or embryo development | 23 |
| Publications unrelated to fertility | 29 |
| Rank | Author | Country | Institution | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CAT & MF | CAT & OS | MF & OS | CAT & MF | CAT & OS | MF & OS | CAT & MF | CAT & OS | MF & OS | |
| 1st | Czerniecki, J. (25%) | Maleki, BH. (17%) | Agarwal, A. (13%) | Japan (33%) | Iran (33%) | USA (26%) | Instituto Valenciano Infertilidad (25%) | Allameh Tabatabai Univ. (17%) | Cleveland Clinic Foundation. (14%) |
| 2nd | Fujii, J. (25%) | Tartibian, B. (17%) | Alves, MG. (7%) | Poland (33%) | Germany (17%) | Italy (12%) | Polish Academy of Sciences (25%) | Justus Liebig Univ. (17%) | Univ. Beira Interior (6%) |
| 3rd | Garrido, N. (25%) | Agarwal, A. (11%) | Oliveira, PF. (7%) | Spain (33%) | USA (17%) | India (11%) | Medical Univ. of Bialystok (25%) | ACECR (11%) | Univ. Do Porto (5%) |
| 4th | Ishii, T. (25%) | Abdollahi, M. (6%) | Rato, L. (4%) | Brazil (11%) | Portugal (11%) | Cleveland clinic foundation (11%) | Mcgill Univ. (4%) | ||
| 5th | Iuchi, Y. (25%) | Aitken, RJ. (6%) | Aitken, RJ. (3%) | China (11%) | Australia (7%) | Tehran Univ. of Medical Sciences (11%) | Sapienza Univ. Rome (4%) | ||
| Ref. | Biological Matrix | CAT Measurement Method | Results | Clinical Implication | Subject of Study |
|---|---|---|---|---|---|
| [60] | Seminal plasma | Measurement of H2O2 degradation by spectrophotometry [83] | Significant CAT increase. Correlation with significant improvement in seminal parameters. | Beneficial effect of PTX on antioxidant capacity of seminal plasma related to improved seminal parameters | Infertile men oligoastenozoospermic (OAT) (25-to-40 years old) |
| [61] | Seminal plasma | Measurement of H2O2 degradation by spectrophotometry [84] | Significant CAT increase. No correlation exists between CAT and an improvement in seminal parameters. | Supplementation with Q10 attenuates OS in seminal plasma and allows improvement of antioxidant enzyme activity | Infertile men OAT (25-to-40 years old) |
| [62] | Seminal plasma | Measurement of H2O2 degradation by spectrophotometry [84] | Significant CAT increase. Correlation with a significant improvement in seminal parameters. | Honey supplementation reduces the increase in OS during high-intensity exercise and increases CAT levels. | Fertile and unmarried men (18-to-28 years old) |
| [66] | Blood (erythrocytes) | Measurement of H2O2 degradation by spectrophotometry [85] | Significant CAT increase. Correlation with seminal parameters not studied. | Bacterial prostatitis causes a decrease of CAT and an increase in ROS resulting in OS damage. | Men with chronic bacterial prostatitis for 1-to-12 years (21-to-30 years old) |
| [70] | Blood (supernatant and plasma) | Commercial kit | Significant CAT increase. Correlation with seminal parameters not studied. | Continued excessive intake of alcohol results in lower CAT activity and increases OS eventually leading to problems in male fertility. | Alcoholic men (20-to-40 years old) |
| [75] | Seminal plasma | Measurement of H2O2 degradation by spectrophotometry [84] | Significant CAT increase. Correlation with a significant improvement in seminal parameters. | Exercise causes improved antioxidant levels, a decrease in OS and better seminal quality, moderate continued exercise representing the best modality. | Unfertile men with sedentary lifestyles (25-to-40 years old) |
| [76] | Seminal plasma | Measurement of H2O2 degradation by spectrophotometry [84] | Significant CAT increase. Correlation with a significant improvement in seminal parameters. | Aerobic and anaerobic exercise results in improved CAT levels, decreased OS and enhanced semen quality. | Infertile married men with sedentary lifestyles (25-to-40 years old) |
| [77] | Seminal plasma | Measurement of H2O2 degradation by spectrophotometry [84] | Significant CAT increase. Correlation with a significant improvement in seminal parameters. | Conducting resistance exercise causes an improvement in CAT levels, a decrease in OS, and an improvement in semen quality. | Infertile married men with sedentary lifestyles (25-40 years old) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Riquelme, N.; Huerta-Retamal, N.; Gómez-Torres, M.J.; Martínez-Espinosa, R.M. Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants 2020, 9, 78. https://doi.org/10.3390/antiox9010078
Rubio-Riquelme N, Huerta-Retamal N, Gómez-Torres MJ, Martínez-Espinosa RM. Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants. 2020; 9(1):78. https://doi.org/10.3390/antiox9010078
Chicago/Turabian StyleRubio-Riquelme, Nuria, Natalia Huerta-Retamal, María José Gómez-Torres, and Rosa María Martínez-Espinosa. 2020. "Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview" Antioxidants 9, no. 1: 78. https://doi.org/10.3390/antiox9010078
APA StyleRubio-Riquelme, N., Huerta-Retamal, N., Gómez-Torres, M. J., & Martínez-Espinosa, R. M. (2020). Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants, 9(1), 78. https://doi.org/10.3390/antiox9010078






