Controls on the Formation and Stability of Siderite (FeCO3) and Chukanovite (Fe2(CO3)(OH)2) in Reducing Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analysis and Calculation
3. Results
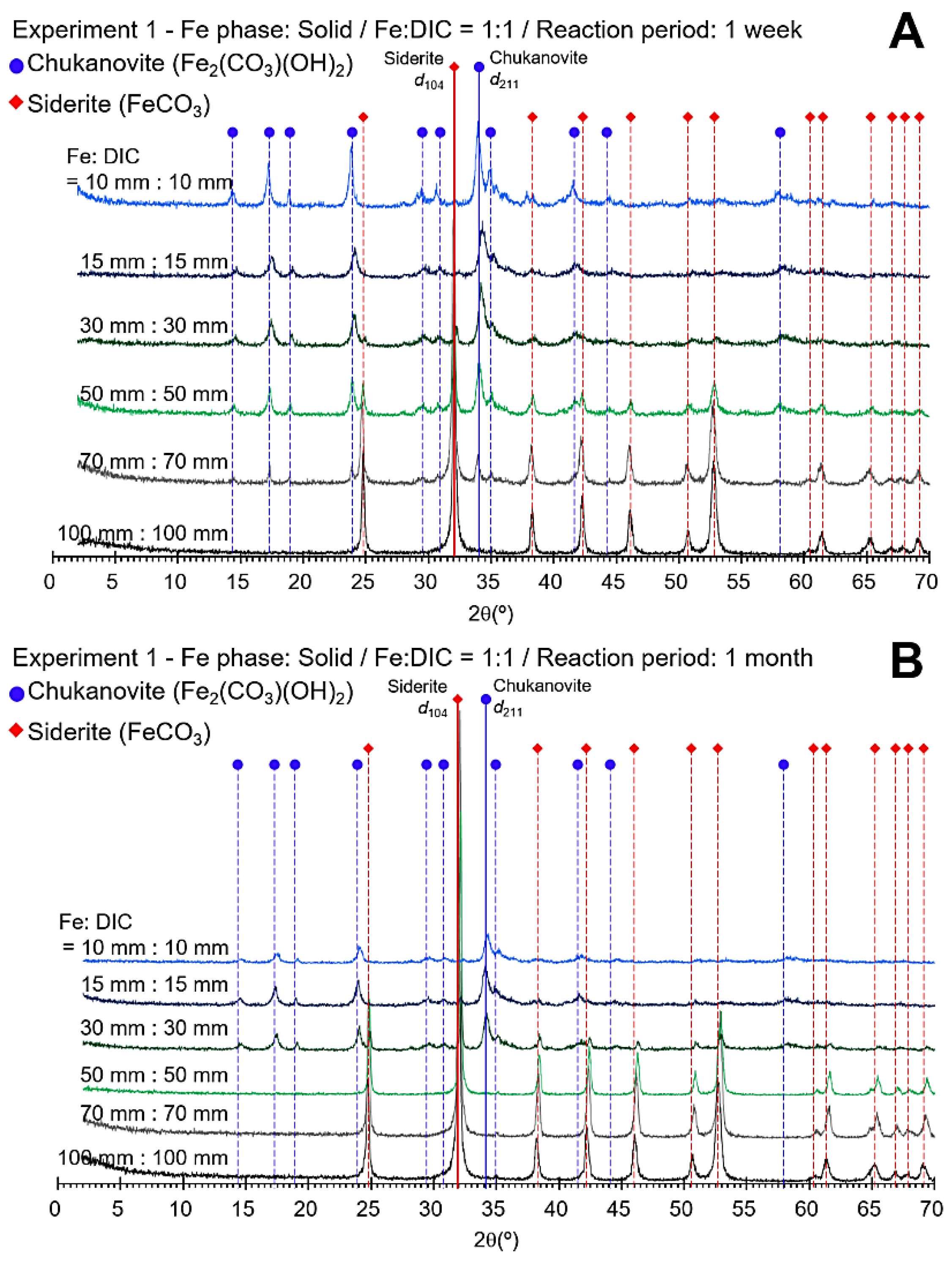
3.1. Concentration Limit of Siderite Formation
3.2. Control Species for Siderite Formation
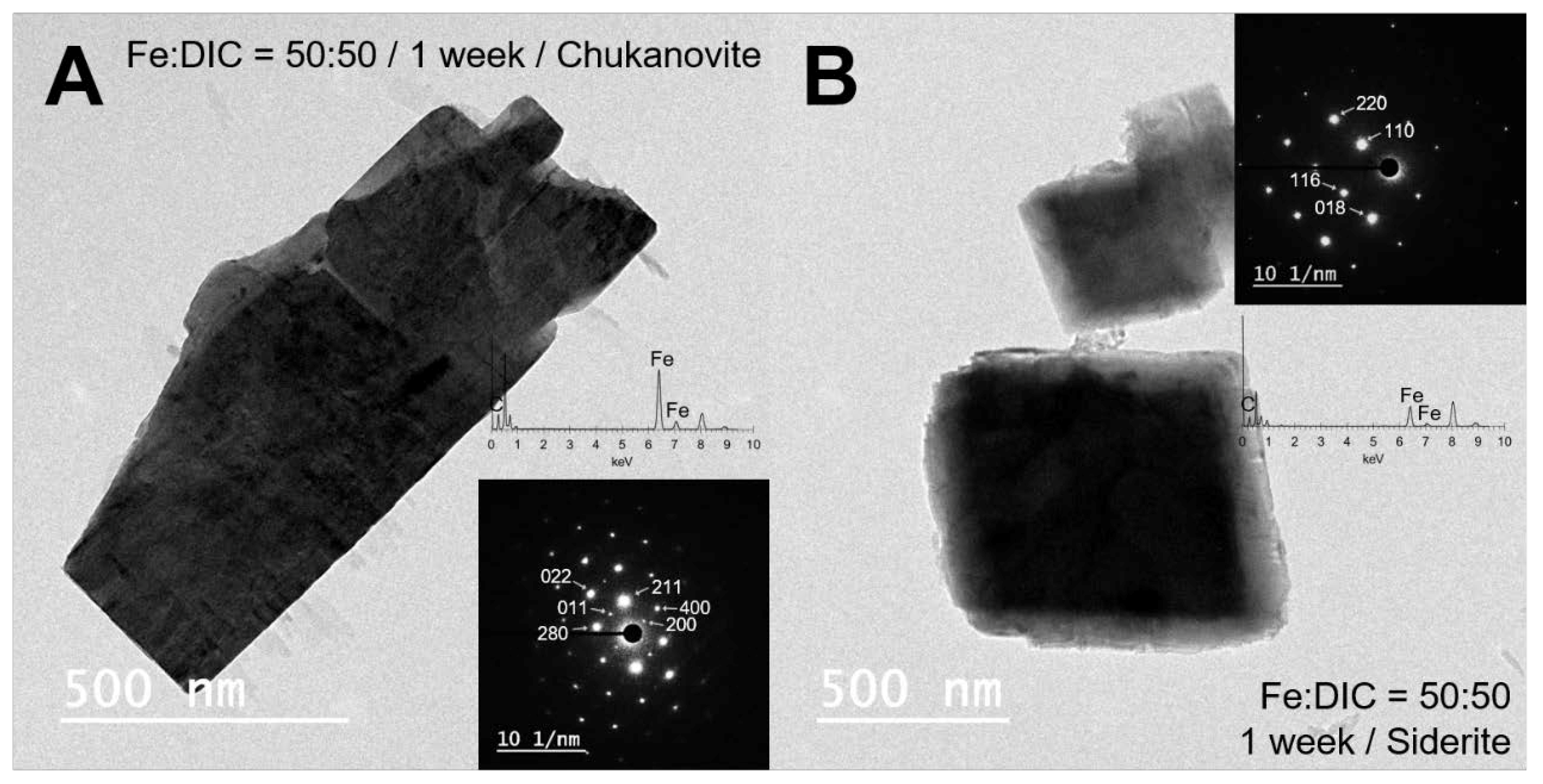
3.3. Electron Microscopic Observation of Synthesized Siderite and Chukanovite
4. Discussion
4.1. Concentration Limit of Siderite Formation
4.2. Control Species for Siderite Formation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anthony, T.; Cline, H. Surface rippling induced by surface-tension gradients during laser surface melting and alloying. J. Appl. Phys. 1977, 48, 3888–3894. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Knoll, A.H. Fossilization potential of iron-bearing minerals in acidic environments of Rio Tinto, Spain: Implications for Mars exploration. Icarus 2008, 194, 72–85. [Google Scholar] [CrossRef]
- Hałas, S.; Chlebowski, R. Unique siderite occurrence in Baltic Sea: A clue to siderite-water oxygen isotope fractionation at low temperatures. Geol. Q. 2004, 48, 317–322. [Google Scholar]
- Romanek, C.S.; Grady, M.M.; Wright, I.; Mittlefehldt, D.; Socki, R.; Pillinger, C.; Gibson, E.K., Jr. Record of fluid–rock interactions on Mars from the meteorite ALH84001. Nature 1994, 372, 655. [Google Scholar] [CrossRef] [PubMed]
- Valley, J.W.; Eiler, J.M.; Graham, C.M.; Gibson, E.K.; Romanek, C.S.; Stolper, E.M. Low-temperature carbonate concretions in the Martian meteorite ALH84001: Evidence from stable isotopes and mineralogy. Science 1997, 275, 1633–1638. [Google Scholar] [CrossRef]
- Treiman, A.H.; Romanek, C.S. Bulk and stable isotopic compositions of carbonate minerals in Martian meteorite Allan Hills 84001: No proof of high formation temperature. Meteorit. Planet. Sci. 1998, 33, 737–742. [Google Scholar] [CrossRef]
- Seguin, M. Instability of FeCO3 in air. Am. J. Sci. 1966, 264, 562–568. [Google Scholar] [CrossRef]
- McMillan, S.; Schwertmann, U. Morphological and genetic relations between siderite, calcite and goethite in a Low Moor Peat from southern Germany. Eur. J. Soil Sci. 1998, 49, 283–293. [Google Scholar] [CrossRef]
- Duckworth, O.W.; Martin, S.T. Role of molecular oxygen in the dissolution of siderite and rhodochrosite. Geochim. Cosmochim. Acta 2004, 68, 607–621. [Google Scholar] [CrossRef]
- Poulton, S.W.; Canfield, D.E. Ferruginous conditions: A dominant feature of the ocean through Earth’s history. Elements 2011, 7, 107–112. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; De Ros, L.F.; Neumann, R.; Borghi, L. Paleoenvironmental implications of early diagenetic siderites of the Paraíba do Sul Deltaic Complex, eastern Brazil. Sediment. Geol. 2015, 323, 15–30. [Google Scholar] [CrossRef]
- McKay, D.S.; Gibson, E.K.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clemett, S.J.; Chillier, X.D.; Maechling, C.R.; Zare, R.N. Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH84001. Science 1996, 273, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Clemett, D.; Cockett, M.; Marsden, C.; Fone, K. Antisense oligonucleotide-induced reduction in 5-hydroxytryptamine7 receptors in the rat hypothalamus without alteration in exploratory behaviour or neuroendocrine function. J. Neurochem. 1998, 71, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, E.I.; Wierzchos, J.; Ascaso, C.; Winklhofer, M. Chains of magnetite crystals in the meteorite ALH84001: Evidence of biological origin. Proc. Natl. Acad. Sci. 2001, 98, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.K., Jr.; McKay, D.S.; Thomas-Keprta, K.L.; Wentworth, S.; Westall, F.; Steele, A.; Romanek, C.S.; Bell, M.; Toporski, J. Life on Mars: Evaluation of the evidence within Martian meteorites ALH84001, Nakhla, and Shergotty. Precambrian Res. 2001, 106, 15–34. [Google Scholar] [CrossRef]
- Dong, H.; Kukkadapu, R.K.; Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Kostandarithes, H.M. Microbial reduction of structural Fe (III) in illite and goethite. Environ. Sci. Technol. 2003, 37, 1268–1276. [Google Scholar] [CrossRef]
- Koo, T.-H.; Lee, G.; Kim, J.-W. Biogeochemical dissolution of nontronite by Shewanella oneidensis MR-1: Evidence of biotic illite formation. Appl. Clay Sci. 2016, 134, 13–18. [Google Scholar] [CrossRef]
- Roh, Y.; Zhang, C.-L.; Vali, H.; Lauf, R.; Zhou, J.; Phelps, T. Biogeochemical and environmental factors in Fe biomineralization: Magnetite and siderite formation. Clays Clay Miner. 2003, 51, 83–95. [Google Scholar] [CrossRef]
- Kim, J.; Dong, H. Application of electron energy-loss spectroscopy (EELS) and energy-filtered transmission electron microscopy (EFTEM) to the study of mineral transformation associated with microbial Fe-reduction of magnetite. Clays Clay Miner. 2011, 59, 176–188. [Google Scholar] [CrossRef]
- Johnson, C.M.; Roden, E.E.; Welch, S.A.; Beard, B.L. Experimental constraints on Fe isotope fractionation during magnetite and Fe carbonate formation coupled to dissimilatory hydrous ferric oxide reduction. Geochim. Cosmochim. Acta 2005, 69, 963–993. [Google Scholar] [CrossRef]
- Kim, J.; Dong, H.; Seabaugh, J.; Newell, S.W.; Eberl, D.D. Role of microbes in the smectite-to-illite reaction. Science 2004, 303, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Romanek, C.S. Precipitation kinetics and carbon isotope partitioning of inorganic siderite at 25 °C and 1 atm. Geochim. Cosmochim. Acta 2004, 68, 557–571. [Google Scholar] [CrossRef]
- Van Dijk, J.; Fernandez, A.; Müller, I.A.; Lever, M.; Bernasconi, S.M. Oxygen isotope fractionation in the siderite-water system between 8.5 and 62 °C. Geochim. Cosmochim. Acta 2018, 220, 535–551. [Google Scholar] [CrossRef]
- Fernandez, A.; Tang, J.; Rosenheim, B.E. Siderite ‘clumped’isotope thermometry: A new paleoclimate proxy for humid continental environments. Geochim. Cosmochim. Acta 2014, 126, 411–421. [Google Scholar] [CrossRef]
- Wiesli, R.A.; Beard, B.L.; Johnson, C.M. Experimental determination of Fe isotope fractionation between aqueous Fe (II), siderite and “green rust” in abiotic systems. Chem. Geol. 2004, 211, 343–362. [Google Scholar] [CrossRef]
- Kim, S.-T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Ahmed, I.S.; Mohamed, T.Y.; Khatab, M. A controlled, template-free, and hydrothermal synthesis route to sphere-like α-Fe2O3 nanostructures for textile dye removal. Rsc Adv. 2016, 6, 20001–20013. [Google Scholar] [CrossRef]
- Pekov, I.V.; Perchiazzi, N.; Merlino, S.; Kalachev, V.N.; Merlini, M.; Zadov, A.E. Chukanovite, Fe(CO3)(OH)2, a new mineral from the weathered iron meteorite Dronino. Eur. J. Mineral. 2007, 19, 891–898. [Google Scholar] [CrossRef]
- Erdös, E.; Altorfer, H. Ein dem Malachit ähnliches basisches Eisenkarbonat als Korrosionsprodukt von Stahl. Mater. Corros. 1976, 27, 304–312. [Google Scholar] [CrossRef]
- Azoulay, I.; Rémazeilles, C.; Refait, P. Determination of standard Gibbs free energy of formation of chukanovite and Pourbaix diagrams of iron in carbonated media. Corros. Sci. 2012, 58, 229–236. [Google Scholar] [CrossRef]
- Pandarinathan, V.; Lepková, K.; Van Bronswijk, W. Chukanovite (Fe2(OH)2CO3) identified as a corrosion product at sand-deposited carbon steel in CO2-saturated brine. Corros. Sci. 2014, 85, 26–32. [Google Scholar] [CrossRef]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Kennedy, D.W. Biotransformation of two-line silica-ferrihydrite by a dissimilatory Fe (III)-reducing bacterium: Formation of carbonate green rust in the presence of phosphate. Geochim. Cosmochim. Acta 2004, 68, 2799–2814. [Google Scholar] [CrossRef]
- Kim, S.; Marrs, C.; Nemer, M.; Je-Hun Jang, J. Solubility Model for Ferrous Iron Hydroxide, Hibbingite, Siderite, and Chukanovite in High Saline Solutions of Sodium Chloride, Sodium Sulfate, and Sodium Carbonate. ACS Earth Space Chem. 2017, 1, 647–663. [Google Scholar] [CrossRef]
- Graf, D.L. Crystallographic tables for the rhombohedral carbonates. Am. Mineral. J. Earth 1961, 46, 1283–1316. [Google Scholar]
- De Marco, R.; Jiang, Z.-T.; John, D.; Sercombe, M.; Kinsella, B. An in situ electrochemical impedance spectroscopy/synchrotron radiation grazing incidence X-ray diffraction study of the influence of acetate on the carbon dioxide corrosion of mild steel. Electrochim. Acta 2007, 52, 3746–3750. [Google Scholar] [CrossRef]
- Curtis, C.; Spears, D. The formation of sedimentary iron minerals. Econ. Geol. 1968, 63, 257–270. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H. The crystal structure and Gibbs free energy of formation of chukanovite as an oxidation product of carbon steel in human liver. Chem. Geol. 2018, 488, 180–188. [Google Scholar] [CrossRef]




| Experiment | FeCl2·4H2O | NaHCO3 | Reaction Time | |
|---|---|---|---|---|
| Phase | Concentration (mmolal) | Concentration (mmolal) | ||
| Experiment 1 (Fe:DIC = 1:1) | Solid (powder) | 10, 15, 30, 50, 70, 100 | 10, 15, 30, 50, 70, 100 | 1 week 1 month |
| Experiment 2 (Fe:DIC = 1:1) | Aqueous | 10, 15, 30, 50, 70, 100 | 10, 15, 30, 50, 70, 100 | 1 week 1 month |
| Experiment 3 (Fe:DIC = X:50) | Aqueous | 15, 30, 70, 100 | 50 | 1 week 1 month |
| Experiment 4 (Fe:DIC = 50:X) | Aqueous | 50 | 15, 30, 70, 100 | 1 week 1 month |
| Experiment | Concentration Fe:DIC (mmolal:mmolal) | 1 Week | 1 Month | ||
|---|---|---|---|---|---|
| Intensity Ratio | Siderite Proportion (%) | Intensity Ratio | Siderite Proportion (%) | ||
| Experiment 1 | 10:10 | 0.128 | 2.8 | 0.164 | 7.0 |
| 15:15 | 0.130 | 3.0 | 0.221 | 12.2 | |
| 30:30 | 0.351 | 20.2 | 1.364 | 43.6 | |
| 50:50 | 1.821 | 48.6 | 55.750 | 100 | |
| 70:70 | 12.104 | 81.2 | 67.229 | 100 | |
| 100:100 | 43.125 | 100 | 388.696 | 100 | |
| Experiment 2 | 10:10 | 0.239 | 13.5 | 0.184 | 9.1 |
| 15:15 | 0.244 | 13.9 | 0.344 | 19.8 | |
| 30:30 | 1.383 | 43.8 | 9.747 | 77.5 | |
| 50:50 | 7.590 | 73.2 | 23.256 | 92.5 | |
| 70:70 | 8.481 | 75.1 | 37.667 | 100 | |
| 100:100 | 24.415 | 93.3 | 35.702 | 100 | |
| Experiment | Concentration Fe:DIC (mmolal:mmolal) | 1 Week | 1 Month | ||
|---|---|---|---|---|---|
| Intensity Ratio | Siderite Proportion | Intensity Ratio | Siderite Proportion | ||
| Experiment 3 | 15:50 | 1.887 | 49.2 | 0.481 | 25.6 |
| 30:50 | 6.541 | 70.6 | 30.493 | 97.2 | |
| 50:50 * | 7.590 | 73.2 | 23.256 | 92.5 | |
| 70:50 | 20.931 | 90.7 | 57.000 | 100 | |
| 100:50 | 21.766 | 91.4 | 54.086 | 100 | |
| Experiment 4 | 50:15 | 0.510 | 26.6 | 2.211 | 51.9 |
| 50:30 | 3.152 | 58.0 | 11.952 | 81.0 | |
| 50:50 * | 7.590 | 73.2 | 23.256 | 92.5 | |
| 50:70 | 25.262 | 93.9 | 29.047 | 96.3 | |
| 50:100 | 37.897 | 100 | 48.484 | 100 | |
| Experiment | Concentration Fe:DIC (mmolal:mmolal) | 1 Week | 1 Month | ||
|---|---|---|---|---|---|
| Initial pH | Final pH | Initial pH | Final pH | ||
| Experiment 3 | 15:50 | 7.19 | 6.39 | 7.21 | 6.31 |
| 30:50 | 6.99 | 5.38 | 6.99 | 5.87 | |
| 50:50 * | 6.93 | 5.55 | 6.93 | 5.88 | |
| 70:50 | 6.78 | 5.63 | 6.78 | 5.38 | |
| 100:50 | 6.67 | 5.50 | 6.68 | 5.30 | |
| Experiment 4 | 50:15 | 6.78 | 6.27 | 6.60 | 5.87 |
| 50:30 | 6.81 | 5.95 | 6.86 | 5.73 | |
| 50:50 * | 6.93 | 5.55 | 6.93 | 5.88 | |
| 50:70 | 6.79 | 5.80 | 6.77 | 6.08 | |
| 50:100 | 6.77 | 5.88 | 6.76 | 5.62 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, T.-h.; Kim, J. Controls on the Formation and Stability of Siderite (FeCO3) and Chukanovite (Fe2(CO3)(OH)2) in Reducing Environment. Minerals 2020, 10, 156. https://doi.org/10.3390/min10020156
Koo T-h, Kim J. Controls on the Formation and Stability of Siderite (FeCO3) and Chukanovite (Fe2(CO3)(OH)2) in Reducing Environment. Minerals. 2020; 10(2):156. https://doi.org/10.3390/min10020156
Chicago/Turabian StyleKoo, Tae-hee, and Jinwook Kim. 2020. "Controls on the Formation and Stability of Siderite (FeCO3) and Chukanovite (Fe2(CO3)(OH)2) in Reducing Environment" Minerals 10, no. 2: 156. https://doi.org/10.3390/min10020156
APA StyleKoo, T.-h., & Kim, J. (2020). Controls on the Formation and Stability of Siderite (FeCO3) and Chukanovite (Fe2(CO3)(OH)2) in Reducing Environment. Minerals, 10(2), 156. https://doi.org/10.3390/min10020156






