Metabolic Profiling of Varronia curassavica Jacq. Terpenoids by Flow Modulated Two-Dimensional Gas Chromatography Coupled to Mass Spectrometry
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Extraction of Essential Oil
2.3. Comprehensive Two-Dimensional Gas Chromatographic Analysis (GC×GC-FID/MS)
2.4. Data Analysis
3. Results and Discussions
3.1. Essential Oil Content
3.2. Characterization of the Essential Oil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilbert, B.; Favoreto, R. Cordia verbenacea DC. Boraginaceae. Revista Fitos 2012, 7, 17–25. [Google Scholar]
- Aché Laboratórios Farmacêuticos Brazil. Available online: http://www.ache.com.br/arquivos/26-05 Helleva chega ao México e o Acheflan tambem.pdf (accessed on 8 January 2020).
- Oliveira, M. Laboratório em renovação. Pesquisa Fapesp 2017, 255, 74–77. [Google Scholar]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.F.; Fernandes, E.S.; Cunha, F.M.; Ferreira, J.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J. Ethnopharmacol. 2007, 110, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Passos, G.F.; Vitor, C.E.; Koepp, J.; Mazzuco, T.L.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldão, E.F.; Witaicenis, A.; Seito, L.N.; Hiruma-Lima, C.A.; Di Stasi, L.C. Evaluation of the antiulcerogenic and analgesic activities of Cordia verbenacea DC. (Boraginaceae). J. Ethnopharmacol. 2008, 119, 94–98. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Luchini, A.C.; Seito, L.N.; Gomes, J.C.; Crespo-López, M.E.; Di Stasi, L.C. Cordia verbenacea and secretion of mast cells in different animal species. J. Ethnopharmacol. 2011, 135, 463–468. [Google Scholar] [CrossRef]
- Michielin, E.M.Z.; Wiese, L.P.L.; Ferreira, E.A.; Pedrosa, R.C.; Ferreira, S.R.S. Radical-scavenging activity of extracts from Cordia verbenacea DC obtained by different methods. J. Supercrit. Fluids 2011, 56, 89–96. [Google Scholar] [CrossRef]
- Parisotto, E.B.; Michielin, E.M.Z.; Biscaro, F.; Ferreira, S.R.S.; Filho, D.W.; Pedrosa, R.C. The antitumor activity of extracts from Cordia verbenacea D.C. obtained by supercritical fluid extraction. J. Supercrit. Fluids 2012, 61, 101–107. [Google Scholar] [CrossRef]
- Pimentel, S.P.; Barrella, G.E.; Casarin, R.C.V.; Cirano, F.R.; Casati, M.Z.; Foglio, M.A.; Figueira, G.F.; Ribeiro, F.V.R. Protective effect of topical Cordia verbenacea in a rat periodontitis model: Immune-inflammatory, antibacterial andmorphometric assays. BMC Complement. Altern. Med. 2012, 12, 224. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.F.; Oliveira, L.G.; Rodrigues, F.F.; Saraiva, M.E.; Almeida, S.C.; Cabral, M.E.; Campos, A.R.; Costa, J.G. Chemical composition, antibacterial and antifungal activities of essential oil from Cordia verbenacea DC leaves. Pharmacogn. Res. 2012, 4, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, E.F.F.; Alves, E.F.; Silva, M.K.N.; Carvalho, V.R.A.; Medeiros, C.R.; Santos, F.A.V.; Bitu, V.C.N.; Souza, C.E.S.; Figueredo, F.G.; Boligon, A.A.; et al. Potentiation of antibiotic activity of aminoglycosides by natural products from Cordia verbenacea DC. Microb. Pathog. 2016, 95, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.R.; Díaz, I.E.C.; Paciencia, M.L.B.; Fana, S.A.; Morais, D.; Eberlin, M.N.; Silva, J.S.; Silveira, E.R.; Barros, M.P.; Suffredini, I.B. Interference of seasonal variation on the antimicrobial and cytotoxic activities of the essential oils from the leaves of Iryanthera polyneura in the Amazon Rain Forest. Chem. Biodivers. 2019, 16, e1900374. [Google Scholar] [CrossRef] [PubMed]
- Zouari-Bouassida, K.; Trigui, M.; Makni, S.; Jlatel, L.; Tounsi, S. Seasonal variation in essential oils composition and the biological and pharmaceutical protective effects of Mentha longifolia leaves grown in Tunisia. BioMed. Res. Int. 2018, e7856517. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, E.O.; Vieira, M.A.R.; Ferreira, M.I.; Fernandes, A., Jr.; Marques, M.O.M.; Minatel, I.O.; Albano, M.; Sambo, P.; Lima, G.P.P. Seasonality effects on chemical composition, antibacterial activity and essential oil yield of three species of Nectandra. PLoS ONE 2018, 13, e0204132. [Google Scholar] [CrossRef]
- Sun, C.X.; Li, M.Q.; Gao, X.X.; Liu, L.N.; Wu, X.F.; Zhou, J.H. Metabolic response of maize plants to multi-factorial abiotic stress. Plant Biol. 2016, 18, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Gholamali, H.; Mahmoudi, Z.; Asghari, M. Diurnal variation of essential of the oil components of Pycnocycla spinosa Decne. ex Boiss. Jundishapur. J. Nat. Pharm. Prod. 2014, 9, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, E.; Figueiredo, A.C.; Barroso, J.G.; Henriques, J.; Sousa, E.; Bonifácio, L. Effect of Monochamus galloprovincialis feeding on Pinus pinaster and Pinus pinea, oleoresin and insect valatiles. Phytochemistry 2020, 169, 112159. [Google Scholar] [CrossRef]
- Medbouhi, A.; Benbelaïd, F.; Djabou, N.; Beaufay, C.; Bendahou, M.; Quetin-Leclercq, J.; Tintaru, A.; Costa, J.; Muselli, A. Essential Oil of Algerian Eryngium campestre: Chemical Variability and Evaluation of Biological Activities. Molecules 2019, 24, 2575. [Google Scholar] [CrossRef] [Green Version]
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; Silva, J.K.R.; Setzer, W.N. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules 2019, 24, 4244. [Google Scholar] [CrossRef] [Green Version]
- Souza, T.S.; Da Silva Ferreira, M.F.; Menini, L.; De Lima Souza, J.R.C.; De Oliveira Bernardes, C.; Ferreira, A. Chemotype diversity of Psidium guajava L. Phytochemistry 2018, 153, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.T.; Dufour, J.P.; Lewis, A.C. Application of comprehensive two-dimensional gas chromatography combined with time of flight mass spectrometry (GCxGC-TOFMS) for high resolution analysis of hop essential oil. J. Sep. Sci. 2004, 27, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.G.; Fukuda, K.; Kato, M.J.; Sartorato, A.; Duarte, M.C.T.; Ruiz, A.L.T.G.; Carvalho, J.E.; Augusto, F. Characterization of the essential oils of two species of Piperaceae by one- and two-dimensional chromatographic techniques with quadrupole mass spectrometric detection. Microchem. J. 2014, 115, 113–120. [Google Scholar] [CrossRef]
- Koek, M.M.; Kloet, F.M.; Kleemann, R.; Kooistra, T.; Verheij, E.R.; Hankemeier, T. Semi-automated non-target processing in GC×GC-MS metabolomics analysis: Applicability for biomedical studies. Metabolomics 2011, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dallüge, J.; Beens, J.; Brinkman, U.A.T. Comprehensive two-dimensional gas chromatography: A powerful and versatile analytical tool. J. Chromatogr. A 2003, 1000, 69–108. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Franchina, F.A.; Mondello, L. Analysis of essential oils through comprehensive two-dimensional gas chromatography: General utility. Flavour Frag. J. 2017, 32, 218. [Google Scholar] [CrossRef]
- Wong, Y.F.; Perlmutter, P.; Marriott, P.J. Untargeted metabolic profiling of Eucalyptus spp. leaf oils using comprehensive two-dimensional gas chromatography with high resolution mass spectrometry: Expanding the metabolic coverage. Metabolomics 2017, 13, 46. [Google Scholar] [CrossRef]
- Seeley, J.V.; Seeley, S.K. Multidimensional gas chromatography: Fundamental advances and new applications. Anal. Chem. 2013, 85, 557–578. [Google Scholar] [CrossRef]
- Meinert, C.; Meierhenrich, U.J. A new dimension in separation science: Comprehensive two-dimensional gas chromatography. Angew. Chem. Int. Ed. Engl. 2012, 51, 10460–10470. [Google Scholar] [CrossRef]
- Pedroso, M.P.; Godoy, L.A.F.; Fidélis, C.H.V.; Ferreira, E.C.; Poppi, R.J.; Augusto, F. Cromatografia gasosa bidimensional abrangente (GC×GC). Quím. Nova 2009, 32, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Duhamel, C.; Cardinael, P.; Peulon-agasse, V.; Firor, R.; Pascaud, L.; Semard-jousset, G.; Giusti, P.; Livadaris, V. Comparison of cryogenic and differential flow (forward and reverse fill/flush) modulators and applications to the analysis of heavy petroleum cuts by high-temperature comprehensive gas. J. Chromatogr. A 2015, 1387, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Rubiolo, P.; Reichenbach, S.E.; Carreta, A.; Cobelli, L.; Giardina, M.; Bicchi, C. Method translation and full metadata transfer from thermal to differential flow modulated comprehensive two dimensional gas chromatography: Profiling of suspected fragrance allergens. J. Chromatogr. A 2017, 1480, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Rubiolo, P.; Cobelli, L.; Stani, G.; Miliazza, A.; Giardina, M.; Firor, R.; Bicchi, C. Potential of the reversed-inject dofferential flow modulator for comprehensive two-dimensional gas chromatography in the quantitative profiling and fingerprinting of essenctial oils of different complexity. J. Chromatogr. A 2015, 1417, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Barbará, J.A.; Nicolli, K.P.; Souza-Silva, E.A.; Biasoto, A.C.T.; Welke, J.E.; Zini, C.A. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Horvat, I.; Vrhovsek, U. Combined targeted and untargeted profiling of volatile aroma compounds with comprehensive two-dimensional gas chromatography for differentiation of virgin olive oils according to variety and geographical. Food Chem. 2019, 270, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.L.; Novaes, A.D.S.; Polidoro, A.D.S.; Barros, M.E.; Mota, J.S.; Lima, D.B.M.; Krause, L.C.; Cardosos, C.A.L.; Jacques, R.A.; Camarão, E.B. Chemical characterisation of Piper amalogo (Piperaceae) essential oil by comprehensive two-dimensional gas chromatography coupled with rapid-scanning quadrupole mass spectrometry (GC×GC/qMS) and their antilithiasic activity and acute toxicity. Phytochem. Anal. 2018, 29, 432–445. [Google Scholar] [CrossRef]
- Yan, D.; Wong, Y.F.; Tedone, L.; Shellie, R.A.; Marriott, P.J.; Whittock, S.P.; Koutoulis, A. Chemotyping of new hop (Humulus lupulus L.) genotypes using comprehensive two-dimensional gas chromatography with quadrupole accurate mass-time-of-flight mass spectrometry. J. Chromatogr. A 2018, 1536, 110–121. [Google Scholar] [CrossRef]
- Filippi, J.J.; Belhassen, E.; Baldovini, N.; Brevard, H.; Meierhenrich, U.J. Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chromatogr. A 2013, 1288, 127–148. [Google Scholar] [CrossRef]
- Fidelis, C.H.V.; Augusto, F.; Sampaio, P.T.B.; Krainovic, P.M.; Barata, L.E.S. Chemical characterization of rosewood (Aniba rosaeodora Ducke) leaf essential oil by comprehensive two-dimensional gas chromatography coupled with quadrupole mass spectrometry. J. Essent. Oil Res. 2012, 24, 245–251. [Google Scholar] [CrossRef]
- Keppler, E.A.H.; Jenkins, C.L.; Davis, T.J.; Bean, H.D. Advances in the application of comprehensive two-dimensional gas chromatography in metabolomics. TrAC Trend Anal. Chem. 2018, 109, 275–286. [Google Scholar] [CrossRef]
- Morimoto, J.; Rosso, M.C.; Kfoury, N.; Bicchi, C.; Cordero, C.; Robbat, A., Jr. Untargeted/Targeted 2D gas chromatography/mass spectrometry detection of the total volatile Tea metabolome. Molecules 2019, 24, 3757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Ros, A.; Masuero, D.; Riccadonna, S.; Bubola, K.B.; Mulinacci, N.; Mattivi, F.; Lukić, I.; Vrhovsek, U. Complementary untargeted and targeted metabolomics for differentiation of extra virgin Olive oils of different origin of purchase based on volatile and phenolic composition and sensory quality. Molecules 2019, 24, 2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciarrone, D.; Giuffrida, D.; Rotondo, A.; Micalizzi, G.; Zoccali, M.; Pantò, S.; Donato, P.; Rodrigues-das-Dores, R.G.; Mondello, L. Quali-quantitative characterization of the volatile constituints in Cordia verbenacea D.C. essential oil exploiting advanced chromatographic approaches and nuclear magnetic resonance analysis. J. Chromatogr. A 2017, 1524, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.P.; Santos, T.A.C.; Moutinho, B.L.; Silva, R.S.; Pinto, V.S.; Blank, A.F.; Corrêa, C.B.; Scher, R.; Fernandes, R.P.M. Using Varronia curassavica (Cordiaceae) essential oil for the biocontrolo f Phytomonas serpens. Ind. Crops Prod. 2019, 139, 111523. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Alves, E.F.; Silva, M.K.N.; Carvalho, V.R.A.; Fegueiredo, F.G.; Ferreira, J.V.A.; Coutinho, H.D.M.; Silva, M.F.L.; Ribeiro-Filho, J.; Costa, J.G.M. Seasonal variation, chemical composition and biological activity of theessential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod. 2016, 87, 45–53. [Google Scholar] [CrossRef]
- Queiroz, T.B.; Mendes, A.D.R.; Silva, J.C.R.L.; Fonseca, F.S.A.; Martins, E.R. Content and chemical composition of the essential oil of ‘erva-baleeira’ (Varronia curassavica Jaqc.) as a function of harvesting times. Rev. Bras. Plantas Med. 2016, 18, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.P.S.; Bonfim, F.P.G.; Dantas, W.F.C.; Puppi, R.J.; Marques, M.O.M. Chemical composition of essential oil from Varronia curassavica Jacq. accessions in different seasons of the year. Ind. Crops Prod. 2019, 140, 111656. [Google Scholar] [CrossRef]
- Santos, A.V.; Defaveri, A.C.A.; Bizzo, H.R.; Gil, R.A.S.S.; Sato, A. In vitro propagation, histochemistry, and analysis of essential oil from conventionally propagated and in vitro-propagated plants of Varronia curassavica Jacq. In Vitro Cell. Dev. Biol. Plant 2013, 49, 405–413. [Google Scholar] [CrossRef]
- CIIAGRO—Centro Integrado de Informações Agrometeorológicas. Instituto Agronômico de Campinas (IAC). Available online: http://www.ciiagro.sp.gov.br/ (accessed on 8 January 2020).
- Adams, R.P. Identification of Essential Oil Components by Gas. Cromatography/Mass Spectroscopy, 4.1 ed.; Allured Publ. Corp: Carol Stream, IL, USA, 2017. [Google Scholar]
- Van Den Dool, H.; Kratz, D.J. A generalization of the retention index system incluing liner temperature programmed gas-liquid partition chromatograpy. J. Cromatogr. 1963, 11, 463–467. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinforma. 2019, 68, e86. [Google Scholar] [CrossRef]
- Fidelis, C.H.V.; Sampaio, P.T.B.; Krainovic, P.M.; Augusto, F.; Barata, L.E.S. Correlation between maturity of tree and GCxGC-qMS chemical profiles of essential oil from leaves of Aniba rosaeodora Ducke. Microchem. J. 2013, 109, 73–77. [Google Scholar] [CrossRef]
- Kaya, D.A.; Arslan, M.; Rusu, L.-C. Effects of harvesting hour on essential oil content and composition of Thymus vulgaris. Farmacia 2013, 61, 1194–1203. [Google Scholar]
- Lakušić, B.S.; Ristić, M.S.; Slavkovska, V.N.; Stojanović, D.L.; Lakušić, D.V. Variations in essential oil yields and compositions of Salvia officinalis (Lamiaceae) at different developmental stages. Botanica Serbica 2013, 37, 127–139. [Google Scholar]
- Mishra, R.; Gupta, A.K.; Kumar, A.; Lal, R.K.; Saikia, D.; Chanotiya, C.S. Genetic diversity, essential oil composition, and in vitro antioxidant and antimicrobial activity of Curcuma longa L. germplasm collections. J. Appl. Res. Med. Aromat. Plants 2018, 10, 75–84. [Google Scholar] [CrossRef]
- Alemu, A.; Garedew, W.; Gebre, A. Essential oil yield and yield components of basil (Ocimum basilicum L.) as affected by genotype and intrarow spacing at Jimma, SW Ethiopia. Acta Agrobot. 2018, 71, 1743. [Google Scholar] [CrossRef]
- Külheim, C.; Padovan, A.; Hefer, C.; Krause, S.T.; Köllner, T.G.; Myburg, A.A.; Degenhardt, J.; Foley, W.F. The Eucalyptus terpene synthase gene family. BMC Genom. 2015, 16, 450. [Google Scholar] [CrossRef] [Green Version]
- Facanali, R.; Colombo, C.A.; Teixeira, J.P.F.; Ming, L.C.; Zucchi, M.I.; Marques, M.O.M. Genetic and chemical diversity of native populations of Ocimum selloi Benth. Ind. Crops Prod. 2015, 76, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Padovan, A.; Keszei, A.; Külheim, C.; Foley, W.J. The evolution of foliar terpene diversity in Myrtaceae. Phytochem. Rev. 2014, 13, 695–716. [Google Scholar] [CrossRef]
- Souza, T.S.; Ferreira, M.F.S.; Menini, L.; Souza, J.R.C.L.; Parreira, L.A.; Cecon, P.R.; Ferreira, A. Essential oil of Psidium guajava: Influence of genotypes and environment. Sci. Hortic. 2017, 216, 38–44. [Google Scholar] [CrossRef]
- Gasparetto, A.; Cruz, A.B.; Wagner, T.M.; Bonomini, T.J.; Correa, R.; Malheiros, A. Seasonal variation in the chemical composition, antimicrobial and mutagenic potential of essential oils from Piper cernuum. Ind. Crops Prod. 2017, 95, 256–263. [Google Scholar] [CrossRef]
- Macêdo, D.G.; Souza, M.M.A.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Santos, A.T.L.; Cruz, R.P.; Costa, J.G.M.; Rodrigues, F.F.G.; Quintans-junior, L.J.; Almeida, J.R.G.S.; et al. Effect of seasonality on chemical profile and antifungal activity of essential oil isolated from leaves Psidium salutare (Kunth) O. Berg. PeerJ 2018, 6, e5476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.F.; Almeida, M.P.; Leite, M.F.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Amaral, J.G.; David, J.M. Seasonal variation in the chemical composition of two chemotypes of Lippia alba. Food Chem. 2019, 273, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.A.O.; Blank, A.F.; Nogueira, P.C.L.; Arrigoni-Blank, M.F.; Andrade, T.M.; Sampaio, T.S.; Pereira, K.L.G. Chemical chracterization of the essential oil from leaves of basil genotypes cultivated in different seasons. Bol. Latinoam. Caribe Plant Med. Aromat. 2019, 18, 58–70. [Google Scholar] [CrossRef]
- Mendes, L.A.; Souza, T.S.; Menini, L.; Guilhen, J.H.S.; Bernardes, C.O.; Ferreira, A.; Ferreira, M.F.S. Spring alterations in the chromatographic profile of leaf essential oils of improved guava genotypes in Brazil. Sci. Hortic. 2018, 238, 295–302. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Shi, J.; Sun, Z.; Lei, Z.; Yin, Z.; Qian, Z.; Tang, H.; Xie, H. Genotypic and Environmental Effects on the Volatile Chemotype of Valeriana jatamansi Jones. Front. Plant Sci. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef] [Green Version]
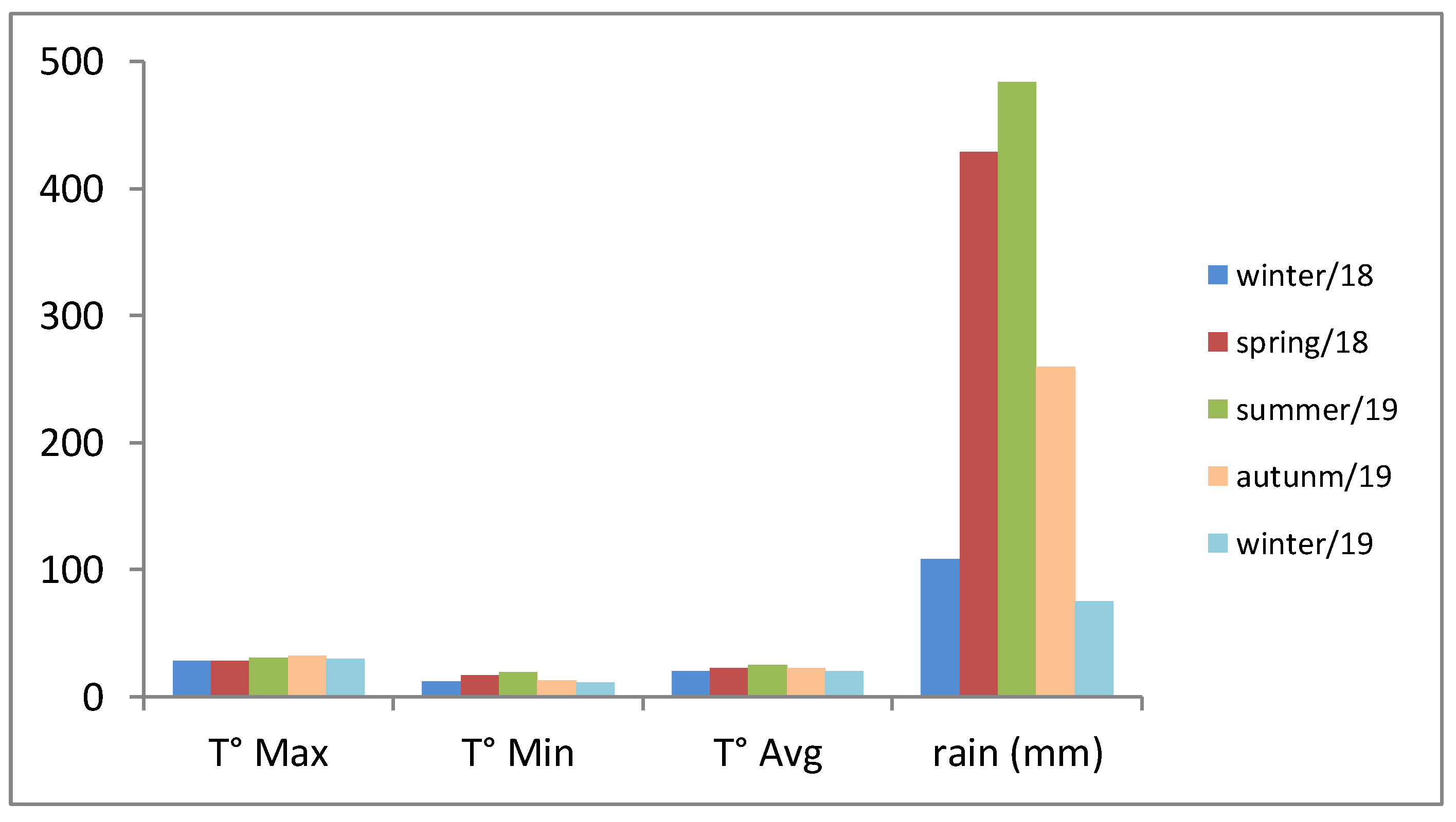



| Genotype | Season | |||
|---|---|---|---|---|
| Winter B | Spring A | Summer AB | Autumn AB | |
| VC1a | 0.22 ± 0.04 | 0.42 ± 0.04 | 0.32 ± 0.08 | 0.41 ± 0.03 |
| VC2a | 0.36 ± 0.06 | 0.60 ± 0.03 | 0.46 ± 0.02 | 0.52 ± 0.02 |
| VC3a | 0.29 ± 0.04 | 0.58 ± 0.02 | 0.45 ± 0.03 | 0.44 ± 0.04 |
| Substance | LTPRI | Genotype/Season | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC1 | VC2 | VC3 | ||||||||||||
| Exp. | Lit. | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | |
| tricyclene | 930 | 926 | 0.464 | ta | ta | ta | 1.074 | ta | ta | ta | 0.091 | ta | ta | ta |
| α-thujene | 939 | 930 | 8.046 | 5.929 | 6.047 | 9.273 | 9.369 | 7.188 | 7.154 | 9.392 | 7.479 | ta | 0.032 | 0.078 |
| α-pinene | 947 | 939 | 28.920 | 35.236 | 33.769 | 48.178 | 27.102 | 29.058 | 33.985 | 33.062 | 33.029 | 41.899 | 45.805 | 58.864 |
| thuja-2,4(10)-diene | 956 | 951 | ta | ta | 0.036 | ta | 0.026 | ta | 0.067 | ta | - | - | - | - |
| camphene | 963 | 954 | 0.233 | 0.212 | 0.161 | 0.142 | 0.288 | 0.252 | 0.181 | 0.182 | 0.245 | 0.172 | 0.158 | 0.133 |
| sabinene | 983 | 975 | 2.048 | 1.353 | 1.401 | 1.621 | 2.557 | 1.387 | 1.321 | 2.333 | 0.614 | 0.372 | 0.414 | 0.305 |
| β-pinene | 990 | 979 | 3.179 | 2.734 | 2.535 | 3.178 | 5.997 | 5.341 | 4.898 | 5.702 | 2.106 | 1.530 | 1.517 | 1.585 |
| myrcene | 998 | 990 | 0.547 | 0.320 | 0.403 | 0.367 | 0.829 | 0.427 | 0.359 | 0.647 | 0.442 | 0.113 | 0.296 | 0.212 |
| α-phellandrene | 1019 | 1002 | 0.038 | 0.079 | 0.079 | 0.124 | 0.088 | 0.079 | 0.074 | 0.128 | 0.027 | 0.019 | 0.043 | ta |
| α-terpinene | 1027 | 1017 | ta | ta | 0.036 | ta | 0.031 | ta | 0.053 | 0.051 | - | - | - | - |
| o-cymene | 1037 | 1022 | 0.122 | ta | 0.044 | ta | 0.123 | ta | 0.049 | ta | 0.026 | ta | 0.006 | ta |
| limonene | 1024 | 1024 | ta | ta | ta | 0.147 | ta | ta | ta | 0.147 | - | - | - | - |
| β-phellandrene | 1029 | 1025 | ta | 0.043 | ta | ta | ta | 0.030 | ta | ta | ta | 0.532 | ta | ta |
| sylvestrene | 1038 | 1025 | 0.492 | 0.556 | 0.590 | 0.310 | 0.356 | 0.367 | 0.392 | 0.349 | 0.635 | ta | 0.721 | 0.399 |
| 1,8-cineole | 1041 | 1026 | 2.016 | 1.412 | 1.019 | 1.455 | 2.269 | 1.112 | 0.702 | 1.855 | 1.039 | 0.853 | 0.854 | 0.600 |
| (E)-β-ocimene | 1044 | 1049 | ta | ta | 0.015 | ta | ta | ta | 0.025 | ta | ta | ta | 0.025 | ta |
| γ-terpinene | 1070 | 1059 | 0.040 | 0.078 | 0.107 | 0.085 | 0.100 | 0.076 | 0.154 | 0.077 | 0.021 | ta | 0.023 | ta |
| cis-sabinene hydrate | 1081 | 1070 | 0.069 | 0.043 | 0.026 | 0.030 | 0.119 | 0.034 | 0.042 | ta | 0.018 | ta | 0.012 | ta |
| terpinolene | 1102 | 1088 | ta | ta | 0.014 | ta | ta | 0.019 | 0.026 | ta | - | - | - | - |
| linalool | 1108 | 1096 | 0.057 | ta | ta | 0.052 | 0.031 | ta | ta | ta | - | - | - | - |
| α-pinene oxide | 1108 | 1097 | 0.078 | ta | ta | ta | 0.021 | ta | ta | ta | 0.105 | tr | tr | tr |
| trans-sabinene hydrate | 1111 | 1098 | 0.029 | 0.034 | ta | ta | 0.108 | 0.034 | 0.027 | ta | 0.009 | tr | tr | tr |
| n-nonanal | 1115 | 1110 | 0.444 | ta | 0.243 | ta | 0.344 | ta | 0.181 | ta | 0.119 | tr | 0.095 | tr |
| (E)-3(10)-caren-4-ol | 1130 | * | 0.018 | ta | ta | ta | 0.043 | ta | ta | ta | - | - | - | - |
| cis-p-menth-2-en-1-ol | 1134 | 1124 | - | - | - | - | 0.023 | ta | ta | ta | - | - | - | - |
| α-campholenal | 1126 | 1139 | ta | ta | 0.033 | ta | 0.025 | ta | 0.028 | ta | 0.015 | 0.012 | 0.018 | tr |
| trans-pinocarveol | 1152 | 1139 | 0.036 | ta | 0.051 | ta | ta | ta | 0.041 | ta | 0.028 | tr | 0.019 | tr |
| isopinocarveol | 1151 | 1140 | ta | 0.034 | 0.016 | ta | ta | 0.026 | 0.019 | ta | tr | 0.032 | 0.019 | tr |
| trans-sabinol | 1157 | 1142 | 0.061 | 0.017 | ta | ta | 0.049 | ta | 0.036 | ta | 0.034 | tr | tr | tr |
| camphor | 1157 | 1146 | 0.029 | 0.034 | 0.028 | ta | - | - | - | - | - | - | - | - |
| pinocarvone | 1174 | 1164 | 0.032 | ta | 0.039 | ta | 0.030 | ta | ta | ta | 0.015 | 0.009 | 0.011 | tr |
| borneol | 1182 | 1169 | ta | ta | 0.009 | ta | ta | 0.012 | ta | ta | 0.010 | 0.015 | 0.008 | tr |
| terpinen-4-ol | 1190 | 1177 | 0.105 | 0.108 | 0.103 | 0.074 | 0.166 | 0.077 | 0.180 | 0.112 | 0.033 | 0.017 | 0.022 | tr |
| α-terpineol | 1209 | 1188 | 0.033 | ta | 0.016 | ta | 0.034 | ta | 0.022 | ta | 0.006 | tr | tr | tr |
| β-cyclocitral | 1229 | 1219 | ta | ta | 0.018 | ta | ta | ta | ta | ta | 0.007 | tr | tr | tr |
| citronellol | 1223 | 1229 | ta | ta | 0.012 | ta | ta | ta | 0.017 | ta | - | - | - | - |
| bornyl acetate | 1290 | 1285 | 0.857 | 0.466 | 0.451 | 0.373 | 1.096 | 0.720 | 0.830 | 0.633 | 0.706 | 0.557 | 0.671 | 0.537 |
| trans-pinocarvyl acetate | 1302 | 1298 | - | - | - | - | - | - | - | - | 0.005 | 0.014 | tr | tr |
| myrtenyl acetate | 1329 | 1326 | - | - | - | - | - | - | - | - | 0.013 | tr | 0.009 | tr |
| δ-elemene | 1338 | 1338 | 0.010 | 0.022 | 0.019 | 0.057 | 0.006 | 0.024 | 0.012 | ta | - | - | - | - |
| α-cubebene | 1351 | 1351 | 0.061 | 0.069 | 0.064 | 0.052 | 0.037 | 0.042 | 0.034 | ta | 0.005 | 0.017 | tr | tr |
| citronellyl acetate | 1353 | 1352 | 0.592 | 0.178 | 0.143 | 0.159 | 0.434 | 0.098 | 0.113 | 0.222 | 0.315 | 0.087 | 0.217 | 0.069 |
| neryl acetate | 1361 | 1365 | - | - | - | - | 0.008 | - | - | - | 0.011 | - | - | - |
| cyclosativene | 1373 | 1371 | 0.909 | 0.669 | ta | 0.420 | ta | 0.472 | ta | 0.356 | 0.072 | 0.061 | ta | ta |
| α-Ylangene | 1375 | 1373 | ta | ta | 0.511 | ta | 0.566 | ta | 0.272 | ta | ta | ta | 0.035 | ta |
| α-copaene | 1380 | 1376 | 1.568 | 1.340 | 1.278 | 0.879 | 0.716 | 0.671 | 0.537 | 0.417 | 0.079 | 0.078 | 0.037 | 0.042 |
| β-bourbonene | 1387 | 1388 | 0.069 | 0.193 | 0.212 | 0.125 | 0.127 | 0.117 | 0.148 | 0.081 | 0.006 | 0.014 | ta | ta |
| β-cubebene | 1393 | 1388 | 1.616 | 1.499 | 1.206 | 1.057 | 0.755 | 0.705 | 0.507 | 0.571 | - | - | - | - |
| 7-epi-sesquithujene | 1389 | 1391 | - | - | - | - | - | - | - | - | 0.036 | 0.019 | 0.051 | 0.022 |
| β-longipinene | 1410 | 1403 | 0.033 | 0.032 | ta | 0.060 | 0.041 | 0.034 | ta | ta | - | - | - | - |
| sesquithujene | 1404 | 1405 | - | - | - | - | - | - | - | - | 1.438 | 1.473 | 1.192 | 1.027 |
| (Z)-caryophyllene | 1410 | 1408 | - | - | - | - | - | - | - | - | 0.017 | 0.008 | 0.010 | ta |
| cis-α-bergamotene | 1418 | 1412 | - | - | - | - | - | - | - | - | 1.125 | 1.179 | 0.998 | 0.860 |
| α-santalene | 1425 | 1417 | - | - | - | - | - | - | - | - | 10.213 | 11.887 | 9.140 | 9.379 |
| (E)-caryophyllene | 1427 | 1419 | 7.481 | 10.997 | 11.679 | 7.119 | 18.026 | 23.999 | 22.384 | 21.548 | 11.917 | 14.800 | 12.330 | 11.453 |
| β-copaene | 1435 | 1432 | 0.111 | - | - | 0.051 | - | - | 0.076 | - | - | - | - | - |
| trans-α-bergamotene | 1435 | 1434 | - | - | - | - | - | - | - | - | 0.197 | 0.271 | 0.145 | 0.082 |
| α-guaiene | 1451 | 1439 | 0.024 | ta | 0.021 | ta | - | - | - | - | - | - | - | - |
| aromadendrene | 1457 | 1441 | 0.020 | 0.172 | 0.070 | ta | 0.110 | 0.197 | 0.021 | ta | - | - | - | - |
| (Z)-β-Farnesene | 1445 | 1442 | - | - | - | - | - | - | - | - | 0.212 | 0.207 | 0.171 | 0.105 |
| epi-β-santalene | 1451 | 1447 | - | - | - | - | - | - | - | - | ta | 0.026 | 0.020 | 0.026 |
| cis-muurola-3,5-diene | 1450 | 1449 | ta | ta | ta | ta | 0.010 | 0.033 | ta | ta | 0.035 | ta | ta | ta |
| trans-muurola-3,5-diene | 1457 | 1453 | ta | 0.030 | ta | ta | ta | 0.034 | ta | ta | ta | ta | ta | ta |
| sesquisabinene | 1454 | 1454 | - | - | - | - | - | - | - | - | ta | ta | 1.652 | ta |
| α-humulene | 1463 | 1454 | 1.470 | 2.383 | 2.442 | 1.378 | 2.649 | 3.821 | 3.934 | 3.284 | 2.892 | 3.456 | 2.880 | 1.574 |
| (E)-β-farnesene | 1457 | 1456 | - | - | - | - | - | - | - | - | 1.862 | 1.835 | 0.057 | 1.004 |
| allo-aromadendrene | 1469 | 1460 | 11.904 | 12.781 | 13.821 | 11.513 | 10.687 | 8.632 | 9.879 | 12.300 | - | - | - | - |
| dehydro-aromadendrene | 1471 | 1462 | 0.541 | 0.312 | 0.573 | ta | 0.218 | ta | 0.276 | ta | 0.154 | 0.232 | ta | ta |
| γ-muurolene | 1480 | 1479 | 0.840 | 0.589 | 0.532 | 0.290 | 0.256 | 0.251 | 0.168 | ta | 0.023 | 0.017 | ta | ta |
| γ-elemene | 1480 | 1488 | ta | ta | 0.036 | 0.204 | - | - | - | - | - | - | - | - |
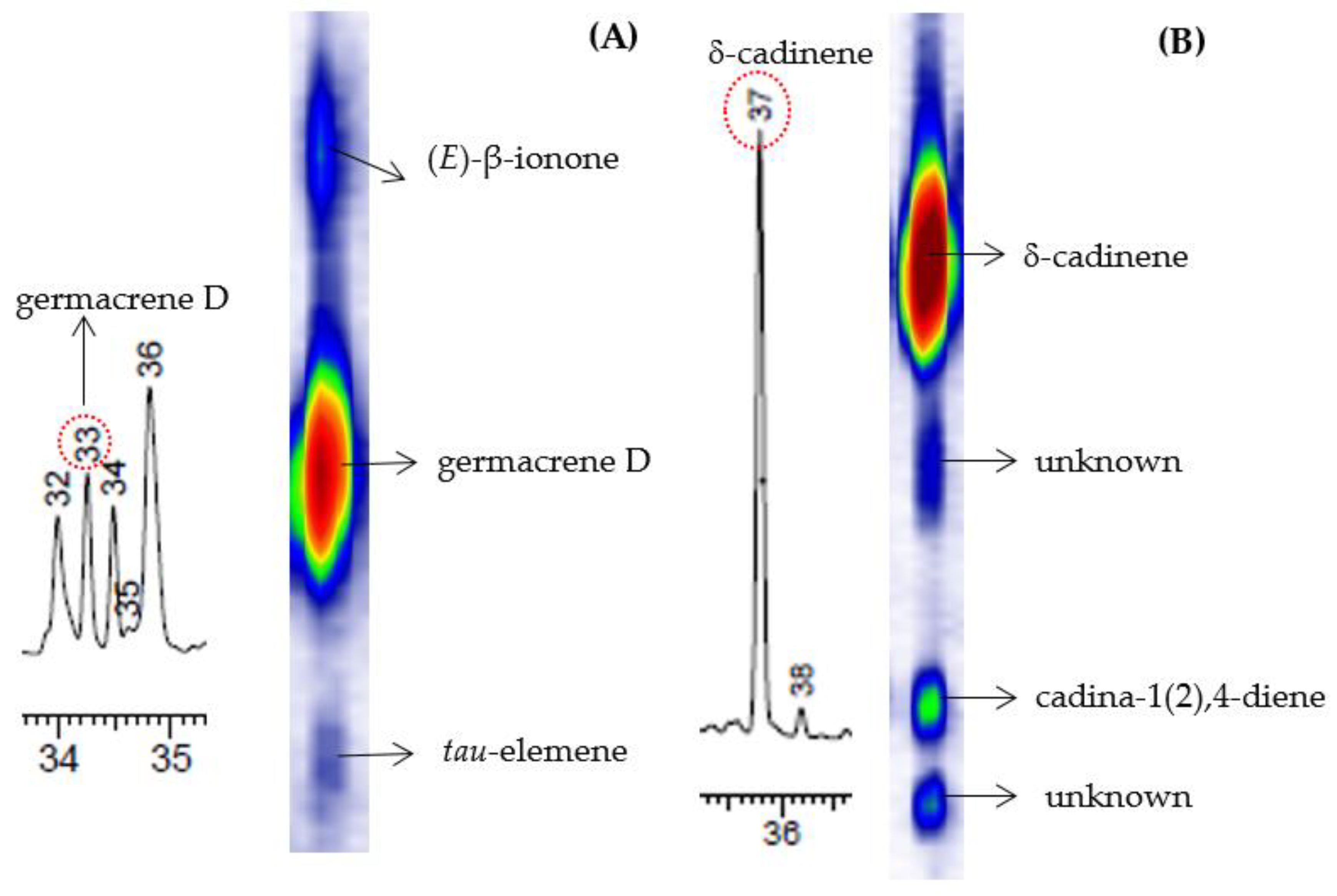
| germacrene D | 1488 | 1481 | 1.716 | 2.965 | 3.879 | 1.585 | 0.015 | 3.548 | 4.855 | 2.066 | 0.592 | 0.498 | 1.170 | 0.383 |
| tau-elemene | 1484 | 1484 | 0.383 | 0.539 | 0.216 | 0.139 | 0.175 | 0.163 | ta | ta | 0.264 | ta | ta | ta |
| (E)-β-ionone | 1488 | 1488 | 0.168 | ta | ta | ta | 0.050 | ta | ta | ta | 0.059 | 0.244 | ta | ta |
| cis-β-guaiene | 1492 | 1490 | ta | ta | 0.085 | 0.087 | ta | ta | 0.042 | ta | ta | ta | ta | ta |
| α-zingiberene | 1500 | 1493 | - | - | - | - | - | - | - | - | 0.020 | ta | 0.054 | 0.037 |
| trans-muurola-4(14),5-diene | 1488 | 1493 | ta | ta | ta | ta | 2.062 | ta | ta | ta | ta | ta | 0.021 | ta |
| γ-amorphene | 1496 | 1493 | ta | ta | ta | ta | 0.069 | ta | ta | 0.084 | ta | ta | ta | ta |
| epi-cubebol | 1493 | 1494 | ta | ta | 0.698 | 0.121 | ta | ta | 0.160 | 0.124 | - | - | - | - |
| biciclogermacrene | 1502 | 1500 | 1.283 | ta | 6.183 | 3.822 | 1.051 | ta | 2.166 | 1.690 | - | - | - | - |
| α-muurolene | 1504 | 1500 | 1.016 | ta | ta | ta | ta | ta | ta | 0.087 | 0.142 | ta | 0.057 | 0.037 |
| trans-β-guaiene | 1504 | 1502 | 0.845 | ta | 0.022 | ta | 0.563 | ta | 0.017 | ta | - | - | - | - |
| β-bisabolene | 1512 | 1505 | - | - | - | - | - | - | - | - | 2.498 | ta | 2.438 | 0.070 |
| guaia-1(10),11-diene | 1516 | 1509 | 0.178 | 0.169 | ta | ta | 0.072 | 0.079 | ta | ta | - | - | - | - |
| (Z)-γ-bisabolene | 1514 | 1514 | - | - | - | - | - | - | - | - | ta | ta | 0.274 | 0.034 |
| cadina-1(2)4-diene | 1524 | 1519 | 0.023 | ta | ta | ta | 0.021 | ta | ta | ta | - | - | - | - |
| cubebol | 1514 | 1521 | ta | ta | 1.186 | 1.107 | ta | ta | 0.552 | 0.317 | - | - | - | - |
| β-sesquiphellandrene | 1521 | 1525 | - | - | - | - | - | - | - | - | ta | ta | 0.287 | 0.208 |
| δ-cadinene | 1524 | 1530 | 7.217 | 5.800 | 3.640 | 2.924 | 2.998 | 1.969 | 0.830 | 0.838 | 0.636 | ta | ta | ta |
| (E)-γ-bisabolene | 1531 | 1531 | - | - | - | - | - | - | - | - | 1.002 | 1.390 | 0.886 | 0.487 |
| trans-cadina-1,4-diene | 1541 | 1534 | 0.045 | 0.067 | 0.029 | 0.037 | 0.026 | 0.032 | 0.016 | ta | - | - | - | - |
| (E)-α-bisabolene | 1545 | 1545 | - | - | - | - | - | - | - | - | 0.023 | ta | 0.020 | 0.038 |
| α-calacorene | 1551 | 1545 | 0.045 | 0.020 | 0.018 | ta | - | - | - | - | - | - | - | - |
| elemol | 1557 | 1549 | 0.035 | 0.047 | 0.017 | ta | - | - | - | - | - | - | - | - |
| cis-muurol-5-en-4-ol | 1561 | 1551 | - | - | - | - | 0.048 | 0.047 | - | - | - | - | - | - |
| cis-sesquisabinene hidrate | 1551 | 1559 | - | - | - | - | - | - | - | - | 0.110 | 0.053 | - | - |
| germacrene B | 1567 | 1561 | 0.277 | 0.185 | 0.063 | 0.083 | 0.133 | 0.086 | 0.036 | ta | - | - | - | - |
| trans-sesquisabinene hydrate | 1561 | 1565 | - | - | - | - | - | - | - | - | 0.486 | 0.345 | ta | ta |
| (E)-nerolidol | 1567 | 1565 | ta | ta | 0.037 | ta | ta | ta | ta | ta | 0.039 | ta | 0.039 | ta |
| β-calacorene | 1573 | 1565 | 0.011 | ta | 0.004 | ta | ta | ta | ta | ta | ta | ta | ta | ta |
| germacrene D-4-ol | 1586 | 1575 | ta | 0.059 | 0.057 | 0.041 | ta | 0.018 | 0.025 | ta | ta | ta | ta | ta |
| spathulenol | 1588 | 1578 | 3.738 | 1.003 | 1.422 | 0.527 | 0.985 | 0.171 | 0.555 | 0.185 | - | - | - | - |
| caryophyllene oxide | 1594 | 1583 | 0.726 | 0.424 | 0.438 | 0.198 | 2.524 | 0.502 | 0.636 | 0.274 | 2.509 | 1.031 | 0.901 | 0.644 |
| 7-epi-trans-sesquisabinene hydrate | 1598 | * | - | - | - | - | - | - | - | - | ta | ta | 0.629 | 0.327 |
| salvial-4(14)-en-1-one | 1604 | 1594 | 0.122 | ta | ta | ta | ta | 0.015 | 0.021 | ta | ta | ta | ta | ta |
| ledol | 1615 | 1602 | 0.716 | 0.437 | 0.410 | ta | 0.370 | 0.254 | 0.282 | ta | - | - | - | - |
| humulene epoxide II | 1621 | 1608 | 0.349 | 0.067 | 0.083 | ta | 0.302 | 0.043 | 0.083 | ta | 0.284 | 0.075 | 0.049 | ta |
| isoaromadendrene epoxide | 1612 | 1620 | ta | ta | ta | 0.237 | ta | ta | ta | 0.192 | - | - | - | - |
| muurola-4,10(14)-dien-1-b-ol | 1636 | 1631 | 0.426 | 0.123 | 0.173 | 0.097 | - | - | - | - | - | - | - | - |
| cis-Cadin-4-en-7-ol | 1636 | 1636 | ta | ta | ta | ta | 0.143 | 0.087 | 0.069 | 0.061 | ta | 0.017 | ta | ta |
| isospathulenol | 1643 | 1639 | 0.263 | 0.162 | 0.100 | ta | 0.165 | 0.027 | 0.073 | ta | - | - | - | - |
| caryophylla-4(12),8(13)-dien-5α-ol | 1649 | 1640 | ta | ta | ta | ta | 0.121 | ta | ta | ta | ta | 0.017 | ta | ta |
| tau-cadinol | 1640 | 1640 | ta | ta | 0.045 | ta | ta | ta | 0.056 | 0.035 | ta | ta | ta | ta |
| allo-aromadendrene epoxide | 1651 | 1641 | 0.054 | 0.108 | ta | ta | - | - | - | - | - | - | - | - |
| cubenol | 1655 | 1646 | ta | ta | 0.015 | ta | ta | 0.064 | ta | ta | - | - | - | - |
| α-muurolol | 1657 | 1646 | 1.067 | 0.500 | 0.333 | 0.200 | 0.366 | 0.134 | 0.160 | 0.093 | 0.070 | 0.034 | ta | ta |
| α-cadinol | 1666 | 1654 | 0.113 | 0.081 | 0.151 | ta | 0.051 | 0.040 | 0.102 | ta | - | - | - | - |
| cis-calamenen-10-ol | 1668 | 1661 | 0.300 | ta | ta | ta | ta | ta | ta | ta | ta | ta | ta | ta |
| intermedeol | 1670 | 1666 | 0.123 | 0.070 | ta | ta | ta | 0.018 | ta | ta | ta | ta | ta | ta |
| trans-10-hydroxycalamenene | 1679 | 1675 | 0.035 | ta | 0.025 | ta | - | - | - | - | - | - | - | - |
| ledene oxide (II) | 1687 | 1682 | 0.093 | 0.045 | 0.045 | ta | - | - | - | - | - | - | - | - |
| (2Z,6Z)-farnesal | 1684 | 1684 | ta | ta | 0.022 | ta | ta | ta | 0.008 | ta | ta | ta | ta | ta |
| Z-α-trans-bergamotol | 1668 | 1690 | - | - | - | - | - | - | - | - | 0.491 | 0.218 | ta | ta |
| (E)-α-bergamotenal | 1679 | ** | - | - | - | - | - | - | - | - | 4.098 | 3.311 | 4.171 | 2.212 |
| (E)-α-Santalal | 1689 | ** | - | - | - | - | - | - | - | - | 6.003 | 5.618 | 7.227 | 3.679 |
| eudesm-7(11)-en-4-ol | 1707 | 1700 | 0.113 | 0.081 | ta | ta | 0.064 | 0.026 | ta | ta | - | - | - | - |
| (Z)-β-santalol | 1716 | 1716 | - | - | - | - | - | - | - | - | 0.048 | 0.021 | 0.034 | ta |
| β-santalol | 1723 | 1727 | - | - | - | - | - | - | - | - | ta | ta | 0.014 | 0.036 |
| (E)-β-santalol | 1732 | 1739 | - | - | - | - | - | - | - | - | 0.030 | 0.172 | 0.168 | 0.126 |
| (Z)-α-santalol acetate | 1780 | 1778 | - | - | - | - | - | - | - | - | 0.296 | 0.139 | 0.132 | 0.092 |
| (Z)-α-trans-bergamotol acetate | 1795 | 1794 | - | - | - | - | - | - | - | - | 0.207 | ta | ta | ta |
| (Z)-β-santalol acetate | 1821 | 1819 | - | - | - | - | - | - | - | - | 0.012 | 0.017 | ta | ta |
| hexahydrofarnesyl acetone | 1845 | 1844 | 0.075 | ta | ta | ta | 0.009 | ta | ta | ta | 0.014 | ta | ta | ta |
| isopimara-9(11),15-diene | 1946 | 1905 | 0.028 | 0.014 | ta | ta | 0.019 | 0.014 | ta | ta | ta | 0.008 | ta | ta |
| isopimara-8,15-diene | 1956 | 1947 | 0.032 | 0.017 | ta | ta | 0.022 | 0.014 | ta | ta | 0.020 | 0.007 | ta | ta |
| sandaracopimara-8(14),15-diene | 1963 | 1969 | 0.075 | ta | ta | ta | 0.014 | ta | ta | ta | 0.021 | ta | ta | ta |
| Monoterpene hydrocarbons | 44.129 | 46.541 | 45.223 | 63.426 | 47.939 | 44.206 | 48.712 | 52.070 | 44.717 | 44.637 | 49.040 | 61.576 | ||
| Oxygenated monoterpenes | 2.561 | 1.681 | 1.382 | 1.611 | 2.918 | 1.315 | 1.141 | 1.967 | 1.319 | 0.937 | 0.964 | 0.600 | ||
| Sesquiterpene hydrocarbons | 39.683 | 40.834 | 46.600 | 31.881 | 41.388 | 44.908 | 46.209 | 43.322 | 35.460 | 37.470 | 33.926 | 26.870 | ||
| Oxygenated sesquiterpenes | 8.284 | 3.208 | 5.262 | 2.527 | 5.137 | 1.445 | 2.781 | 1.282 | 14.680 | 11.068 | 13.364 | 7.117 | ||
| Others | 2.271 | 0.676 | 0.837 | 0.532 | 1.996 | 0.847 | 1.124 | 0.855 | 1.281 | 0.916 | 0.993 | 0.607 | ||
| Total identified | 96.93 | 92.94 | 99.30 | 99.98 | 99.38 | 92.72 | 99.97 | 99.50 | 97.46 | 95.03 | 98.29 | 96.77 | ||
| Genotype | α-Humulene | |||
|---|---|---|---|---|
| Winter AB | Spring A | Summer A | Autumn B | |
| VC1b | 1.47 ± 0.45 | 2.38 ± 0.39 | 2.44 ± 0.19 | 1.38 ± 0.49 |
| VC2a | 2.65 ± 0.06 | 3.82 ± 0.31 | 3.93 ± 0.10 | 3.28 ± 0.21 |
| VC3ab | 2.89 ± 0.14 | 3.46 ± 0.09 | 2.88 ± 0.16 | 1.57 ± 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facanali, R.; Marques, M.O.M.; Hantao, L.W. Metabolic Profiling of Varronia curassavica Jacq. Terpenoids by Flow Modulated Two-Dimensional Gas Chromatography Coupled to Mass Spectrometry. Separations 2020, 7, 18. https://doi.org/10.3390/separations7010018
Facanali R, Marques MOM, Hantao LW. Metabolic Profiling of Varronia curassavica Jacq. Terpenoids by Flow Modulated Two-Dimensional Gas Chromatography Coupled to Mass Spectrometry. Separations. 2020; 7(1):18. https://doi.org/10.3390/separations7010018
Chicago/Turabian StyleFacanali, Roselaine, Marcia Ortiz Mayo Marques, and Leandro Wang Hantao. 2020. "Metabolic Profiling of Varronia curassavica Jacq. Terpenoids by Flow Modulated Two-Dimensional Gas Chromatography Coupled to Mass Spectrometry" Separations 7, no. 1: 18. https://doi.org/10.3390/separations7010018
APA StyleFacanali, R., Marques, M. O. M., & Hantao, L. W. (2020). Metabolic Profiling of Varronia curassavica Jacq. Terpenoids by Flow Modulated Two-Dimensional Gas Chromatography Coupled to Mass Spectrometry. Separations, 7(1), 18. https://doi.org/10.3390/separations7010018





