Purification of Curcumin from Ternary Extract-Similar Mixtures of Curcuminoids in a Single Crystallization Step
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.3. Solubility and Metastable Zone Width Measurements
3. Results and Discussion
3.1. Selection of Solvents for Crystallization
3.1.1. CUR Solubility in Acetone, Acetonitrile, Methanol, Ethanol and 2-propanol
3.1.2. Effect of DMC and BDMC on CUR Solubility in the Selected Process Solvents
3.2. Design of the Seeded Cooling Crystallization for Separation of CUR
3.3. Implementation of the Purification Process
3.4. Improvement of the Total Yield by Means of Anti-Solvent Addition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdul, R.; Hatifah, P.L.; Ratna, W.; Muhammad, K. Use of Thin Layer Chromatography and FTIR Spectroscopy Along with Multivariate Calibration for Analysis of Individual Curcuminoid in Turmeric (Curcuma longa Linn) Powder. Int. J. Pharm. Clin. Res. 2016, 8, 419–424. [Google Scholar]
- Bagchi, A. Extraction of Curcumin. IOSR J. Environ. Sci. Toxicol. Food Technol. 2012, 1, 1–16. [Google Scholar] [CrossRef]
- Tanaka, K.; Kuba, Y.; Sasaki, T.; Hiwatashi, F.; Komatsu, K. Quantitation of curcuminoids in curcuma rhizome by near-infrared spectroscopic analysis. J. Agric. Food Chem. 2008, 56, 8787–8792. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [PubMed]
- Esatbeyoglu, T.; Hübbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.B.; Taralkar, S.V.; Sakpal, V.S.; Shewale, S.P.; Sakpal, R.S. Extraction, isolation, and evaluation of anti-inflammatory activity of curcuminoids from Curcuma longa. Int. J. Chem. Sci. Appl. 2011, 2, 172–174. [Google Scholar]
- Esatbeyouglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, D.; Shirai, N.; Amatsubo, T.; Taguchi, H.; Hirao, K.; Urushitani, M.; Morikawa, S.; Inubushi, T.; Kato, M.; Kato, F.; et al. Relationship between the tautomeric structures of curcumin derivatives and their Abeta-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 2010, 31, 4179–4185. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. J. Pharm. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Salem, M.; Rohani, S.; Gillies, E.R. Curcumin, a promising anti-cancer therapeutic: A review of its chemical properties, bioactivity and approaches to cancer cell delivery. Rsc Adv. 2014, 4, 10815. [Google Scholar] [CrossRef]
- Chin, D.; Huebbe, P.; Pallauf, K.; Rimbach, G. Neuroprotective Properties of Curcumin in Alzheimer’s Disease–Merits and Limitations. Curr. Med. Chem. 2013, 20, 3955–3985. [Google Scholar] [CrossRef]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; Abdel-Kader, R.; Muller, W.E.; Rimbach, G.; Frank, J. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.J.; Mukhtar, H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007, 255, 170–181. [Google Scholar] [CrossRef]
- Simon, A. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationship. Cancer Lett. 1998, 129, 111–116. [Google Scholar] [CrossRef]
- Ruby, A.J.; Kuttan, G.; Dinesch Badu, K.; Rajasekharan, K.N.; Kuttan, R. Anti-tumor and antioxidant activity of natural corcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Luis, P.B.; Boeglin, W.E.; Schneider, C. Thiol Reactivity of Curcumin and Its Oxidation Products. Chem. Res. Toxicol. 2018, 31, 269–276. [Google Scholar] [CrossRef]
- Modasiya, M.K.; Patel, V.M. Studies on solubility of curcumin. Int. J. Pharm. Life Sci. 2012, 3, 1490–1497. [Google Scholar]
- Carvalho, D.M.; Takeuchi, K.P.; Geraldine, R.M.; Moura, C.J.; Torres, M.C.L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin - synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandakumar, D.N.; Nagaraj, V.A.; Vathsala, P.G.; Rangarajan, P.; Padmanaban, G. Curcumin-artemisinin combination therapy for malaria. Antimicrob. Agents Chemother. 2006, 50, 1859–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaitanya Mannava, M.K.; Suresh, K.; Bommaka, M.K.; Konga, D.B.; Nangia, A. Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study. Pharmaceutics 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabon, H.J.J. A synthesis of curcumin and related compounds. Recl. Trav. Chim. Pays-Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Kim, E.J.; Cha, M.H.; Kim, B.-G.; Ahn, J.-H. Production of Curcuminoids in Engineered Escherichia coli. J. Microbiol. Biotechnol. 2017, 27, 975–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grynkiewicz, G.; Ślifirski, P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim. Pol. 2012, 59, 201–212. [Google Scholar] [CrossRef]
- Pothitirat, W.; Gritsanapan, W. Quantitative analysis of curcumin, demethoxycurcumin and bisdemethoxycurcumin in the crude curcuminoid extract from Curcuma Longa L. in Thailand by TLC-Densitometry. Mahidol Univ. J. Pharm. Sci. 2005, 32, 23–30. [Google Scholar]
- Aggarwal, B.B.; Bhatt, I.D.; Ichikawa, H.; Ahn, K.S.; Sethi, G.; Sandur, S.K.; Sundaram, C.; Seeram, N.; Shishodia, S. Curcumin-Biological and medicinal properties. In Turmeric the Genus Curcuma; Ravindran, P.N., Babu, K.N., Sivaraman, K., Eds.; CRC Press: Abingdon, UK, 2007; pp. 297–368. [Google Scholar]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.M.; Mitchell, M.S.; Mohan, R.S. Isolation of curcumin from turmeric. J. Chem. Educ. 2000, 77, 359–360. [Google Scholar] [CrossRef]
- Revathy, S.; Elumalai, S.; Benny, M.; Antony, B. Isolation, Purification and Identification of Curcuminoids from Turmeric (Curcuma longa L.) by Column Chromatography. J. Exp. Sci. 2011, 2, 21–25. [Google Scholar]
- Péret-Almeida, L.; Cherubino, A.P.F.; Alves, R.J.; Dufossé, L.; Glória, M.B.A. Separation and determination of the physico-chemical characteristics of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Res. Int. 2005, 38, 1039–1044. [Google Scholar] [CrossRef]
- Liu, J.; Svard, M.; Hippen, P.; Rasmuson, A.C. Solubility and crystal nucleation in organic solvents of two polymorphs of curcumin. J. Pharm. Sci. 2015, 104, 2183–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukrainczyk, M.; Hodnett, B.K.; Rasmuson, A.C. Process Parameters in the Purification of Curcumin by Cooling Crystallization. Org. Process Res. Dev. 2016, 20, 1593–1602. [Google Scholar] [CrossRef]
- Horvath, Z.; Horosanskaia, E.; Lee, J.W.; Lorenz, H.; Gilmore, K.; Seeberger, P.H.; Seidel-Morgenstern, A. Recovery of Artemisinin from a Complex Reaction Mixture Using Continuous Chromatography and Crystallization. Org. Process Res. Dev. 2015, 19, 624–634. [Google Scholar] [CrossRef]
- Heffernan, C.; Ukrainczyk, M.; Gamidi, R.K.; Hodnett, B.K.; Rasmuson, A.C. Extraction and Purification of Curcuminoids from Crude Curcumin by a Combination of Crystallization and Chromatography. Org. Process Res. Dev. 2017, 21, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Horosanskaia, E.; Triemer, S.; Seidel-Morgenstern, A.; Lorenz, H. Purification of Artemisinin from the Product Solution of a Semisynthetic Reaction within a Single Crystallization Step. Org. Process Res. Dev. 2019, 23, 2074–2079. [Google Scholar] [CrossRef] [Green Version]
- Horosanskaia, E.; Nguyen, T.M.; Vu, T.D.; Seidel-Morgenstern, A.; Lorenz, H. Crystallization-Based Isolation of Pure Rutin from Herbal Extract of Sophora japonica L. Org. Process Res. Dev. 2017, 21, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Wiekhusen, D. Development of batch crystallizations. In Crystallization—Basic Concepts and Industrial Applications; Beckmann, W., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 187–202. [Google Scholar]
- Jadhav, B.K.; Mahadik, K.R.; Paradkar, A.R. Development and validation of improved reversed phase HPLC method for simultaneous determination of curcumin, demethoxycurcumin and bis-demethoxycurcumin. Chromatographia 2007, 65, 483–488. [Google Scholar] [CrossRef]
- Lorenz, H. Solubility and solution equilibria in crystallization. In Crystallization – Basic Concepts and Industrial Applications; Beckmann, W., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 35–74. [Google Scholar]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013–5015. [Google Scholar] [CrossRef]
- Gately, S.; Triezenberg, S.J. Solid Forms of Curcumin. U.S. Patent WO2012138907A2, 20 September 2012. [Google Scholar]
- Mishra, M.K.; Sanphui, P.; Ramamurty, U.; Desiraju, G.R. Solubility-Hardness Correlation in Molecular Crystals: Curcumin and Sulfathiazole Polymorphs. Cryst. Growth Des. 2014, 14, 3054–3061. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Solid-State Phase Transformations and Storage Stability of Curcumin Polymorphs. Cryst. Growth Des. 2015, 15, 1757–1770. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J.; Mostad, A. Structural Studies of Curcuminoids. I. The Crystal Structure of Curcumin. Acta Chem. Scand. 1982, 36, 475–479. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J.; Mostad, A.; Pedersen, U.; Rasmussen, P.B.; Lawesson, S.-O. Structural Studies of Curcuminoids. II. Crystal Structure of 1,7-Bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione-Methanol Complex. Acta Chem. Scand. 1983, 37, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, J.; Mostad, A.; Tonnesen, H.H. Structural studies of curcuminoids. VI. Crystal structure of 1,7_bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione hydrate. Acta Chem. Scand. 1988, 42, 23–27. [Google Scholar] [CrossRef]
- Yuan, L.; Horosanskaia, E.; Engelhardt, F.; Edelmann, F.T.; Couvrat, N.; Sanselme, M.; Cartigny, Y.; Coquerel, G.; Seidel-Morgenstern, A.; Lorenz, H. Solvate formation of bis(demethoxy)curcumin: Crystal structure analyses and stability investigations. Cryst. Growth Des. 2019, 19, 854–867. [Google Scholar] [CrossRef]
- Yuan, L.; Lorenz, H. Solvate formation of bis(demethoxy)curcumin: Screening and characterization. Crystals 2018, 8, 407. [Google Scholar] [CrossRef] [Green Version]


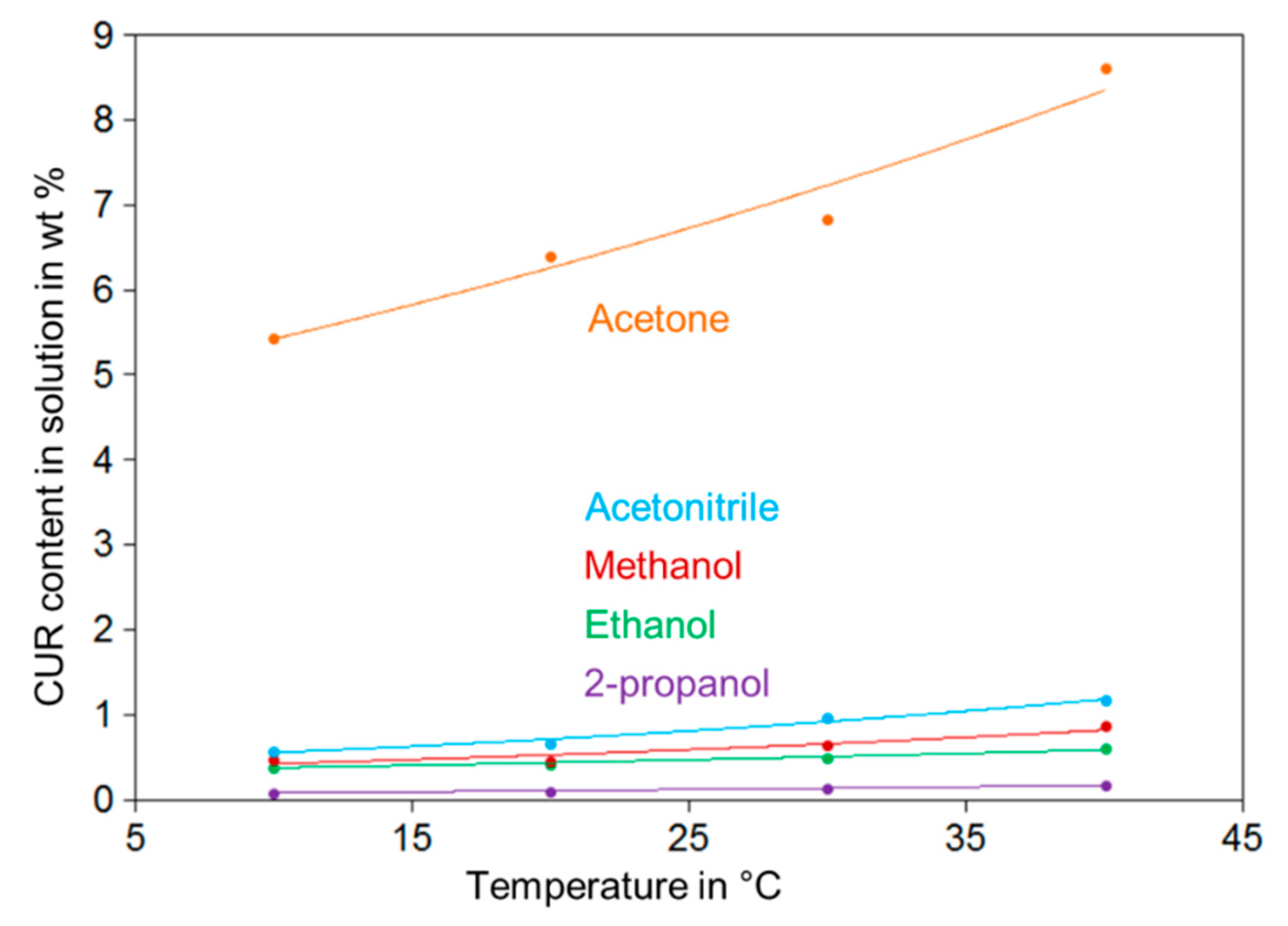

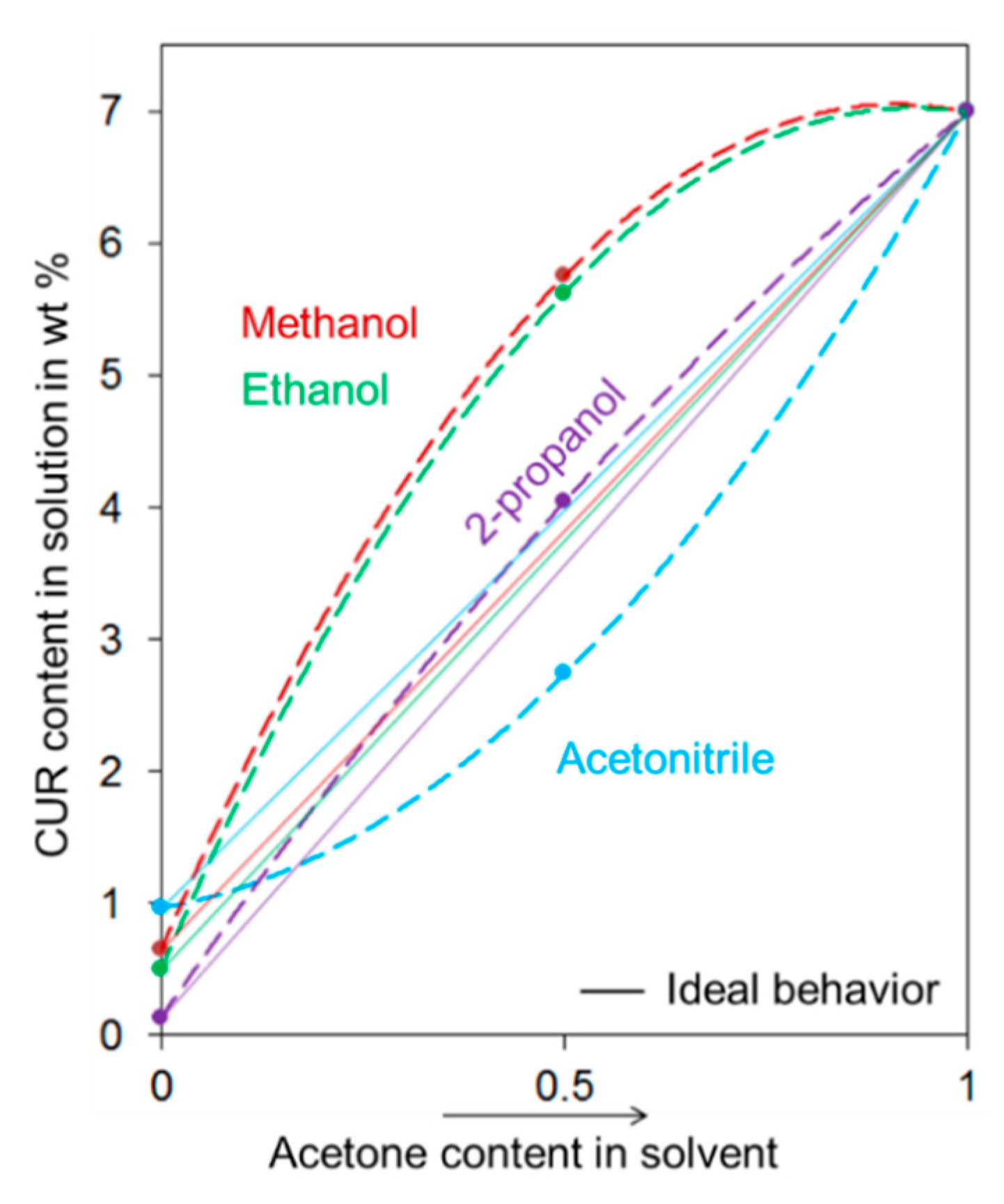



| Reference | Raw Mixture Content of | Solvent | Crystallization Method | No. of Crystallization Steps | Product Content of | Total Yield % | ||
|---|---|---|---|---|---|---|---|---|
| CUR % | DMC % | CUR % | DMC % | |||||
| 1 | / 1 | / 1 | Methanol | Anti-solvent addition, water | 3 | 92.2 | 7.8 | 40 |
| 2 | 82.0 | 16.0 | Ethanol | Cooling, 70 °C to 5 °C | 2 | 96.0 | 4.0 | / 1 |
| 3 | 78.6 | 17.7 | 2-Propanol | Cooling, 60 °C to 20 °C | 3 | >98/ 99.12 | <2/ 0.92 | 50 2 |
| Crude Solid No. | CUR Content wt% | DMC Content wt% | BDMC Content wt% |
|---|---|---|---|
| 1 | 67.2 | 25.5 | 7.3 |
| 2 | 70.8 | 23.5 | 5.7 |
| 3 | 75.0 | 19.2 | 5.8 |
| 4 | 80.7 | 16.5 | 2.8 |
| Process | Process Solvent | Crude Solid |
|---|---|---|
| No. | No. | |
| 1 | Acetone | 3 |
| 2 | 50/50 (wt/wt) acetone/2-propanol | 2 |
| 3 | 50/50 (wt/wt) acetone/acetonitrile | 1 |
| 4 | Acetonitrile | 1 |
| Process | Process Solvent | Tstart | Tsat | Tseeds | Tend | Cooling Rate |
|---|---|---|---|---|---|---|
| No. | °C | °C | °C | °C | K/h | |
| 1 | Acetone | 45 | 37 | 30 | 0 | 10 |
| 2 | 50/50 acetone/2-propanol | 60 | 51 | 46 | 0 | 10 |
| 3 | 50/50 acetone/acetonitrile | 60 | 57 | 51 | 0 | 10 |
| 4 | Acetonitrile | 60 | 56 | 51 | 0 | 10 |
| Process | m (CURD) | m (Solvent) | cstart (CUR) | cend = csat (CUR) | ΔcTD (CUR) | mmax (CUR) |
|---|---|---|---|---|---|---|
| No. | g | g | wt% | wt% | wt% | g |
| 1 | 19.0 | 150 | 8.4 | 5.1 | 3.3 | 5.7 |
| 2 | 14.0 | 150 | 6.4 | 3.2 | 3.2 | 5.1 |
| 3 | 11.5 | 140 | 5.1 | 1.5 | 3.6 | 5.5 |
| 4 | 5.2 | 150 | 2.2 | 0.5 | 1.7 | 2.7 |
| Ternary Mixture of Curcuminoids | Crystalline Product | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Process No. | Process Solvent | mstart (CUR) | CUR Content | DMC Content | BDMC Content | mmax (CUR) | m (Product) | CUR Content | DMC Content | BDMC Content | ηTD (CUR) | η (CUR) |
| g | % | % | % | g | g | % | % | % | % | % | ||
| 1 | Acetone | 14.3 | 75.0 | 19.2 | 5.8 | 5.7 | 4.6 | 95.7 | 4.3 | 0 | 77 | 31 |
| 2 | 50/50 acetone/ 2-propanol | 9.9 | 70.8 | 23.5 | 5.7 | 5.1 | 1.3 | 99.4 | 0.6 | 0 | 25 | 13 |
| 3 | 50/50 acetone/ acetonitrile | 7.7 | 67.2 | 25.5 | 7.3 | 5.5 | 5.3 | 90.1 | 9.9 | 0 | 87 | 62 |
| 4 | Acetonitrile | 3.5 | 67.2 | 25.5 | 7.3 | 2.7 | 2.1 | 92.3 | 7.7 | 0 | 72 | 55 |
| Ternary Mixture of Curcuminoids | Crystalline Product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Process No. | Solvent | mstart (CUR) | CUR Content | DMC Content | BDMC Content | m (Product) | CUR Content | DMC Content | BDMC Content | η (CUR) |
| g | % | % | % | g | % | % | % | % | ||
| 1-1 | 25/75 acetone/water | 2.9 | 67.2 | 25.5 | 7.3 | 2.7 | 85.5 | 13.4 | 1.1 | 79 |
| 1-2 | 25/75 acetone/2-propanol | 2.9 | 67.2 | 25.5 | 7.3 | 1.1 | 96.2 | 3.7 | 0.1 | 36 |
| 1-3 | 25/75 acetone/acetonitrile | 2.9 | 67.2 | 25.5 | 7.3 | 2.3 | 88.3 | 10.8 | 0.9 | 70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horosanskaia, E.; Yuan, L.; Seidel-Morgenstern, A.; Lorenz, H. Purification of Curcumin from Ternary Extract-Similar Mixtures of Curcuminoids in a Single Crystallization Step. Crystals 2020, 10, 206. https://doi.org/10.3390/cryst10030206
Horosanskaia E, Yuan L, Seidel-Morgenstern A, Lorenz H. Purification of Curcumin from Ternary Extract-Similar Mixtures of Curcuminoids in a Single Crystallization Step. Crystals. 2020; 10(3):206. https://doi.org/10.3390/cryst10030206
Chicago/Turabian StyleHorosanskaia, Elena, Lina Yuan, Andreas Seidel-Morgenstern, and Heike Lorenz. 2020. "Purification of Curcumin from Ternary Extract-Similar Mixtures of Curcuminoids in a Single Crystallization Step" Crystals 10, no. 3: 206. https://doi.org/10.3390/cryst10030206
APA StyleHorosanskaia, E., Yuan, L., Seidel-Morgenstern, A., & Lorenz, H. (2020). Purification of Curcumin from Ternary Extract-Similar Mixtures of Curcuminoids in a Single Crystallization Step. Crystals, 10(3), 206. https://doi.org/10.3390/cryst10030206






