Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation
Abstract
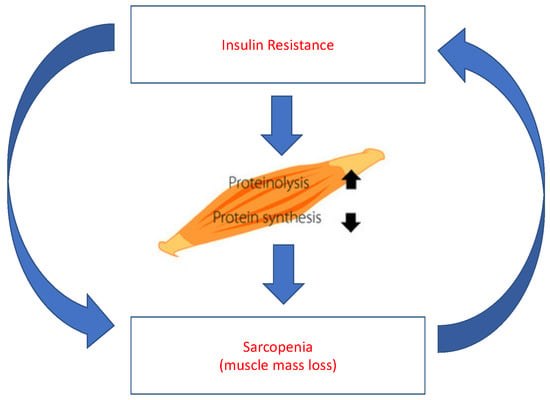
:1. Introduction
2. General Considerations for HMB, Gln, Arg, Leu
2.1. Supplementation with HMB
2.2. Supplementation with Leu
2.3. Supplementation with Arg
2.4. Supplementation with Gln
2.5. Supplementation with HMB/Arg/Gln
2.6. Supplementation with Leu/Arg/Gln
3. Discussion
3.1. HMB
3.2. Leu
3.3. HMB and Leu
3.4. Arg—Arg and Gln
3.5. HMB, Gln and Arg
4. Concurrent Therapies for Type 2 Diabetes Mellitus and Sarcopenia
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tayfur, M. Yaşlı Diyabetik Erkeklerde Sarkopeni; Uzmanlık tezi, İstanbul Üniversitesi Tıp Fakültesi İç Hastalıkları Anabilim Dalı: İstanbul, Türkiye, 2010. [Google Scholar]
- Jong, N. Nutrition and Senescence: Healthy Aging for All in the New Millennium. Nutrition 2000, 16, 537–541. [Google Scholar] [CrossRef]
- Diet, Nutrition and The Prevention of Chronic Diseases; WHO Technical Report Series DSÖ Raporu; Benefits of Physical Activity; Report of a Joint WHO/FAO Expertconsultation: Geneva, Switzerland, 2002; p. 73.
- World Health Organization. Healthy Ageing. In Practical Pointers on Keeping Well; WHO Western Pacific Regional Office: Geneva, Switzerland, 2005. [Google Scholar]
- van Abellan Kan, G. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 2009, 13, 708–712. [Google Scholar] [CrossRef]
- Fleg, J.L.; Lakatta, E.G. Role of muscle loss in the age-associated reduction in VO2max. J. Appl. Physiol. 1988, 65, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Lindle, R.S.; Metter, E.J.; Lynch, N.A.; Fleg, J.L.; Fozard, J.L.; Tobin, J.; Roy, T.A.; Hurley, B.F. Age and gender comparison of muscle strength in 654 women and men age 20–93 yr. J. Appl. Physiol. 1997, 83, 1581–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical Definition of Sarcopenia. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Marcell, T.J. Sarcopenia: Causes, consequences, and preventions. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M911–M916. [Google Scholar] [CrossRef]
- Öztürk, Z.A. Tip II Diyabetes Mellituslu Sarkopenik Obez Kişilerde Kan Şekeri Regülasyonunun Sarkopeni Parametreleri Üzerine Etkisi; yan dal uzmanlık tezi, Gaziantep Üniversitesi Tıp Fakültesi İç Hastalıkları Anabiliim Dalı Geriatri Bilim Dalı: Gaziantep, Turkey, 2014. [Google Scholar]
- Boirie, Y. Physiopathological mechanism of sarcopenia. J. Nutr. Heal. Aging 2009, 13, 717–723. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Devjit, T. Skeletal Muscle Insulin Resistance ıs the Primary Defect in Type 2 Diabetes, Diabetes Care; American Diabetes Association: Arlington, VA, USA, 2009. [Google Scholar]
- Hickson, M. Nutritional interventions in sarcopenia: A critical review. Proc. Nutr. Soc. 2015, 74, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Junior, H.J.; Bossola, M.; Urbani, A.; et al. A Distinct Pattern of Circulating Amino Acids Characterizes Older Persons with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2018, 10, 1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candow, D.G.; Forbes, S.C.; Little, J.P.; Cornish, S.M.; Pinkoski, C.; Chilibeck, P.D. Effect of nutritional interventions and resistance exercise on aging muscle mass and strength. Biogerontology 2012, 13, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Wilson, J.M.; Manninen, A.H. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr. Metab. 2008, 5, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noe, J.E. L-Glutamine use in the treatment and prevention of mucositis and cachexia: A naturopathic perspective. Integr. Cancer Ther. 2009, 8, 409–415. [Google Scholar] [CrossRef]
- Ogawa, M.; Yoshida, N.; Satomi-Kobayashi, S.; Tsuboi, Y.; Komaki, K.; Wakida, K.; Gotake, Y.; Inoue, T.; Tanaka, H.; Yamashita, T.; et al. Efficacy of preoperative amino acid supplements on postoperative physical function and complications in open heart surgery patients: A study protocol for a randomized controlled trial. J. Cardiol. 2019, 74, 360–365. [Google Scholar] [CrossRef]
- Clark, R.H.; Feleke, G.; Din, M.; Yasmin, T.; Singh, G.; Khan, F.A.; Rathmacher, J.A. Nutritional treatment for acquired immunodeficiency virus-associated wasting using β-hyroxy-β-methylbutyrate, glutamine, and arginin: A randomized, double-blind, placebo-controlled study. J. Parenter. Enter. Nutr. 2000, 24, 133–139. [Google Scholar] [CrossRef]
- Hsieh, L.C.; Chow, C.J.; Chang, W.C.; Liu, T.H.; Chang, C.K. Effect of beta hydroxybeta methyl butyrate on protein metabolism in bed ridden elderly receiving tube feeding. Asia Pac. J. Clin. Nutr. 2010, 19, 200–208. [Google Scholar]
- May, P.E.; Barber, A.; D’Olimpio, J.T. Reversal of cancer-related wasting using oral supplementation with a combination of β-hyroxy-β-methylbutyrate, arginine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef]
- Mero, A. Leucine Supplementation and Intensive Training. Sports Med. 1999, 27, 347–358. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W H Freeman: New York, NY, USA, 2002; p. 16. [Google Scholar]
- Fukagawa, N.K. Protein and amino acid supplementation in older humans. Amino Acids 2013, 44, 1493–1509. [Google Scholar] [CrossRef]
- Wittmann, F.; Prix, N.; Mayr, S.; Angele, P.; Wichmann, M.W.; van den Engel, N.K.; Hernandez-Richter, T.; Chaudry, I.H.; Jauch, K.W.; Angele, M.K.; et al. l-Arginine Improves Wound Healing after Trauma-Hemorrhage by Increasing Collagen Synthesis. J. Trauma Acute Care Surg. 2005, 59, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.Z.; Abumrad, N.; Barbul, A. Effect of a specialized amino acid mixture on human collagen deposition. Ann. Surg. 2002, 236, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Walrand, S.; Guillet, C.; Salles, J.; Cano, N.; Boirie, Y. Physiopathological mechanism of sarcopenia. Clin. Geriatr. Med. 2011, 27, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.C.; Chien, S.L.; Huang, S.; Tseng, H.F.; Chang, C.K. Anti-inflammatory and anticatabolic effects of short-term β-hyroxy- β-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac. J. Clin. Nutr. 2006, 15, 544–550. [Google Scholar] [PubMed]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Heal. Aging 2018, 23, 145–150. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of Leu is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Metab. 2006, 291, E381–E387. [Google Scholar]
- Koopman, R.; Verdijk, L.; Manders, R.J.F.; Gijsen, A.P.; Gorselink, M.; Pijpers, E.; Wagenmakers, A.J.; van Loon, L.J.C. Co-ingestion of protein and Leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am. J. Clin. Nutr. 2006, 84, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Dardevet, D.; Sornet, C.; Balage, M.; Grizard, J. Stimulation of in vitro rat muscle protein synthesis by Leucine decreases with age. J. Nutr. 2000, 130, 2630–2635. [Google Scholar] [CrossRef]
- Rieu, I.; Sornet, C.; Bayle, G.; Prugnaud, J.; Pouyet, C.; Balage, M.; Papet, I.; Grizard, J.; Dardevet, D. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J. Nutr. 2003, 133, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef]
- Debras, E.; Prod’homme, M.; Rieu, I.; Balage, M.; Dardevet, D.; Grizard, J. Postprandial Leucine deficiency failed to alter muscle protein synthesis in growing and adult rats. Nutrition 2007, 23, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Breen, L.; Burd, N.; Hector, A.; Churchward-Venne, T.; Josse, A.; Tarnopolsky, M.A.; Phillips, S. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Nutrition 2007, 23, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, K.E.; Snijders, T.; Zulyniak, M.; Kumbhare, D.; Parise, G.; Chabowski, A.; Phillips, S.M. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: A randomized controlled trial. PLOS ONE 2017, 12, e0181387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am. J. Cardiol. 2008, 101, S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Paddon-Jones, D.; Hays, N.P.; Kortebein, P.; Ronsen, O.; Williams, R.H.; McComb, A.; Symons, T.B.; Wolfe, R.R.; Evans, W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin. Nutr. 2010, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Opizzi, A.; Antoniello, N.; Boschi, F.; Iadarola, P.; Pasini, E.; Aquilani, R.; Dioguardi, F.S. Effect of amino acid supplementation on quality of life, amino acid profile and strength in institutionalized elderly patients. Clin. Nutr. 2011, 30, 571–577. [Google Scholar] [CrossRef]
- Xu, Z.E.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. The effectiveness of Leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: A systematic review and meta-analysis. Br. J. Nutr. 2014, 113, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.H.; Saddler, N.I.; Devries, M.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: A parallel-group crossover study. Am. J. Clin. Nutr. 2016, 104, 1594–1606. [Google Scholar] [CrossRef]
- Backx, E.M.P.; Horstman, A.M.H.; Marzuca-Nassr, G.N.; van Kranenburg, J.; Smeets, J.S.; Fuchs, C.J.; Janssen, A.A.W.; de Groot, L.C.P.G.M.; Snijders, T.; Verdijk, L.B.; et al. Leucine Supplementation Does Not Attenuate Skeletal Muscle Loss during Leg Immobilization in Healthy, Young Men. Nutrients 2018, 10, 635. [Google Scholar] [CrossRef] [Green Version]
- Trappe, S.; Creer, A.; Slivka, D.; Minchev, K.; Trappe, T. Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. J. Appl. Physiol. 2007, 103, 1242–1250. [Google Scholar] [CrossRef] [Green Version]
- Trappe, T.A.; Burd, N.A.; Louis, E.S.; Lee, G.A.; Trappe, S.W. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol. 2007, 191, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.; Creer, A.; Minchev, K.; Slivka, D.; Louis, E.; Luden, N.; Trappe, T. Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. J. Physiol. Integr. Comp. Physiol. 2008, 294, R939–R947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komar, B.; Schwingshackl, L.; Hoffmann, G. Effects of Leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: A systematic review and meta-analysis. J. Nutr. Heal. Aging 2014, 19, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cholewa, J.M.; Dardevet, D.; Lima-Soares, F.; de Araújo Pessôa, K.; Oliveira, P.H.; Dos Santos Pinho, J.R.; Nicastro, H.; Xia, Z.; Cabido, C.E.; Zanchi, N.E. Dietary proteins and amino acids in the control of the muscle mass during immobilization and aging: Role of the MPS response. Amino Acids 2017, 49, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, E.; Bui, Q.U.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalacker-Mercer, A.E.; Drummond, M.J. The importance of dietary protein for muscle health in inactive, hospitalized older adults. Ann. N. Y. Acad. Sci. 2014, 1328, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, M.K.; Moraes, M.R.; Rosa, T.S.; Passos, C.S.; Neves, R.V.P.; Haro, A.S.; Cenedeze, M.A.; Arias, S.C.A.; Fujihara, C.K.; Teixeira, S.A.; et al. l-Arginine Supplementation Blunts Resistance Exercise Improvement in Rats with Chronic Kidney Disease. Life Sci. 2019, 232, 116604. [Google Scholar] [CrossRef]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of β-hydroxy-β-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Haba, Y.; Fujimura, T.; Oyama, K.; Kinoshita, J.; Miyashita, T.; Fushida, S.; Harada, S.; Ohta, T. Effect of Oral Branched-Chain Amino Acids and Glutamine Supplementation on Skeletal Muscle Atrophy After Total Gastrectomy in Rat Model. J. Surg. Res. 2019, 243, 281–288. [Google Scholar] [CrossRef]
- Mignon, M.; Lêvêque, L.; Bonnel, E.; Meynial-Denis, D. Does Glutamine Supplementation Decrease the Response of Muscle Glutamine Synthesis to Fasting in Muscle in Adult and Very Old Rats? J. Parenter. Enter. Nutr. 2007, 31, 26–31. [Google Scholar] [CrossRef]
- Meynial-Denis, D.; Patureau Mirand, P. Is Glutamine the Cornerstone of Sarcopenia in Very Old Individuals? J. Nutr. Health Aging 2011, 15, 507. [Google Scholar]
- Tatti, P.; Barber, A.E. The use of a specialized nutritional supplement for diabetic foot ulcers reduces the use of antibiotics. J. Endocrinol. Metab. 2012, 2, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Baier, S.; Johannsen, D.; Abumrad, N.; Rathmacher, J.A.; Nissen, S.; Flakoll, P. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of betahydroxy-beta-methylbutyrate (HMB), L-arginine, and L-lysine. JPEN 2009, 33, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Mesa, M.D.; Poyatos, R.M.; Aguilera, C.M.; Moreno-Torres, R.; Perez de la Cruz, A.; Gil, A. A specific protein-enriched enteral formula decreases cortisolemia and improves plasma albumin and amino acid concentrations in elderly patients. Nutr. Metab. 2010, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Nissen, S.; Sharp, R.; Ray, M.; Rathmacher, J.A.; Rice, D.; Fuller, J.C., Jr.; Connelly, A.S.; Abumrad, N. Effect of Leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar] [CrossRef]
- Nissen, S.L.; Sharp, R.L. Effect of dietary supplements on lean mass and strength gains with resistance exercise: A meta-analysis. J. Appl. Physiol. 2003, 94, 651–659. [Google Scholar] [CrossRef]
- Marzani, B.; Balage, M.; Vénien, A.; Astruc, T.; Papet, I.; Dardevet, D.; Mosoni, L. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J. Nutr. 2008, 138, 2205–2211. [Google Scholar] [CrossRef] [Green Version]
- Garlick, P.J.; Grant, I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem. J. 1988, 254, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Stancliffe, R.A. Role of beta-hydroxy-beta-methylbutyrate (HMB) in Leucine stimulation of mitochondrial biogenesis and fatty acid oxidation. FASEB J. 2012. [Google Scholar] [CrossRef]
- Rathmacher, J.; Nissen, S.; Panton, L.; Clark, R.; May, P.; Barber, A.; D’Olimpio, J.; Abumrad, N. Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. J. Parenter. Enter. Nutr. 2004, 28, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38–53. [Google Scholar] [PubMed]
- Johnson, C.D. Nutrition, Muscle Mass, and Muscular Performance in Middle Age and Beyond. In Proceedings of the The 110th Abbott Nutrition Research Conference, Columbus, OH, USA, 23–25 June 2009. [Google Scholar]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2000, 49 (Suppl. 1), 3–8. [Google Scholar] [CrossRef]
- Hong, S.; Chang, Y.; Jung, H.S.; Yun, K.E.; Shin, H.; Ryu, S. Relative muscle mass and the risk of incident type 2 diabetes: A cohort study. PLOS ONE 2017, 12, e0188650. [Google Scholar] [CrossRef] [Green Version]
- Mesinovic, J.; McMillan, L.B.; Shore-Lorenti, C.; De Courten, B.; Ebeling, P.R.; Scott, D. Metabolic Syndrome and Its Associations with Components of Sarcopenia in Overweight and Obese Older Adults. J. Clin. Med. 2019, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Clavel, S. Atrophy-related ubiquıtın ligases, Atrogin-1 and MuRF1 are upregulated in aged rat tibialis anterior muscle. Mech. Ageing Dev. 2006, 127, 794–801. [Google Scholar] [CrossRef]
- Scott, D.; de Courten, B.; Ebeling, P.R. Sarcopenia: A potential cause and consequence of type 2 diabetes in Australia’s ageing population? Med. J. Aust. 2016, 205, 329–333. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Oh, C.; No, J. Does Protein Intake Affect Metabolic Risk Factors among Older Adults in Korea? J. Obes. Metab. Syndr. 2017, 26, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Sikalidis, A.K.; Stipanuk, M.H. Growing rats respond to a sulfur amino acid-deficient diet by phosphorylation of eIF2α and induction of adaptive components of the integrated stress. J. Nutr. 2010, 140, 1080–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikalidis, A.K.; Mazor, K.M.; Kang, M.; Liu, H.; Stipanuk, M.H. Total 4E-BP1 Is Elevated in Liver of Rats in Response to Low Sulfur Amino Acid Intake. J. Amino Acids 2013, 864757. [Google Scholar] [CrossRef] [Green Version]

| Treatment | Inflammation Prevention | Increased Muscle Protein Synthesis | Reduced Muscle Deterioration | Increased Glucose Tolerance | Other Supplementation Required? | References |
|---|---|---|---|---|---|---|
| HMB | Yes | Yes | Yes | No | Yes | 22, 23, 30, 31 |
| Leu | No | Undetermined | No | Yes | Yes | 32, 33, 34, 35, 36, 37, 38, 45 |
| Gln | No | Yes | Yes | No | Yes | 22, 23, 57 |
| Arg | No | Yes | No | No | Yes | 22, 23, 52, 54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maykish, A.; Sikalidis, A.K. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. J. Pers. Med. 2020, 10, 19. https://doi.org/10.3390/jpm10010019
Maykish A, Sikalidis AK. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. Journal of Personalized Medicine. 2020; 10(1):19. https://doi.org/10.3390/jpm10010019
Chicago/Turabian StyleMaykish, Adeline, and Angelos K. Sikalidis. 2020. "Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation" Journal of Personalized Medicine 10, no. 1: 19. https://doi.org/10.3390/jpm10010019
APA StyleMaykish, A., & Sikalidis, A. K. (2020). Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. Journal of Personalized Medicine, 10(1), 19. https://doi.org/10.3390/jpm10010019