Effect of Quamoclit angulata Extract Supplementation on Oxidative Stress and Inflammation on Hyperglycemia-Induced Renal Damage in Type 2 Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Quamoclit Angulata Extracts (QAE)
2.2. Identification of Candidate Compounds of QAE
2.3. Animals Experiments
2.4. Hemoglobin A1c (HbA1c) and Plasma Insulin Assay
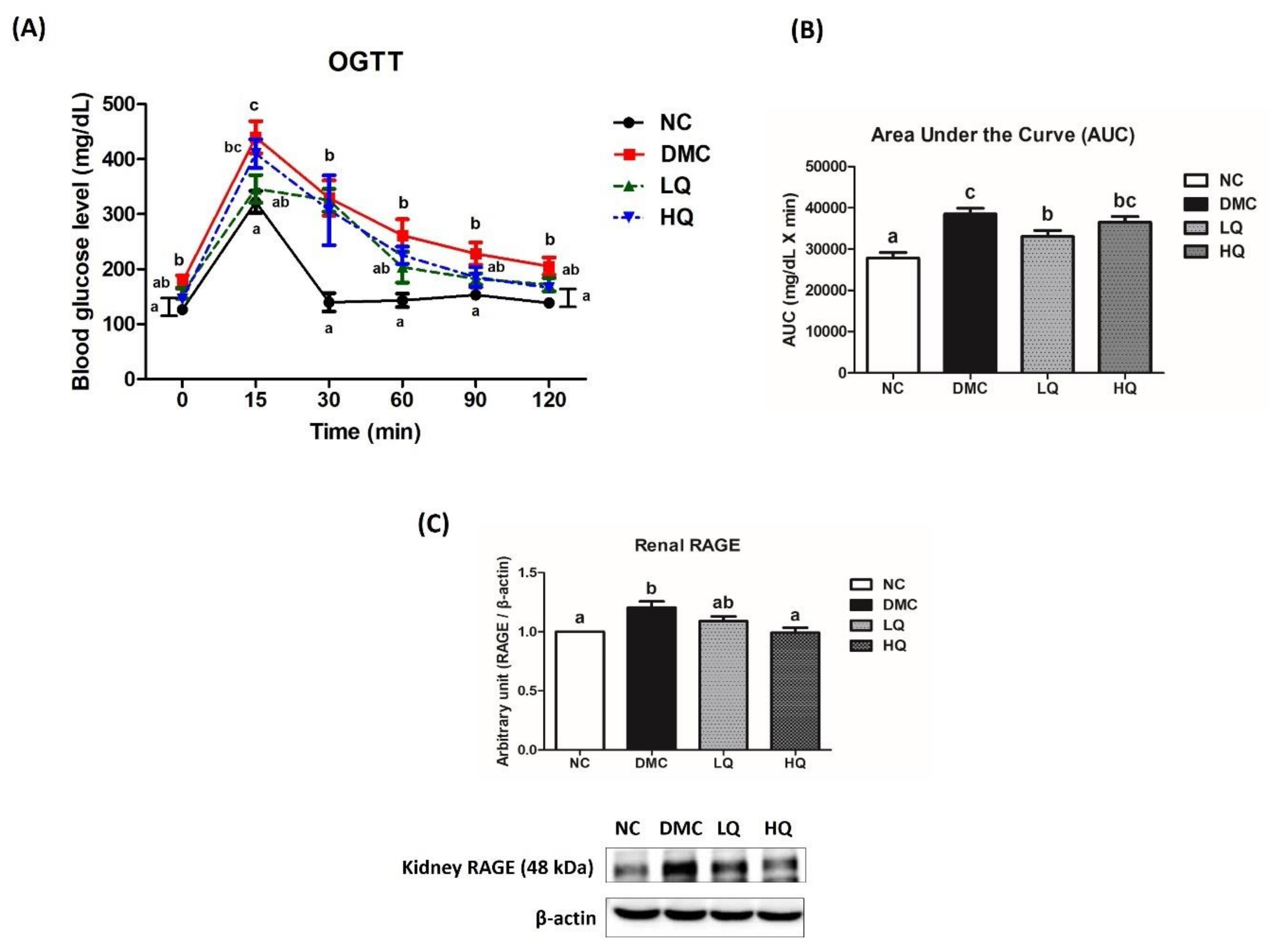
2.5. Oral Glucose Tolerance Test (OGTT)
(i = time sequence)
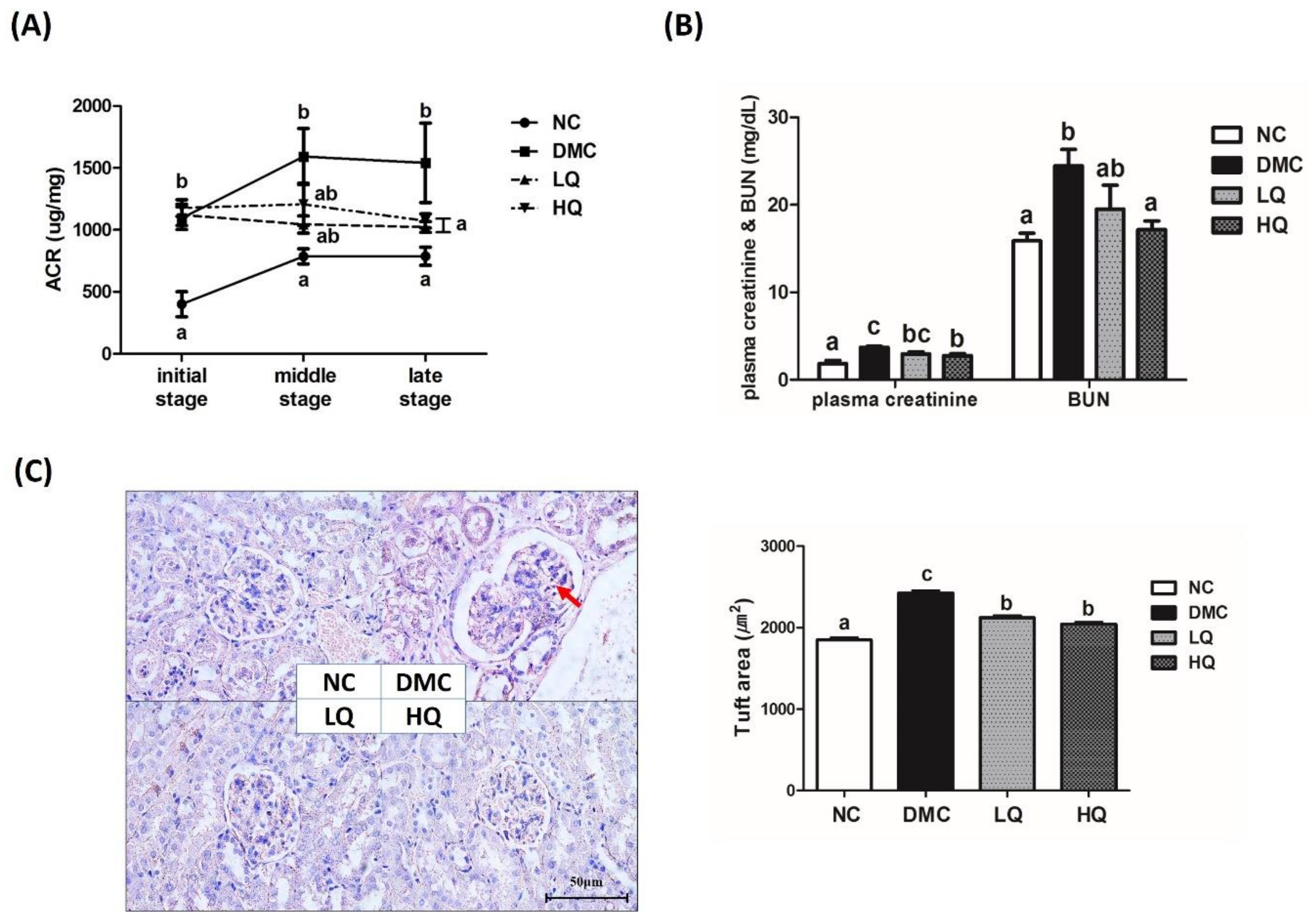
2.6. Renal Function Test
2.7. Histological Observation of Kidney
2.8. Protein Extraction and Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Identification of Major Natural Compounds in QAE
3.2. Effect of QAE Supplementation on Body Weight, Food Intake, and Kidney Weight in T2DM Mice
3.3. Effect of QAE Supplementation on Fasting Blood Glucose and Plasma Insulin Levels, Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), and Hemoglobin A1c (HbA1c) in T2DM Mice
3.4. Effect of QAE Supplementation on Glucose Homeostasis in T2DM Mice
3.5. Effect of QAE Supplementation on Kidney Function in T2DM Mice
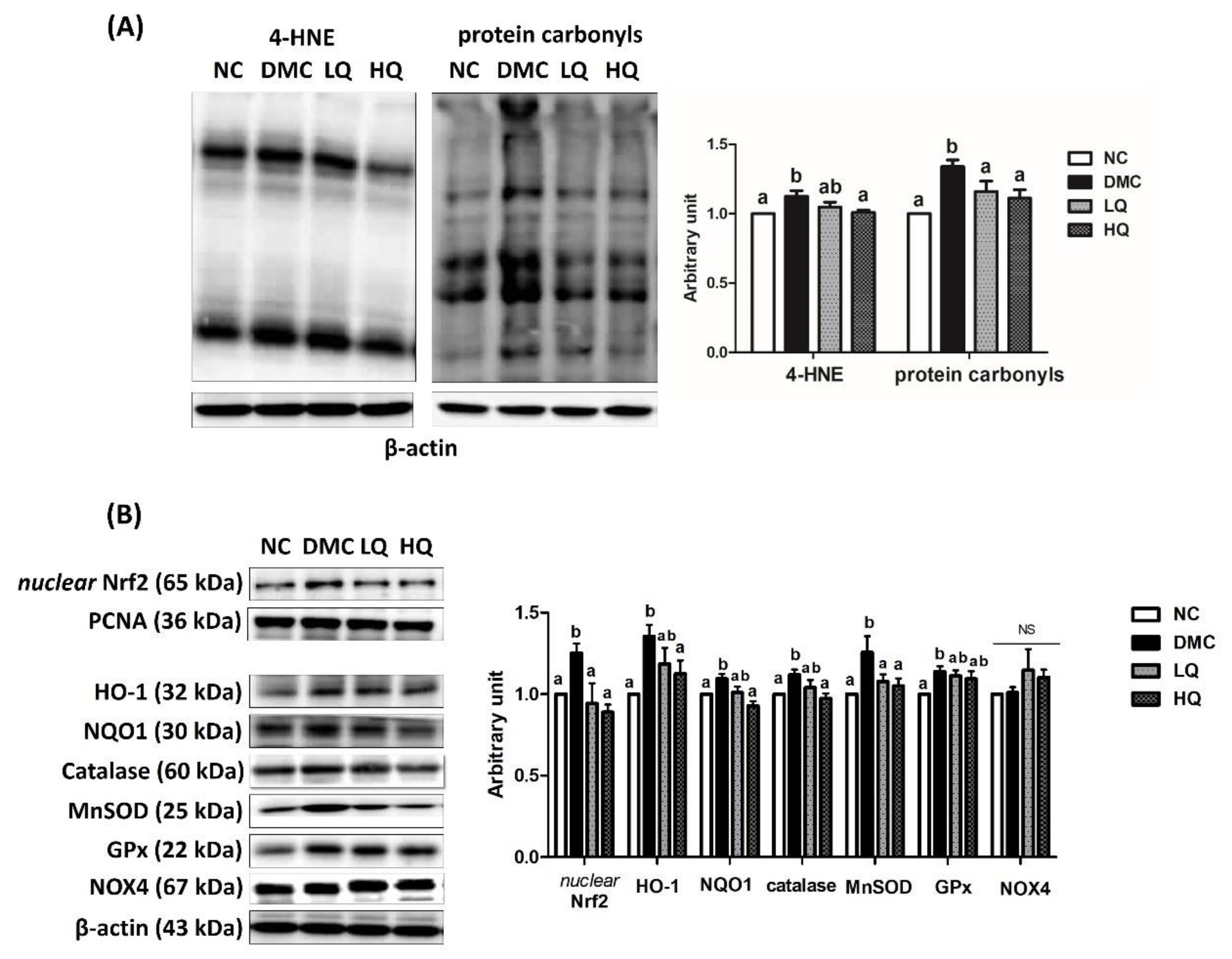
3.6. Effect of QAE Supplementation on Oxidative Stress in T2DM Mice
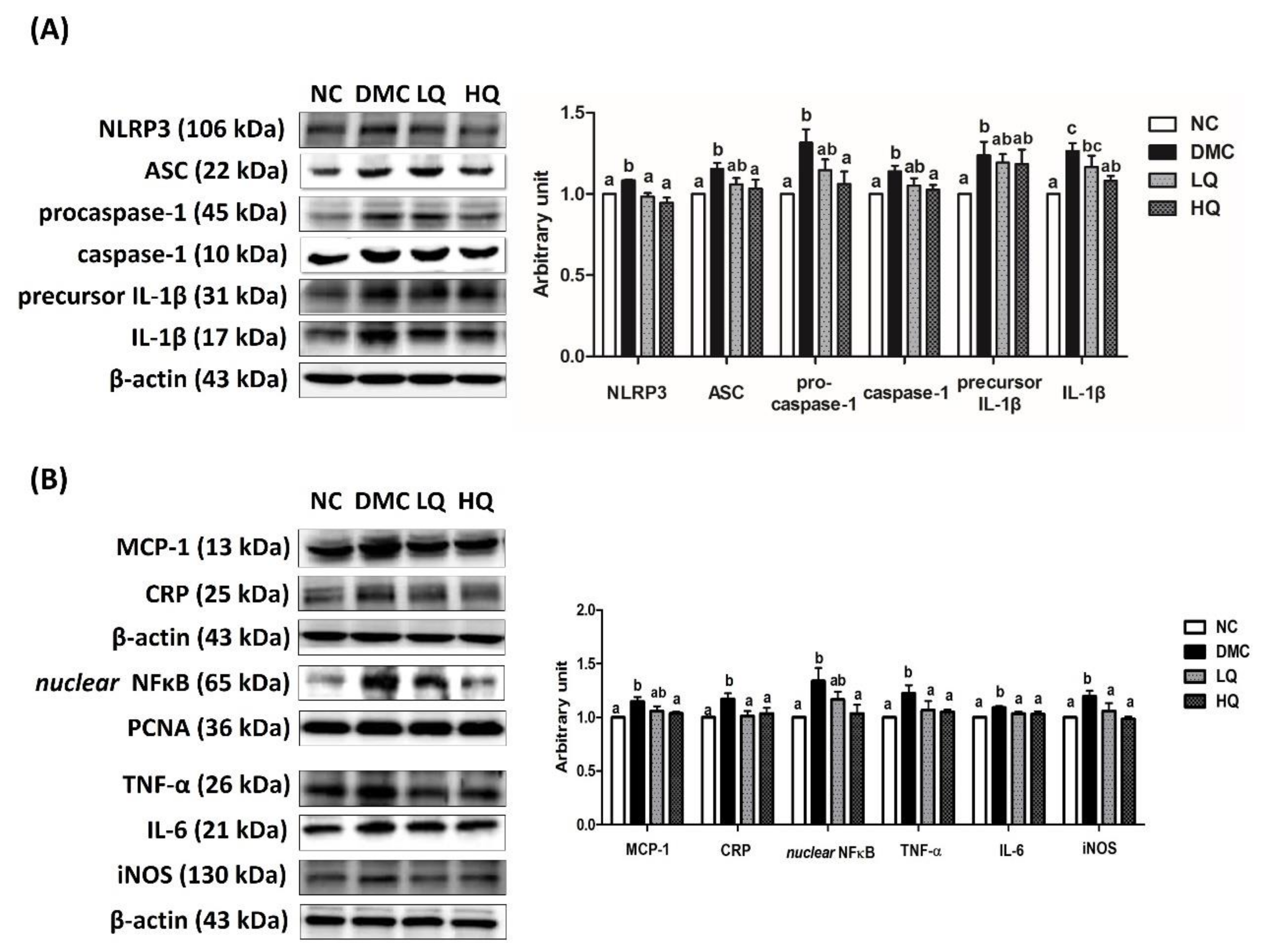
3.7. Effect of QAE Supplementation on Inflammation in T2DM Mice
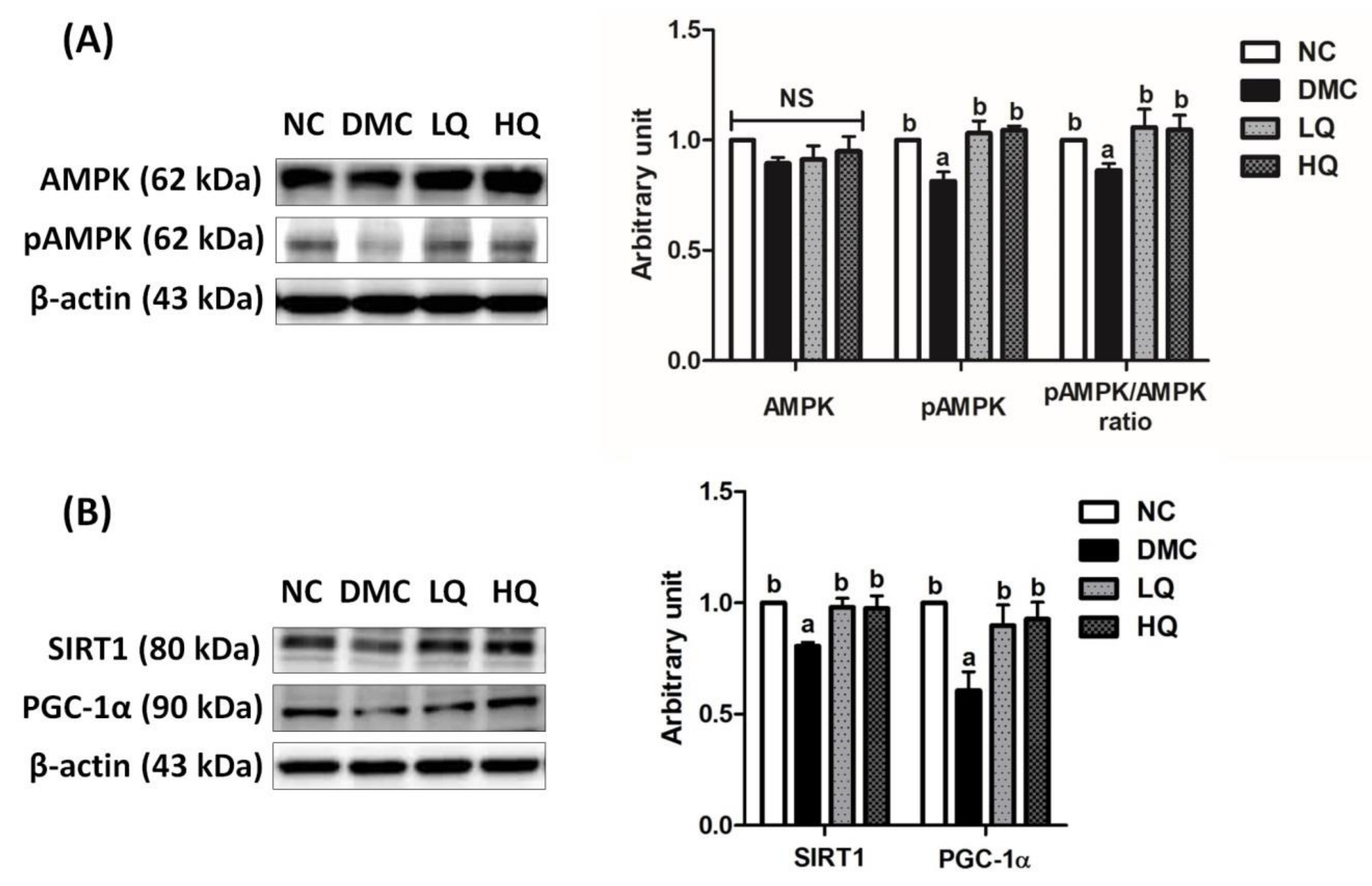
3.8. Effect of QAE Supplementation on Energy Metabolism in T2DM Mice
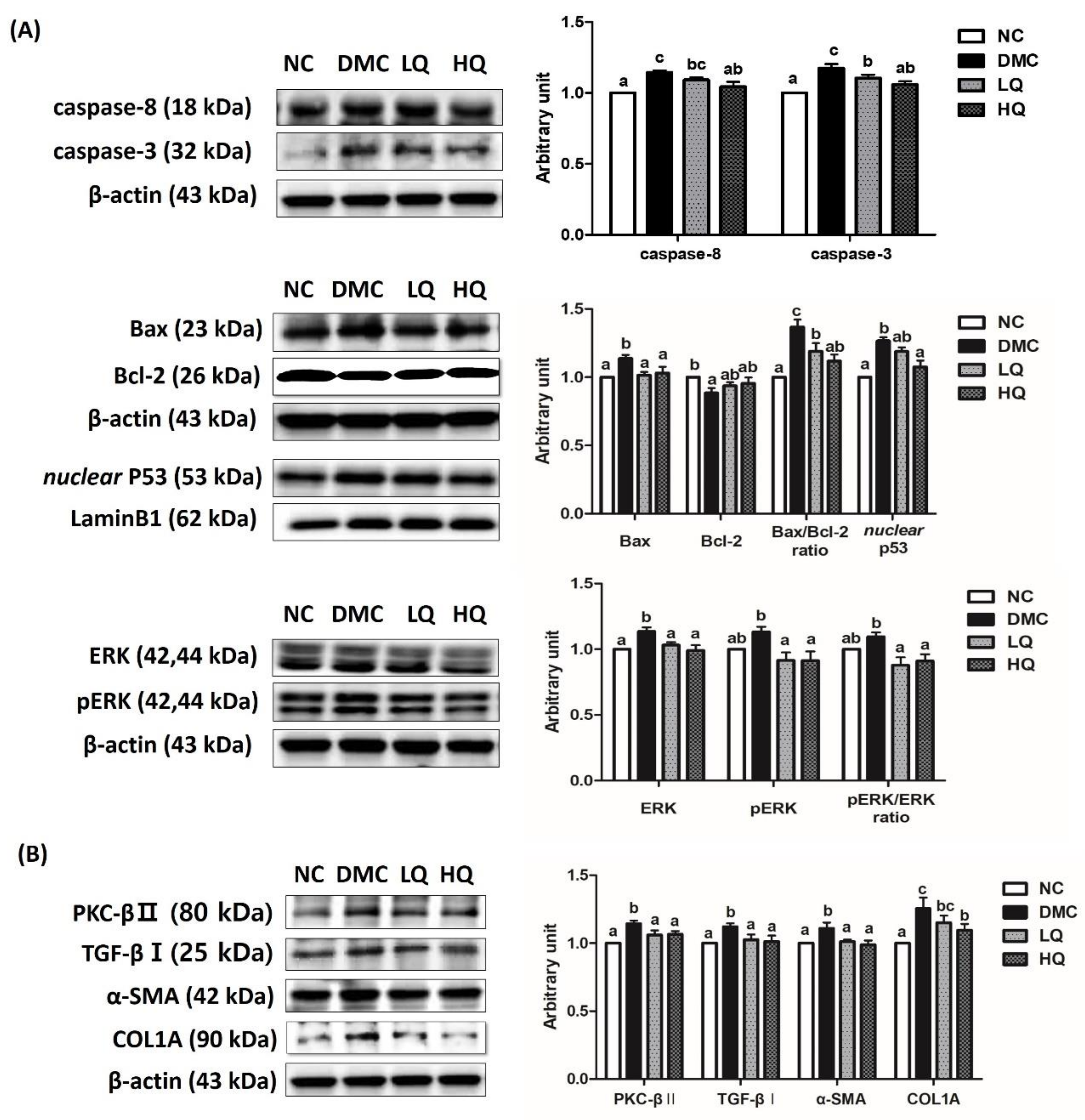
3.9. Effect of QAE Supplementation on Apoptosis and Fibrosis in T2DM Mice
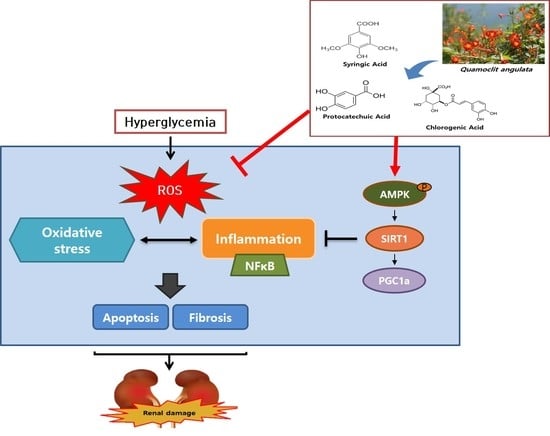
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACR | Albumin/Creatinine ratio |
| AGEs | Advanced glycation end products |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| ASC | Apoptosis-associated speck-like protein containing a caspase recruitment domain |
| AUC | Area under the curve |
| BUN | Blood urea nitrogen |
| DM | Diabetes mellitus |
| DN | Diabetic nephropathy |
| FBG | Fasting blood glucose |
| GPx | Glutathione peroxidase |
| 4-HNE | 4-hydroxynonenal |
| HO-1 | Hemeoxygenase-1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| NC | Normal control |
| NF-κB | Nuclear factor-κB |
| NLRP3 | NOD-like pyrin domain containing receptor 3 |
| NQO1 | NAD(P)H quinone dehydrogenase-1 |
| PKC | Protein kinase C |
| RAGE | Receptor for advanced glycation end products |
| ROS | Reactive oxygen species |
| SIRT1 | Sirtuin1 |
| SMA | Smooth muscle actin |
| SOD | Superoxide dismutase |
| T2DM | Type 2 diabetes mellitus |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| QA | Quamoclit angulata |
| QAE | Quamoclit angulata extract |
References
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Wright, E., Jr.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H.; Allen, T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology 2007, 12, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E. Diabetic nephropathy. Saudi J. Kidney Dis. Transpl. 2006, 17, 481–490. [Google Scholar]
- Turkmen, K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: The Four Horsemen of the Apocalypse. Int. Urol. Nephrol. 2017, 49, 837–844. [Google Scholar] [CrossRef]
- Eo, H.; Lee, H.J.; Lim, Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem. Biophys. Res. Commun. 2016, 478, 1021–1027. [Google Scholar] [CrossRef]
- Newsholme, P. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Habib, S.L. Diabetes and renal tubular cell apoptosis. World J. Diabetes 2013, 4, 27–30. [Google Scholar] [CrossRef]
- Noh, H.; Ha, H. Reactive oxygen species and oxidative stress. Contrib. Nephro. 2011, 170, 102–112. [Google Scholar]
- Lu, M. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. Biomed. Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Park, J.E.; Jeon, Y.J.; Lim, Y. Ameliorative Effect of Ecklonia cava Polyphenol Extract on Renal Inflammation Associated with Aberrant Energy Metabolism and Oxidative Stress in High Fat Diet-Induced Obese Mice. J. Agric. Food Chem. 2017, 65, 3811–3818. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, L.; Jiang, N.; Mou, S.; Zhang, M.; Gu, L.; Shao, X.; Wang, Q.; Qi, C.; Li, S.; et al. NLRP3 inflammasome mediates contrast media-induced acute kidney injury by regulating cell apoptosis. Sci. Rep. 2016, 6, 34682. [Google Scholar] [CrossRef]
- Klen, J.; Goricar, K.; Janez, A.; Dolzan, V. NLRP3 Inflammasome Polymorphism and Macrovascular Complications in Type 2 Diabetes Patients. J. Diabetes Res. 2015, 2015, 616747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wagener, F.A.; Dekker, D.; Berden, J.H.; Scharstuhl, A.; van der Vlag, J. The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis 2009, 14, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Balasescu, E.; Ion, D.A.; Cioplea, M.; Zurac, S. Caspases, Cell Death and Diabetic Nephropathy. Rom. J. Intern. Med. 2015, 53, 296–303. [Google Scholar]
- Dakshinamurty, K.V. Pathophysiology and pathology of Diabetic Nephropathy. In Diabetic Kidney Disease—ECAB; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; p. 223. [Google Scholar]
- Chen, S.; Hong, S.W.; Iglesias-de la Cruz, M.C.; Isono, M.; Casaretto, A.; Ziyadeh, F.N. The key role of the transforming growth factor-beta system in the pathogenesis of diabetic nephropathy. Ren. Fail. 2001, 23, 471–481. [Google Scholar] [CrossRef]
- Kitada, M.; Koya, D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C.W. Adenosine monophosphate-activated protein kinase in diabetic nephropathy. Kidney Res. Clin. Pract. 2016, 35, 69–77. [Google Scholar] [CrossRef]
- Chun, B.H. Pharmaceutical Composition for Treating Diabetes Containing Quamoclit Angulate Extracts. U.S. Patent Application 13/579,743, 3 January 2011. [Google Scholar]
- Zhang, M.; Lv, X.Y.; Li, J.; Xu, Z.G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef]
- Han, B.G.; Hao, C.M.; Tchekneva, E.E.; Wang, Y.Y.; Lee, C.A.; Ebrahim, B.; Harris, R.C.; Kern, T.S.; Wasserman, D.H.; Breyer, M.D.; et al. Markers of glycemic control in the mouse: Comparisons of 6-h- and overnight-fasted blood glucoses to Hb A1c. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E981–E986. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Kukongviriyapan, U.; Kongyingyoes, B.; Thukhammee, W.; Pannangpetch, P. Protocatechuic Acid Restores Vascular Responses in Rats With Chronic Diabetes Induced by Streptozotocin. Phytother. Res. 2016, 30, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Morley, A.L.; Koitka, A.; Samuel, P.; Coughlan, M.T.; Penfold, S.A.; Thomas, M.C.; Bierhaus, A.; Nawroth, P.P.; Yamamoto, H.; et al. Receptor for AGEs (RAGE) blockade may exert its renoprotective effects in patients with diabetic nephropathy via induction of the angiotensin II type 2 (AT2) receptor. Diabetologia 2010, 53, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Macedonio, G.; Carradori, S.; Sobolev, A.P.; De Salvador, R.F.; Monti, S.M.; Buonanno, M.; Zengin, G.; Angeli, A.; et al. Microwave-assisted extraction, HPLC analysis, and inhibitory effects on carbonic anhydrase I, II, VA, and VII isoforms of 14 blueberry Italian cultivars. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. S4), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Yamamoto, N.; Matsushima, S.; Yamamoto, T.; Takada-Takatori, Y.; Akaike, A.; Kume, T. Compensatory role of the Nrf2-ARE pathway against paraquat toxicity: Relevance of 26S proteasome activity. J. Pharmacol. Sci. 2015, 129, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Fasina, O.B.; Ayeni, M.F.; Ajayi, O.M.; Farombi, E.O. Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem. Toxicol. 2019, 125, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R. Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Shariati, H.; Hassanpour, M.; Sharifzadeh, G.; Zarban, A.; Samarghandian, S.; Saeedi, F. Evaluation of diuretic and antioxidant properties in aqueous bark and fruit extracts of pine. Curr. Drug Discov. Technol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Towns, R.; Pietropaolo, M.; Wiley, J.W. Stimulation of autophagy by autoantibody-mediated activation of death receptor cascades. Autophagy 2008, 4, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Fu, J.; Lee, K.; Chuang, P.Y.; Liu, Z.; He, J.C. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am. J. Physiol. Renal Physiol. 2015, 308, F287–F297. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F.; Yang, S.; Chen, B.; Shi, J. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Biomed. Pharmacother. 2018, 98, 18–22. [Google Scholar] [CrossRef]
- Wang, K.; Tang, Z.; Wang, J.; Cao, P.; Li, Q.; Shui, W.; Wang, H.; Zheng, Z.; Zhang, Y. RETRACTED: Polysaccharide from Angelica sinensis ameliorates high-fat diet and STZ-induced hepatic oxidative stress and inflammation in diabetic mice by activating the Sirt1-AMPK pathway. J. Nutr. Biochem. 2017, 43, 88–97. [Google Scholar] [CrossRef]
- Yacoub, R.; Lee, K.; He, J.C. The Role of SIRT1 in Diabetic Kidney Disease. Front. Endocrinol. 2014, 5, 166. [Google Scholar] [CrossRef]
- Wan, X.; Wen, J.J.; Koo, S.J.; Liang, L.Y.; Garg, N.J. SIRT1-PGC1alpha-NFkappaB Pathway of Oxidative and Inflammatory Stress during Trypanosoma cruzi Infection: Benefits of SIRT1-Targeted Therapy in Improving Heart Function in Chagas Disease. PLoS Pathog. 2016, 12, e1005954. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, Q.; Ma, W.; Li, J.; Qu, L.; Wang, M. Protocatechuic Acid Ameliorated Palmitic-Acid-Induced Oxidative Damage in Endothelial Cells through Activating Endogenous Antioxidant Enzymes via an Adenosine-Monophosphate-Activated-Protein-Kinase-Dependent Pathway. J. Agric. Food Chem. 2018, 66, 10400–10409. [Google Scholar] [CrossRef] [PubMed]
- Guclu, A.; Erdur, F.M.; Turkmen, K. The Emerging Role of Sirtuin 1 in Cellular Metabolism, Diabetes Mellitus, Diabetic Kidney Disease and Hypertension. Exp. Clin. Endocrinol. Diabetes 2016, 124, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef]
- Kong, L.; Wu, H.; Zhou, W.; Luo, M.; Tan, Y.; Miao, L.; Cai, L. Sirtuin 1: A Target for Kidney Diseases. Mol. Med. 2015, 21, 87–97. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.S.; Yang, S.J. Interaction between NLRP3 Inflammasome and Sirt1/6: Metabolomics Approach. FASEB J. 2015, 29, 913.12. [Google Scholar]
- Li, Y.; Yang, X.; He, Y.; Wang, W.; Zhang, J.; Zhang, W.; Jing, T.; Wang, B.; Lin, R. Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology 2017, 222, 552–561. [Google Scholar] [CrossRef]
- Guo, K.; Lu, J.; Huang, Y.; Wu, M.; Zhang, L.; Yu, H.; Zhang, M.; Bao, Y.; He, J.C.; Chen, H.; et al. Protective role of PGC-1alpha in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS ONE 2015, 10, e0125176. [Google Scholar]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, H.; Kim, S.Y.; Lim, Y. Lespedeza bicolor Extract Ameliorated Renal Inflammation by Regulation of NLRP3 Inflammasome-Associated Hyperinflammation in Type 2 Diabetic Mice. Antioxidants 2020, 9, 148. [Google Scholar] [CrossRef]
| Samples | Content (µg/g) | ||||
|---|---|---|---|---|---|
| Protocatechuic Acid | Chlorogenic Acid | Syringic Acid | Myricetin | Quercetin | |
| QAE | 198.86 ± 2.26 | 54.11 ± 1.81 | 56.38 ± 0.57 | 12.42 ± 0.09 | 13.41 ± 0.08 |
| Group | NC | DMC | LQ | HQ |
|---|---|---|---|---|
| Body Weight (g) | ||||
| before treatment | 26.58 ± 0.45 a | 32.04 ± 1.04 b | 31.90 ± 1.25 b | 30.80 ± 0.68 b |
| after treatment | 30.42 ± 0.61 a | 40.93 ± 1.57 b | 40.20 ± 2.20 b | 39.40 ± 1.89 b |
| Gain | 3.85 ± 0.33 a | 8.89 ± 0.70 b | 8.31 ± 1.15 b | 8.60 ± 1.33 b |
| Kidney Weight (mg) | 151.00 ± 5.34 a | 183.00 ± 14.37 b | 175.00 ± 11.18 a,b | 176.00 ± 4.30 a,b |
| Food Intake (g/day) | 2.32 ± 0.21 a | 3.48 ± 0.25 b | 3.3 ± 0.12 b | 3.76 ± 0.12 b |
| Plasma Indices | NC | DMC | LQ | HQ |
|---|---|---|---|---|
| FBG (mg/dL) | 122 ± 7.51 a | 173 ± 14.30 b | 156 ± 9.94 a,b | 150 ± 12.04 a,b |
| Insulin (uU/mL) | 1.62 ± 0.30 a | 2.62 ± 0.23 b | 1.80 ± 0.38 a,b | 1.61 ± 0.20 a |
| HOMA-IR | 2.82 ± 0.58 a | 8.45 ± 0.79 b | 3.80 ± 1.01 a | 4.19 ± 0.62 a |
| HbA1c (%) | 6.61 ± 0.51 a | 12.18 ± 2.82 b | 8.64 ± 0.76 a,b | 7.13 ± 0.39 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.E.; Lee, H.; Rho, H.; Hong, S.M.; Kim, S.Y.; Lim, Y. Effect of Quamoclit angulata Extract Supplementation on Oxidative Stress and Inflammation on Hyperglycemia-Induced Renal Damage in Type 2 Diabetic Mice. Antioxidants 2020, 9, 459. https://doi.org/10.3390/antiox9060459
Park JE, Lee H, Rho H, Hong SM, Kim SY, Lim Y. Effect of Quamoclit angulata Extract Supplementation on Oxidative Stress and Inflammation on Hyperglycemia-Induced Renal Damage in Type 2 Diabetic Mice. Antioxidants. 2020; 9(6):459. https://doi.org/10.3390/antiox9060459
Chicago/Turabian StylePark, Ji Eun, Heaji Lee, Hyunkyung Rho, Seong Min Hong, Sun Yeou Kim, and Yunsook Lim. 2020. "Effect of Quamoclit angulata Extract Supplementation on Oxidative Stress and Inflammation on Hyperglycemia-Induced Renal Damage in Type 2 Diabetic Mice" Antioxidants 9, no. 6: 459. https://doi.org/10.3390/antiox9060459
APA StylePark, J. E., Lee, H., Rho, H., Hong, S. M., Kim, S. Y., & Lim, Y. (2020). Effect of Quamoclit angulata Extract Supplementation on Oxidative Stress and Inflammation on Hyperglycemia-Induced Renal Damage in Type 2 Diabetic Mice. Antioxidants, 9(6), 459. https://doi.org/10.3390/antiox9060459