Actin and Myosin in Non-Neuronal Exocytosis
Abstract
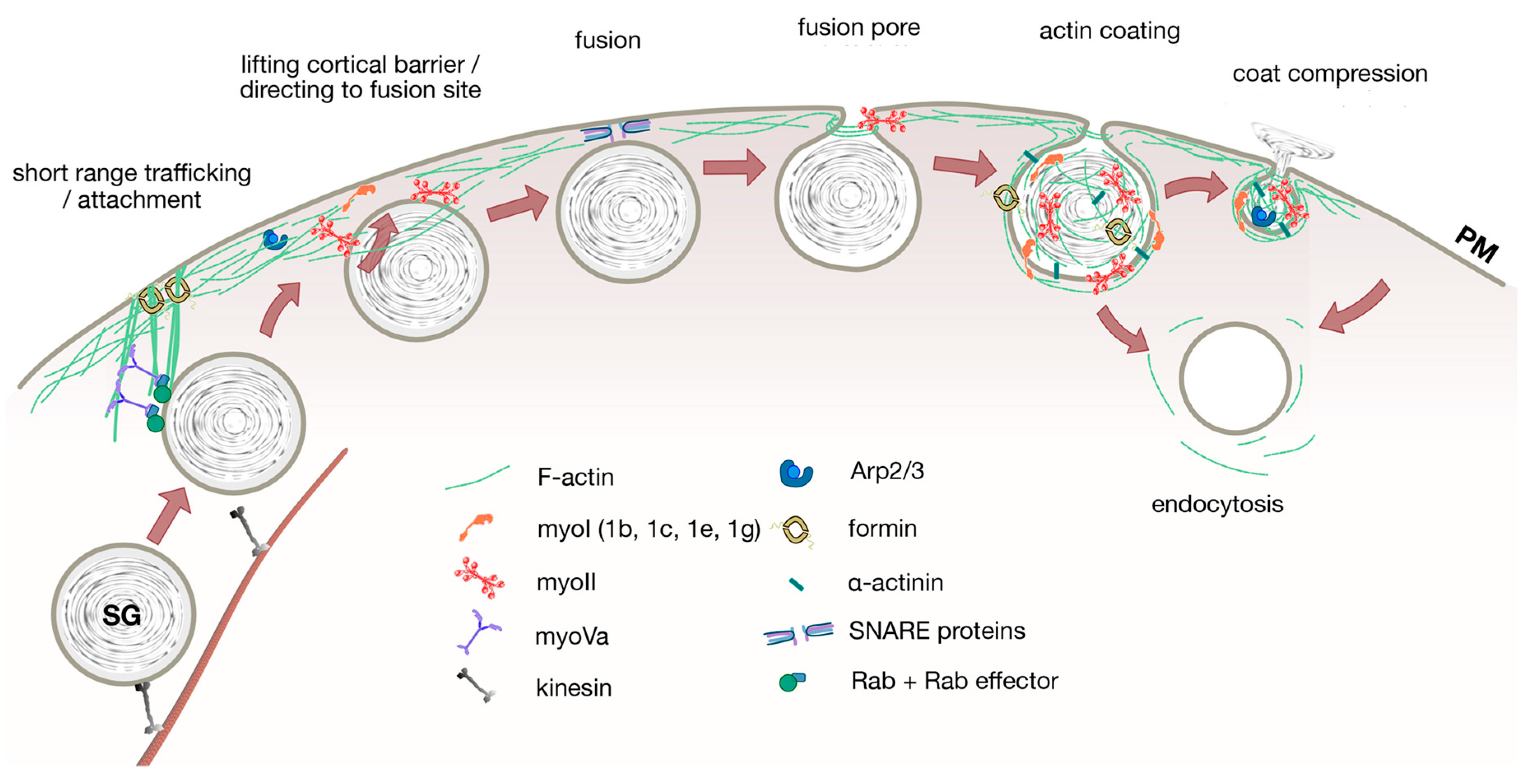
:1. Introduction
2. Vesicle Transport
3. Docking
4. Fusion Pore
5. Post Fusion
5.1. Vesicle Content Release
5.2. Endocytosis
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Burgoyne, R.D.; Morgan, A. Secretory granule exocytosis. Physiol. Rev. 2003, 83, 581–632. [Google Scholar] [CrossRef]
- Rudolf, R.; Kögel, T.; Kuznetsov, S.A.; Salm, T.; Schlicker, O.; Hellwig, A.; Hammer, J.A.; Gerdes, H.H. Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J. Cell Sci. 2003, 116, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Pulido, I.R.; Nightingale, T.D.; Darchen, F.; Seabra, M.C.; Cutler, D.F.; Gerke, V. Myosin Va acts in concert with Rab27a and MyRIP to regulate acute von-Willebrand factor release from endothelial cells. Traffic 2011, 12, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bademosi, A.T.; Luo, J.; Meunier, F.A. Actin Remodeling in Regulated Exocytosis: Toward a Mesoscopic View. Trends Cell Biol. 2018, 28, 685–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, L.M.; Villanueva, J. The role of F-actin in the transport and secretion of chromaffin granules: An historic perspective. Pflugers Arch. Eur. J. Physiol. 2018, 470, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Meunier, F.A.; Gutiérrez, L.M. Captivating New Roles of F-Actin Cortex in Exocytosis and Bulk Endocytosis in Neurosecretory Cells. Trends Neurosci. 2016, 39, 605–613. [Google Scholar] [CrossRef]
- Papadopulos, A. Membrane shaping by actin and myosin during regulated exocytosis. Mol. Cell. Neurosci. 2017, 84, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopulos, A.; Tomatis, V.M.; Kasula, R.; Meunier, F.A. The Cortical Acto-Myosin Network: From Diffusion Barrier to Functional Gateway in the Transport of Neurosecretory Vesicles to the Plasma Membrane. Front. Endocrinol. (Lausanne) 2013, 4, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Porat-Shliom, N.; Milberg, O.; Masedunskas, A.; Weigert, R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell. Mol. Life Sci. 2013, 70, 2099–2121. [Google Scholar] [CrossRef] [Green Version]
- Barlan, K.; Gelfand, V.I. Microtubule-based transport and the distribution, tethering, and organization of organelles. Cold Spring Harb. Perspect. Biol. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Noordstra, I.; Akhmanova, A. Linking cortical microtubule attachment and exocytosis [version 1; referees: 2 approved]. F1000Research 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Conte, I.L.; Hellen, N.; Bierings, R.; Mashanov, G.I.; Manneville, J.B.; Kiskin, N.I.; Hannah, M.J.; Molloy, J.E.; Carter, T. Interaction between MyRIP and the actin cytoskeleton regulates Weibel-Palade body trafficking and exocytosis. J. Cell Sci. 2016, 129, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, T.; Cutler, D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. J. Thromb. Haemost. 2013, 11, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Geron, E.; Schejter, E.D.; Shilo, B.Z. Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1. Proc. Natl. Acad. Sci. USA 2013, 110, 10652–10657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massarwa, R.; Schejter, E.D.; Shilo, B.Z. Apical Secretion in Epithelial Tubes of the Drosophila Embryo Is Directed by the Formin-Family Protein Diaphanous. Dev. Cell 2009, 16, 877–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geron, E.; Schejter, E.D.; Shilo, B.Z. Targeting secretion to the apical surface by mDia1-built actin tracks. Commun. Integr. Biol. 2013, 6, e25660-1–e25660-2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, J.A.; Sellers, J.R. Walking to work: Roles for class v myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 2012, 13, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck-Peterson, S.; Provance, D.; Mooseker, M.; Mercer, J. Class V myosins. Biochim. Biophys. Acta 2000, 1496, 36–51. [Google Scholar] [CrossRef] [Green Version]
- Dolce, L.G.; Ohbayashi, N.; da Silva, D.F.C.; Ferrari, A.J.R.; Pirolla, R.A.S.; de A.P. Schwarzer, A.C.; Zanphorlin, L.M.; Cabral, L.; Fioramonte, M.; Ramos, C.H.I.; et al. Unveiling the interaction between the molecular motor Myosin Vc and the small GTPase Rab3A. J. Proteom. 2020, 212, 1–9. [Google Scholar] [CrossRef]
- Hsueh, P.Y.; Edman, M.C.; Sun, G.; Shi, P.; Xu, S.; Lin, Y.A.; Cui, H.; Hamm-Alvarez, S.F.; Mackay, J.A. Tear-mediated delivery of nanoparticles through transcytosis of the lacrimal gland. J. Control. Release 2015, 208, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Brozzi, F.; Diraison, F.; Lajus, S.; Rajatileka, S.; Philips, T.; Regazzi, R.; Fukuda, M.; Verkade, P.; Molnár, E.; Váradi, A. Molecular mechanism of Myosin Va recruitment to dense core secretory granules. Traffic 2012, 13, 54–69. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Welz, T.; Kerkhoff, E. Exploring the iceberg: Prospects of coordinated myosin V and actin assembly functions in transport processes. Small GTPases 2019, 10, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Membrane traffic in the secretory pathway: Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 2008, 65, 2801–2813. [Google Scholar] [CrossRef]
- Tolmachova, T.; Anders, R.; Stinchcombe, J.; Bossi, G.; Griffiths, G.; Huxley, C.; Seabra, M. A General Role for Rab27a in Secretory Cells. Mol. Biol. Cell 2004, 15, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, T.D.; Pattni, K.; Hume, A.N.; Seabra, M.C.; Cutler, D.F. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood 2009, 113, 5010–5018. [Google Scholar] [CrossRef] [Green Version]
- Cheeseman, L.P.; Boulanger, J.; Bond, L.M.; Schuh, M. Two pathways regulate cortical granule translocation to prevent polyspermy in mouse oocytes. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Bustos, M.A.; Lucchesi, O.; Ruete, M.C.; Mayorga, L.S.; Tomes, C.N. Rab27 and Rab3 sequentially regulate human sperm dense-core granule exocytosis. Proc. Natl. Acad. Sci. USA 2012, 109, E2057–E2066. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Rao, K.; Bowers, M.B.; Copeland, N.G.; Jenkins, N.A.; Hammer, J.A. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 2001, 114, 1091–1100. [Google Scholar]
- Stinchcombe, J.C.; Barral, D.C.; Mules, E.H.; Booth, S.; Hume, A.N.; Machesky, L.M.; Seabra, M.C.; Griffiths, G.M. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001, 152, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, K.; Torii, S.; Yi, Z.; Igarashi, M.; Okamoto, K.; Takeuchi, T.; Izumi, T. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 2002, 517, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Schuh, M. An actin-dependent mechanism for long-range vesicle transport. Nat. Cell Biol. 2011, 13, 1431–1436. [Google Scholar] [CrossRef]
- Chugh, P.; Paluch, E.K. The actin cortex at a glance. J. Cell Sci. 2018, 131, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, K.; Yoshida, S.; Hatano, R.; Asano, S. Pathophysiological roles of ezrin/radixin/moesin proteins. Biol. Pharm. Bull. 2017, 40, 381–390. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, B.B.; Ostap, E.M. Myosin-I molecular motors at a glance. J. Cell Sci. 2016, 129, 2689–2695. [Google Scholar] [CrossRef] [Green Version]
- Chaigne, A.; Campillo, C.; Gov, N.S.; Voituriez, R.; Azoury, J.; Umaña-Diaz, C.; Almonacid, M.; Queguiner, I.; Nassoy, P.; Sykes, C.; et al. A soft cortex is essential for asymmetric spindle positioning in mouse oocytes. Nat. Cell Biol. 2013, 15, 958–966. [Google Scholar] [CrossRef]
- Courtemanche, N. Mechanisms of formin-mediated actin assembly and dynamics. Biophys. Rev. 2018, 10, 1553–1569. [Google Scholar] [CrossRef]
- Rottner, K.; Faix, J.; Bogdan, S.; Linder, S.; Kerkhoff, E. Actin assembly mechanisms at a glance. J. Cell Sci. 2017, 130, 3427–3435. [Google Scholar] [CrossRef] [Green Version]
- Eghiaian, F.; Rigato, A.; Scheuring, S. Structural, mechanical, and dynamical variability of the actin cortex in living cells. Biophys. J. 2015, 108, 1330–1340. [Google Scholar] [CrossRef] [Green Version]
- Clausen, M.P.; Colin-York, H.; Schneider, F.; Eggeling, C.; Fritzsche, M. Dissecting the actin cortex density and membrane-cortex distance in living cells by super-resolution microscopy. J. Phys. D Appl. Phys. 2017, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Eitzen, G. Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta-Mol. Cell Res. 2003, 1641, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, L.M. New Insights into the Role of the Cortical Cytoskeleton in Exocytosis from Neuroendocrine Cells. Int. Rev. Cell Mol. Biol. 2012, 295, 109–137. [Google Scholar] [CrossRef]
- Nightingale, T.D.; White, I.J.; Doyle, E.L.; Turmaine, M.; Harrison-Lavoie, K.J.; Webb, K.F.; Cramer, L.P.; Cutler, D.F. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J. Cell Biol. 2011, 194, 613–629. [Google Scholar] [CrossRef]
- Singh, R.K.; Mizuno, K.; Wasmeier, C.; Wavre-Shapton, S.T.; Recchi, C.; Catz, S.D.; Futter, C.; Tolmachova, T.; Hume, A.N.; Seabra, M.C. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS J. 2013, 280, 892–903. [Google Scholar] [CrossRef]
- Klein, O.; Krier-Burris, R.A.; Lazki-Hagenbach, P.; Gorzalczany, Y.; Mei, Y.; Ji, P.; Bochner, B.S.; Sagi-Eisenberg, R. Mammalian diaphanous-related formin 1 (mDia1) coordinates mast cell migration and secretion through its actin-nucleating activity. J. Allergy Clin. Immunol. 2019, 144, 1074–1090. [Google Scholar] [CrossRef]
- Carisey, A.F.; Mace, E.M.; Saeed, M.B.; Davis, D.M.; Orange, J.S. Nanoscale Dynamism of Actin Enables Secretory Function in Cytolytic Cells. Curr. Biol. 2018, 28, 489–502.e9. [Google Scholar] [CrossRef] [Green Version]
- Jerdeva, G.V.; Wu, K.; Yarber, F.A.; Rhodes, C.J.; Kalman, D.; Schechter, J.E.; Hamm-Alvarez, S.F. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J. Cell Sci. 2005, 118, 4797–4812. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Chang, J.; Tong, J.; Ho, U.; Yau, B.; Kebede, M.A.; Thorn, P. Arp2/3 nucleates F-actin coating of fusing insulin granules in pancreatic β cells to control insulin secretion. J. Cell Sci. 2020, 133, 1–11. [Google Scholar] [CrossRef]
- Colin-York, H.; Li, D.; Korobchevskaya, K.; Chang, V.T.; Betzig, E.; Eggeling, C.; Fritzsche, M. Cytoskeletal actin patterns shape mast cell activation. Commun. Biol. 2019, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, Y.; Cichocki, F.; Sieben, A.; Sforza, F.; Karim, R.; Coughlin, K.; Vogel, R.I.; Gavioli, R.; McCullar, V.; Lenvik, T.; et al. UNC-45A Is a Nonmuscle Myosin IIA Chaperone Required for NK Cell Cytotoxicity via Control of Lytic Granule Secretion. J. Immunol. 2015, 195, 4760–4770. [Google Scholar] [CrossRef] [Green Version]
- Steppan, X.D.; Pan, L.; Gross, K.W.; Kurtz, A. Analysis of the calcium paradox of renin secretion. Am. J. Physiol. Ren. Physiol. 2018, 315, F834–F843. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.; Robida, S.; Furcinitti, P.S.; Chawla, A.; Fogarty, K.; Corvera, S.; Czech, M.P. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol. Cell. Biol. 2004, 24, 5447–5458. [Google Scholar] [CrossRef] [Green Version]
- Kittelberger, N.; Breunig, M.; Martin, R.; Knölker, H.J.; Miklavc, P.; Knölker, H.-J.; Miklavc, P. The role of myosin 1c and myosin 1b in surfactant exocytosis. J. Cell Sci. 2016, 129, 1685–1696. [Google Scholar] [CrossRef] [Green Version]
- Maravillas-Montero, J.L.; López-Ortega, O.; Patiño-López, G.; Santos-Argumedo, L. Myosin 1g regulates cytoskeleton plasticity, cell migration, exocytosis, and endocytosis in B lymphocytes. Eur. J. Immunol. 2014, 44, 877–886. [Google Scholar] [CrossRef]
- Saarikangas, J.; Zhao, H.; Lappalainen, P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 2010, 90, 259–289. [Google Scholar] [CrossRef] [Green Version]
- Janmey, P.A.; Bucki, R.; Radhakrishnan, R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2019, 506, 307–314. [Google Scholar] [CrossRef]
- Bader, M.F.; Doussau, F.; Chasserot-Golaz, S.; Vitale, N.; Gasman, S. Coupling actin and membrane dynamics during calcium-regulated exocytosis: A role for Rho and ARF GTPases. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1742, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Ory, S.; Gasman, S. Rho GTPases and exocytosis: What are the molecular links? Semin. Cell Dev. Biol. 2011, 22, 27–32. [Google Scholar] [CrossRef]
- Sit, S.T.; Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 2011, 124, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Martin, L.D.; Vargaftig, B.B.; Park, J.; Gruber, A.D.; Li, Y.; Adler, K.B. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat. Med. 2004, 10, 193–196. [Google Scholar] [CrossRef]
- Agrawal, A.; Rengarajan, S.; Adler, K.B.; Ram, A.; Ghosh, B.; Fahim, M.; Dickey, B.F. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J. Appl. Physiol. 2007, 102, 399–405. [Google Scholar] [CrossRef]
- Sheats, M.K.; Yin, Q.; Fang, S.; Park, J.; Crews, A.L.; Parikh, I.; Dickson, B.; Adler, K.B. Marcks and lung disease. Am. J. Respir. Cell Mol. Biol. 2019, 60, 16–27. [Google Scholar] [CrossRef]
- Malacombe, M.; Bader, M.F.; Gasman, S. Exocytosis in neuroendocrine cells: New tasks for actin. Biochim. Biophys. Acta-Mol. Cell Res. 2006, 1763, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.T.; Masedunskas, A.; Weigert, R.; Hagen, K.G.T. Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Verhage, M.; Sørensen, J.B. Vesicle docking in regulated exocytosis. Traffic 2008, 9, 1414–1424. [Google Scholar] [CrossRef]
- Varadi, A.; Tsuboi, T.; Rutter, G. Myosin Va Transports Dense Core Secretory Vesicles in Pancreatic MIN6 β-Cells. Mol. Biol. Cell 2005, 16, 2670–2680. [Google Scholar] [CrossRef] [Green Version]
- Südhof, T. The Presynaptic Active Zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Matsunaga, K.; Wang, H.; Ishizaki, R.; Kobayashi, E.; Kiyonari, H.; Mukumoto, Y.; Okunishi, K.; Izumi, T. Exophilin-8 assembles secretory granules for exocytosis in the actin cortex via interaction with RIM-BP2 and myosin-VIIa. Elife 2017, 6, 1–23. [Google Scholar] [CrossRef]
- Encarnação, M.; Espada, L.; Escrevente, C.; Mateus, D.; Ramalho, J.; Michelet, X.; Santarino, I.; Hsu, V.W.; Brenner, M.B.; Barral, D.C.; et al. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J. Cell Biol. 2016, 213, 631–640. [Google Scholar] [CrossRef]
- Machado, E.; White-Gilbertson, S.; Van De Vlekkert, D.; Janke, L.; Moshiach, S.; Campos, Y.; Finkelstein, D.; Gomero, E.; Mosca, R.; Qiu, X.; et al. Regulated lysosomal exocytosis mediates cancer progression. Sci. Adv. 2015, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gerelsaikhan, T.; Chen, X.L.; Chander, A. Secretagogues of lung surfactant increase annexin A7 localization with ABCA3 in alveolar type II cells. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 2017–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knop, M.; Aareskjold, E.; Bode, G.; Gerke, V. Rab3D and annexin A2 play a role in regulated of vWF, but not tPA, from endothelial cells. EMBO J. 2004, 23, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Chehab, T.; Santos, N.C.; Holthenrich, A.; Koerdt, S.N.; Disse, J.; Schuberth, C.; Nazmi, A.R.; Neeft, M.; Koch, H.; Man, K.N.M.; et al. A novel Munc13-4/S100A10/annexin A2 complex promotes Weibel-Palade body exocytosis in endothelial cells. Mol. Biol. Cell 2017, 28, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Hoffman, L.M.; Beckerle, M.C. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 2014, 24, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Li, P.; Yang, Z.; Huang, X.; Wei, G.; Sun, Y.; Kang, X.; Hu, X.; Deng, Q.; Chen, L.; et al. Zyxin regulates endothelial von Willebrand factor secretion by reorganizing actin filaments around exocytic granules. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McLeish, K.R.; Merchant, M.L.; Creed, T.M.; Tandon, S.; Barati, M.T.; Uriarte, S.M.; Ward, R.A. Frontline Science: Tumor necrosis factor-α stimulation and priming of human neutrophil granule exocytosis. J. Leukoc. Biol. 2017, 102, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.D.; Shelby, S.A.; Holowka, D.; Baird, B. Rab11 Regulates the Mast Cell Exocytic Response. Traffic 2016, 17, 1027–1041. [Google Scholar] [CrossRef]
- Südhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef] [Green Version]
- Jorgačevski, J.; Kreft, M.; Zorec, R. Exocytotic fusion pores as a target for therapy. Cell Calcium 2017, 66, 71–77. [Google Scholar] [CrossRef]
- Miklavc, P.; Hecht, E.; Hobi, N.; Wittekindt, O.H.; Dietl, P.; Kranz, C.; Frick, M. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion: A role for Rho, formins and myosin II. J. Cell Sci. 2012, 125, 2765–2774. [Google Scholar] [CrossRef] [Green Version]
- Miklavc, P.; Mair, N.; Wittekindt, O.H.; Haller, T.; Dietl, P.; Felder, E.; Timmler, M.; Frick, M. Fusion-activated Ca 2+ entry via vesicular P2X 4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 14503–14508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larina, O.; Bhat, P.; Pickett, J.A.; Launikonis, B.S.; Shah, A.; Kruger, W.A.; Edwardson, J.M.; Thorn, P. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol. Biol. Cell 2007, 18, 3502–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Peruchena, C.; Navas, S.; Montes, M.A.; Álvarez De Toledo, G. Fusion pore regulation of transmitter release. Brain Res. Rev. 2005, 49, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Ge, L.; Arpino, G.; Villarreal, S.A.; Hamid, E.; Liu, H.; Zhao, W.D.; Wen, P.J.; Chiang, H.C.; Wu, L.G. Visualization of Membrane Pore in Live Cells Reveals a Dynamic-Pore Theory Governing Fusion and Endocytosis. Cell 2018, 173, 934–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, T.; Dietl, P.; Pfaller, K.; Frick, M.; Mair, N.; Paulmichl, M.; Hess, M.W.; Fürst, J.; Maly, K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 2001, 155, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Doreian, B.W.; Fulop, T.G.; Smith, C.B. Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. J. Neurosci. 2008, 28, 4470–4478. [Google Scholar] [CrossRef] [Green Version]
- Ñeco, P.; Fernández-Peruchena, C.; Navas, S.; Gutiérrez, L.M.; Álvarez De Toledo, G.; Alés, E. Myosin II contributes to fusion pore expansion during exocytosis. J. Biol. Chem. 2008, 283, 10949–10957. [Google Scholar] [CrossRef] [Green Version]
- Bhat, P.; Thorn, P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol. Biol. Cell 2009, 20, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Anantharam, A.; Bittner, M.A.; Aikman, R.L.; Stuenkel, E.L.; Schmid, S.L.; Axelrod, D.; Holz, R.W. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol. Biol. Cell 2011, 22, 1907–1918. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Chakrabarti, S.; Andrews, N.W.; Simon, S.M. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004, 2, E233. [Google Scholar] [CrossRef]
- Turvey, M.R.; Thorn, P. Lysine-fixable dye tracing of exocytosis shows F-actin coating is a step that follows granule fusion in pancreatic acinar cells. Pflugers Arch. 2004, 448, 552–555. [Google Scholar] [CrossRef]
- Thorn, P. New insights into the control of secretion. Commun. Integr. Biol. 2009, 2, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bretou, M.; Jouannot, O.; Fanget, I.; Pierobon, P.; Larochette, N.; Gestraud, P.; Guillon, M.; Emiliani, V.; Gasman, S.; Desnos, C.; et al. Cdc42 controls the dilation of the exocytotic fusion pore by regulating membrane tension. Mol. Biol. Cell 2014, 25, 3195–3209. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, M.F.; Lucchesi, O.; Bustos, M.A.; Pocognoni, C.A.; De La Iglesia, P.X.; Tomes, C.N. The rab3A-22A chimera prevents sperm exocytosis by stabilizing open fusion pores. J. Biol. Chem. 2016, 291, 23101–23111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Südhof, T.C. THE SYNAPTIC VESICLE CYCLE. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [Green Version]
- Heuser, J.E.; Reese, T.S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 1973, 57, 315–344. [Google Scholar] [CrossRef]
- Sharma, S.; Lindau, M. The fusion pore, 60 years after the first cartoon. FEBS Lett. 2018, 592, 3542–3562. [Google Scholar] [CrossRef] [Green Version]
- Miklavc, P.; Albrecht, S.; Wittekindt, O.H.; Schullian, P.; Haller, T.; Dietl, P. Existence of exocytotic hemifusion intermediates with a lifetime of up to seconds in type II pneumocytes. Biochem. J. 2009, 424, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklavc, P.; Wittekindt, O.H.; Felder, E.; Dietl, P. Ca2+-dependent actin coating of lamellar bodies after exocytotic fusion: A prerequisite for content release or kiss-and-run. Ann. N. Y. Acad. Sci. 2009, 1152, 43–52. [Google Scholar] [CrossRef]
- Chiang, H.C.; Shin, W.; Zhao, W.D.; Hamid, E.; Sheng, J.; Baydyuk, M.; Wen, P.J.; Jin, A.; Momboisse, F.; Wu, L.G. Post-fusion structural changes and their roles in exocytosis and endocytosis of dense-core vesicles. Nat. Commun. 2014, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.J.; Grenklo, S.; Arpino, G.; Tan, X.; Liao, H.-S.; Heureaux, J.; Peng, S.-Y.; Chiang, H.-C.; Hamid, E.; Zhao, W.-D.; et al. Actin dynamics provides membrane tension to merge fusing vesicles into the plasma membrane. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gasman, S.; Chasserot-Golaz, S.; Malacombe, M.; Way, M.; Bader, M.F. Regulated Exocytosis in Neuroendocrine Cells: A Role for Subplasmalemmal Cdc42/N-WASP-induced Actin Filaments. Mol. Biol. Cell 2004, 15, 520–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berberian, K.; Torres, A.J.; Fang, Q.; Kisler, K.; Lindau, M. F-actin and myosin II accelerate catecholamine release from chromaffin granules. J. Neurosci. 2009, 29, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Segawa, A.; Yamashina, S. Roles of Microfilaments in Exocytosis: A New Hypothesis. Cell Struct. Funct. 1989, 14, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilibary, E.; Williams, M. Actin Structural in Peripheral Changes Rat Induced Lung: By S, Labeling Cytochalasin form accepted. J. Histochem. Cytochem. 1983, 31, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokac, A.M.; Co, C.; Taunton, J.; Bement, W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 2003, 5, 727–732. [Google Scholar] [CrossRef]
- Nemoto, T.; Kojima, T.; Oshima, A.; Bito, H.; Kasai, H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J. Biol. Chem. 2004, 279, 37544–37550. [Google Scholar] [CrossRef] [Green Version]
- Masedunskas, A.; Sramkova, M.; Parente, L.; Sales, K.U.; Amornphimoltham, P.; Bugge, T.H.; Weigert, R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 13552–13557. [Google Scholar] [CrossRef] [Green Version]
- Sokac, A.M.; Bement, W.M. Kiss-and-coat and compartment mixing: Coupling exocytosis to signal generation and local actin assembly. Mol. Biol. Cell 2006, 17, 1495–1502. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.Y.E.; Bement, W.M. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat. Cell Biol. 2007, 9, 149–159. [Google Scholar] [CrossRef]
- Rousso, T.; Schejter, E.D.; Shilo, B.Z. Orchestrated content release from Drosophila glue-protein vesicles by a contractile actomyosin network. Nat. Cell Biol. 2016, 18, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Shitara, A.; Malec, L.; Ebrahim, S.; Chen, D.; Bleck, C.; Hoffman, M.P.; Weigert, R. Cdc42 negatively regulates endocytosis during apical membrane maintenance in live animals. Mol. Biol. Cell 2019, 30, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, T.D.; Cutler, D.F.; Cramer, L.P. Actin coats and rings promote regulated exocytosis. Trends Cell Biol. 2012, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Otomo, T.; Tomchick, D.R.; Otomo, C.; Panchal, S.C.; Machius, M.; Rosen, M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 2005, 433, 488–494. [Google Scholar] [CrossRef]
- Romero, S.; Clainche, C.L.; Didry, D.; Egile, C.; Pantaloni, D.; Carlier, M.F. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 2004, 119, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Vavylonis, D.; Kovar, D.R.; O’Shaughnessy, B.; Pollard, T.D. Model of formin-associated actin filament elongation. Mol. Cell 2006, 21, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-Y.E.; Bement, W.M. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol. Biol. Cell 2007, 18, 4096–4105. [Google Scholar] [CrossRef]
- Nightingale, T.D.; McCormack, J.J.; Grimes, W.; Robinson, C.; da Silva, M.L.; White, I.J.; Vaughan, A.; Cramer, L.P.; Cutler, D.F. Tuning the endothelial response: Differential release of exocytic cargos from Weibel-Palade bodies. J. Thromb. Haemost. 2018, 16, 1873–1886. [Google Scholar] [CrossRef]
- Milberg, O.; Shitara, A.; Ebrahim, S.; Masedunskas, A.; Tora, M.; Tran, D.T.; Chen, Y.; Conti, M.A.; Adelstein, R.S.; Hagen, K.G.T.; et al. Concerted actions of distinct nonmuscle myosin II isoforms drive intracellular membrane remodeling in live animals. J. Cell Biol. 2017, 216, 1925–1936. [Google Scholar] [CrossRef] [Green Version]
- Miklavc, P.; Ehinger, K.; Sultan, A.; Felder, T.; Paul, P.; Gottschalk, K.-E.E.; Frick, M. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J. Cell Sci. 2015, 128, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.; Chen, D.; Weiss, M.; Malec, L.; Ng, Y.; Rebustini, I.; Krystofiak, E.; Hu, L.; Liu, J.; Masedunskas, A.; et al. Dynamic polyhedral actomyosin lattices remodel micron-scale curved membranes during exocytosis in live mice. Nat. Cell Biol. 2019, 21, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Liu, J.; Weigert, R. The Actomyosin Cytoskeleton Drives Micron-Scale Membrane Remodeling In Vivo Via the Generation of Mechanical Forces to Balance Membrane Tension Gradients. BioEssays 2018, 40, 1–7. [Google Scholar] [CrossRef]
- Sokac, A.M.; Schietroma, C.; Gundersen, C.B.; Bement, W.M. Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev. Cell 2006, 11, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giardini, P.A.; Fletcher, D.A.; Theriot, J.A. Compression forces generated by actin comet tails on lipid vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 6493–6498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.; Soekmadji, C.; Mitchell, J.M.; Thomas, W.G.; Thorn, P. Real-time measurement of f-actin remodelling during exocytosis using lifeact-EGFP transgenic animals. PLoS ONE 2012, 7, e39815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engisch, K.L.; Nowycky, M.C. Compensatory and excess retrieval: Two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J. Physiol. 1998, 506, 591–608. [Google Scholar] [CrossRef]
- Mooren, O.L.; Galletta, B.J.; Cooper, J.A. Roles for Actin Assembly in Endocytosis. Annu. Rev. Biochem. 2012, 81, 661–686. [Google Scholar] [CrossRef]
- Gómez-Elías, M.D.; Fissore, R.A.; Cuasnicú, P.S.; Cohen, D.J. Compensatory endocytosis occurs after cortical granule exocytosis in mouse eggs. J. Cell. Physiol. 2020, 235, 4351–4360. [Google Scholar] [CrossRef]
- Thorn, P.; Fogarty, K.E.; Parker, I. Zymogen granule exocytosis is characterized by long fusion pore openings and preservation of vesicle lipid identity. Proc. Natl. Acad. Sci. USA 2004, 101, 6774–6779. [Google Scholar] [CrossRef] [Green Version]
- Shitara, A.; Weigert, R. Imaging membrane remodeling during regulated exocytosis in live mice. Exp. Cell Res. 2015, 337, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Sramkova, M.; Masedunskas, A.; Weigert, R. Plasmid DNA is internalized from the apical plasma membrane of the salivary gland epithelium in live animals. Histochem. Cell Biol. 2012, 138, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, N.L.; White, I.J.; McCormack, J.J.; Robinson, C.; Cutler, D.F.; Nightingale, T.D. Clathrin-mediated post-fusion membrane retrieval influences the exocytic mode of endothelial Weibel-Palade bodies. J. Cell Sci. 2017, 130, 2591–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, N.; Haller, T.; Dietl, P. Exocytosis in alveolar type II cells revealed by cell capacitance and fluorescence measurements. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 276, L376–L382. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Leong, N.T.; Wong, T.; Drubin, D.G. A Pan1/End3/Sla1 complex links Arp2/3-mediated actin assembly to sites of clathrinmediated endocytosis. Mol. Biol. Cell 2015, 26, 3841–3856. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, L.; Frank, R.A.W.; García-Nafría, J.; Colussi, A.; Gunawardana, N.; Johnson, C.M.; Yu, M.; Howard, G.; Andrews, B.; Vallis, Y.; et al. A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits. Cell 2018, 174, 325–337. [Google Scholar] [CrossRef]
- Sun, Y.; Jaldin-Fincati, J.; Liu, Z.; Bilan, P.J.; Klip, A. A complex of Rab13 with MICAL-L2 and α-actinin-4 is essential for insulin-dependent GLUT4 exocytosis. Mol. Biol. Cell 2016, 27, 75–89. [Google Scholar] [CrossRef]
- Wang, Z.; Oh, E.; Thurmond, D.C. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J. Biol. Chem. 2007, 282, 9536–9546. [Google Scholar] [CrossRef] [Green Version]
- Megnagi, B.; Finkelstein, M.; Shabtay, O.; Breitbart, H. The role and importance of cofilin in human sperm capacitation and the acrosome reaction. Cell Tissue Res. 2015, 362, 665–675. [Google Scholar] [CrossRef]
- Gawden-Bone, C.M.; Frazer, G.L.; Richard, A.C.; Ma, C.Y.; Strege, K.; Griffiths, G.M. PIP5 Kinases Regulate Membrane Phosphoinositide and Actin Composition for Targeted Granule Secretion by Cytotoxic Lymphocytes. Immunity 2018, 49, 427–437.e4. [Google Scholar] [CrossRef] [Green Version]
- Reid, A.T.; Lord, T.; Stanger, S.J.; Roman, S.D.; McCluskey, A.; Robinson, P.J.; Aitken, R.J.; Nixon, B. Dynamin regulates specific membrane fusion events necessary for acrosomal exocytosis in mouse spermatozoa. J. Biol. Chem. 2012, 287, 37659–37672. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Anderson, A.L.; Turner, A.P.; De Iuliis, G.N.; McCluskey, A.; McLaughlin, E.A.; Nixon, B. Characterization of a novel role for the dynamin mechanoenzymes in the regulation of human sperm acrosomal exocytosis. Mol. Hum. Reprod. 2017, 23, 657–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Ji, C.; Wu, Y.; Ferguson, S.M.; Tamarina, N.; Philipson, L.H.; Lou, X. Dynamin 2 regulates biphasic insulin secretion and plasma glucose homeostasis. J. Clin. Investig. 2015, 125, 4026–4041. [Google Scholar] [CrossRef] [Green Version]
- Ye, D.; Wang, X.; Wei, C.; He, M.; Wang, H.; Wang, Y.; Zhu, Z.; Sun, Y. Marcksb plays a key role in the secretory pathway of zebrafish Bmp2b. PLoS Genet. 2019, 15, 1–28. [Google Scholar] [CrossRef]
- Satoh, K.; Narita, T.; Katsumata-Kato, O.; Sugiya, H.; Seo, Y. Involvement of myristoylated alanine-rich C kinase substrate phosphorylation and translocation in cholecystokinin-induced amylase release in rat pancreatic acini. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G399–G409. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.L.; Evans, R.D.; Sivarasa, K.; Ramalho, J.S.; Briggs, D.A.; Hume, A.N. The adaptor protein melanophilin regulates dynamic myosin-Va:cargo interaction and dendrite development in melanocytes. Mol. Biol. Cell 2019, 30, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Oberhofer, A.; Spieler, P.; Rosenfeld, Y.; Stepp, W.L.; Cleetus, A.; Hume, A.N.; Mueller-Planitz, F.; Ökten, Z. Myosin Va’s adaptor protein melanophilin enforces track selection on the microtubule and actin networks in vitro. Proc. Natl. Acad. Sci. USA 2017, 114, E4714–E4723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wei, G.; Cao, Y.; Deng, Q.; Han, X.; Huang, X.; Huo, Y.; He, Y.; Chen, L.; Luo, J. Myosin IIa is critical for cAMP-mediated endothelial secretion of von Willebrand factor. Blood 2018, 131, 686–698. [Google Scholar] [CrossRef] [Green Version]
- Vogel, G.F.; Klee, K.M.C.; Janecke, A.R.; Müller, T.; Hess, M.W.; Huber, L.A. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J. Cell Biol. 2015, 211, 587–604. [Google Scholar] [CrossRef]
- Ritter, A.T.; Kapnick, S.M.; Murugesan, S.; Schwartzberg, P.L.; Griffiths, G.M.; Lippincott-Schwartz, J. Cortical actin recovery at the immunological synapse leads to termination of lytic granule secretion in cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 2017, 114, E6585–E6594. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.H.; Cui, Q.; Zhang, T.; Wang, Z.B.; Ouyang, Y.C.; Shen, W.; Ma, J.Y.; Schatten, H.; Sun, Q.Y. Rab3A, Rab27A, and Rab35 regulate different events during mouse oocyte meiotic maturation and activation. Histochem. Cell Biol. 2016, 145, 647–657. [Google Scholar] [CrossRef]
- Quevedo, M.F.; Bustos, M.A.; Masone, D.; Roggero, C.M.; Bustos, D.M.; Tomes, C.N. Grab recruitment by Rab27A-Rabphilin3a triggers Rab3A activation in human sperm exocytosis. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 612–622. [Google Scholar] [CrossRef]
- Ramadass, M.; Johnson, J.L.; Catz, S.D. Rab27a regulates GM-CSF-dependent priming of neutrophil exocytosis. J. Leukoc. Biol. 2017, 101, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Taoka, M.; Isobe, T.; Izumi, T. Rab2a and Rab27a cooperatively regulate the transition from granule maturation to exocytosis through the dual effector Noc2. J. Cell Sci. 2017, 130, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Sheshachalam, A.; Baier, A.; Eitzen, G. The effect of Rho drugs on mast cell activation and degranulation. J. Leukoc. Biol. 2017, 102, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Mietkowska, M.; Schuberth, C.; Wedlich-Söldner, R.; Gerke, V. Actin dynamics during Ca2+ -dependent exocytosis of endothelial Weibel-Palade bodies. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1218–1229. [Google Scholar] [CrossRef]
- Bierings, R.; Hellen, N.; Kiskin, N.; Knipe, L.; Fonseca, A.V.; Patel, B.; Meli, A.; Rose, M.; Hannah, M.J.; Carter, T. The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood 2012, 120, 2757–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masedunskas, A.; Appaduray, M.A.; Lucas, C.A.; Cagigas, M.L.; Heydecker, M.; Holliday, M.; Meiring, J.C.M.; Hook, J.; Kee, A.; White, M.; et al. Parallel assembly of actin and tropomyosin, but not myosin II, during de novo actin filament formation in live mice. J. Cell Sci. 2018, 131, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pre-Fusion | Post-Fusion | ||||
|---|---|---|---|---|---|
| Trafficking | Cortical attachment/actin remodeling | Fusion pore dynamics | Actin coat formation, stabilization, contraction | Endocytosis | |
| α-Actinin | Muscle cells (R.n.) [136] | Endothelial cells (H.s.) [75] Alveolar type II cells (R.n.) [120] | |||
| Annexin | Alveolar type II cells (R.n.) [71] Endothelial cells (H.s.) [72,73] | ||||
| Arp2/3 | NK cells (H.s.) [46] | Salivary gland acinar cells (D.m.) [64] Beta cells (M.m.) [48] | |||
| Cdc42 | Beta cells (M.m.) [137] | Oocytes (X.l.) [106,110] | |||
| Clathrin | Beta cells (M.m.) [48] Endothelial cells (H.s.) [132] | ||||
| Cofilin | Sperm (H.s.) [138] | Alveolar type II cells (R.n.) [120] | |||
| DAG | CT lymphocytes (M.m.) [139] | Oocytes (X.l.) [110] | |||
| Dynamin | Sperm (M.m.; H.s.) [140,141] | Beta cells (M.m.) [48,142] Endothelial cells (H.s.) [132] | |||
| Formins | Epithelial cells (D.m.) [15] Pancreatic acinar cells (M.m.) [14] | Mast cells (M.m.) [45] | Beta cells (M.m.) [48] | Alveolar type II cells (R.n.) [80] Beta cells (M.m.) [48] Salivary gland acinar cells (D.m.) [111] | |
| MARCKS | Airway epithelial cells (M.m.) [60] Embryonic cells (D.r.) [143] Pancreatic acinar cells (R.n.) [144] | ||||
| Melanophilin | Melanocytes (M.m.) [29,145,146] | Melanocytes (M.m.) [29] | |||
| MLCK | Renin-secreting cells (M.m.) [51] | Alveolar type II cells (R.n.) [120] Salivary gland acinar cells (M.m.) [119] | |||
| MyoI | Fibroblasts (M.m.) [52] Alveolar type II cells (R.n.) [53] B lymphocytes (M.m.) [54] | Alveolar type II cells (R.n.) [53] Oocytes (X.l.) [123] Oocytes (X.l.) [117] | Oocytes (X.l.) [123] | ||
| MyoII | NK cells (H.s.) [46] Renin-secreting cells (M.m.) [51] Endothelial cells (H.s.) [147] | Pancreatic acinar cells (M.m.) [88] | Alveolar type II cells (R.n.) [120] Salivary gland acinar cells (M.m.; D.m.) [108,111] Oocytes (X.l.) [117] Lacrimal gland acinar cells (O.c.) [47] Endothelial cells (H.s.) [43,147] | ||
| MyoV | Endothelial cells (H.s.) [26] Melanocytes (M.m.) [29,145] Epithelial cells (H.s.) [148] Beta cells (M.m.) [66] | Endothelial cells (H.s.) [3] Beta cells (M.m.) [66] Mast cells (M.m.) [44] | |||
| MyoVII | Beta cells (M.m.) [68] | ||||
| MyRIP (Exophilin 8) | Endothelial cells (H.s.) [12,26] | Endothelial cells (H.s.) [12,26] Beta cells (M.m.) [68] | |||
| PIP2 | CT lymphocytes (M.m) [139,149] | Salivary gland acinar cells (D.m.) [64] | |||
| PKC | Oocytes (X.l.) [110] Endothelial cells (H.s.) [118] | ||||
| Rab3 | Sperm (H.s.) [28] Endothelial cells (H.s.) [72] Oocytes (M.m.) [150] | Epithelial cells (H.s.) [69] Sperm (H.s.) [151] | Sperm (H.s.) [94] | ||
| Rab11 | Epithelial cells (H.s.) [148] Oocytes (M.m.) [27] | Mast cells (M.m.) [77] | |||
| Rab27 | Endothelial cells (H.s.) [26] Oocytes (M.m.) [27] Sperm (H.s.) [28] Melanocytes (M.m.) [29,145] | Endothelial cells (H.s.) [26] Sperm (H.s.) [28,151] Oocytes (M.m.) [150] Melanocytes (M.m.) [29] CT lymphocytes (M.m.) [30,149] Mast cells (M.m.) [44] Neutrophils (M.m.) [152] Beta cells (M.m.) [153] | |||
| Rho | Mast cells (R.n.) [154] | Oocytes (X.l.) [117] Alveolar type II cells (R.n.) [80] Salivary gland acinar cells (D.m.) [111] Pancreatic acinar cells (M.m.) [107] Endothelial cells (H.s.) [155] | |||
| ROCK | Alveolar type II cells (R.n.) [120] | ||||
| SLP4 (Granuphilin) | Endothelial cells (H.s.) [156] Epithelial cells (H.s.) [69,148] | ||||
| Tropomyosin | Salivary gland acinar cells (M.m.) [157] | ||||
| UNC-45 | NK cells (H.s.) [50] | ||||
| WASP | Salivary gland (D.m.) [64] Oocytes (X.l.) [106] | ||||
| Zyxin | Endothelial cells (H.s.) [75] | Endothelial cells (H.s.) [75] | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklavc, P.; Frick, M. Actin and Myosin in Non-Neuronal Exocytosis. Cells 2020, 9, 1455. https://doi.org/10.3390/cells9061455
Miklavc P, Frick M. Actin and Myosin in Non-Neuronal Exocytosis. Cells. 2020; 9(6):1455. https://doi.org/10.3390/cells9061455
Chicago/Turabian StyleMiklavc, Pika, and Manfred Frick. 2020. "Actin and Myosin in Non-Neuronal Exocytosis" Cells 9, no. 6: 1455. https://doi.org/10.3390/cells9061455
APA StyleMiklavc, P., & Frick, M. (2020). Actin and Myosin in Non-Neuronal Exocytosis. Cells, 9(6), 1455. https://doi.org/10.3390/cells9061455





