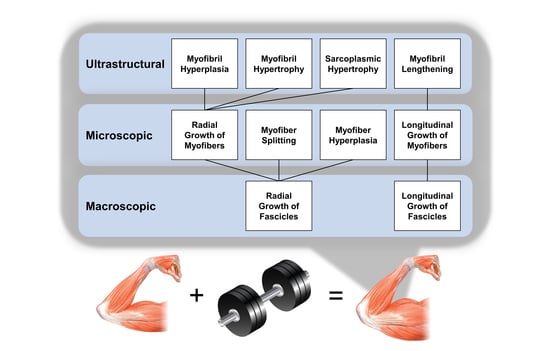
Identifying the Structural Adaptations that Drive the Mechanical Load-Induced Growth of Skeletal Muscle: A Scoping Review
Abstract
1. Introduction
2. Overview of Skeletal Muscle Structure
3. Mechanical Load-Induced Growth of Skeletal Muscle at the Macroscopic Level
3.1. Whole Muscle
3.2. Muscle Fascicles
4. Mechanical Load-Induced Growth of Skeletal Muscle at the Microscopic Level
4.1. Longitudinal Growth of Fascicles
4.2. Radial Growth of Fascicles
4.2.1. Myofiber Hypertrophy
4.2.2. Myofiber Splitting
4.2.3. Hyperplasia
5. Mechanical Load-Induced Growth of Skeletal Muscle at the Ultrastructural Level
5.1. Longitudinal Growth of Myofibers
5.2. Radial Growth of Myofibers
5.2.1. Sarcoplasmic Hypertrophy
5.2.2. Expansion of the Force-Generating Elements
5.2.3. Myofibril Hypertrophy
5.2.4. Myofibril Hyperplasia
5.2.5. The Radial Growth of Myofibers—Closing Remarks
6. Take Home Messages
Author Contributions
Funding
Conflicts of Interest
References
- McLeod, M.; Breen, L.; Hamilton, D.L.; Philp, A. Live strong and prosper: The importance of skeletal muscle strength for healthy ageing. Biogerontology 2016, 17, 497–510. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited Review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; A quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.-Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, J.; Liu, A.; Cui, Y.; Ma, X. The association between sarcopenia and fracture in middle-aged and elderly people: A systematic review and meta-analysis of cohort studies. Injury 2020, 51, 804–811. [Google Scholar] [CrossRef]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The Healthcare Costs of Sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- UN. World Population Prospects 2019: Highlights; ST/ESA/SER.A/423; Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Todd, J. From Milo to Milo: A History of Barbells, Dumbells, and Indian Clubs. Iron Game History 1995, 3, 4–16. [Google Scholar]
- Abe, T.; Kojima, K.; Kearns, C.F.; Yohena, H.; Fukuda, J. Whole body muscle hypertrophy from resistance training: Distribution and total mass. Br. J. Sports Med. 2003, 37, 543–545. [Google Scholar] [CrossRef]
- Trappe, T.A.; Carroll, C.C.; Dickinson, J.M.; LeMoine, J.K.; Haus, J.M.; Sullivan, B.E.; Lee, J.D.; Jemiolo, B.; Weinheimer, E.M.; Hollon, C.J. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R655–R662. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Atherton, P.J.; Reeves, N.D.; Fluck, M.; Williams, J.; Mitchell, W.K.; Selby, A.; Beltran Valls, R.M.; Narici, M.V. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol. 2014, 210, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Mandić, M.; Rullman, E.; Widholm, P.; Lilja, M.; Dahlqvist Leinhard, O.; Gustafsson, T.; Lundberg, T.R. Automated assessment of regional muscle volume and hypertrophy using MRI. Sci. Rep. 2020, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Churchward-Venne, T.A.; Parise, G.; Bellamy, L.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS ONE 2014, 9, e89431. [Google Scholar] [CrossRef]
- Aagaard, P.; Andersen, J.L.; Dyhre-Poulsen, P.; Leffers, A.M.; Wagner, A.; Magnusson, S.P.; Halkjaer-Kristensen, J.; Simonsen, E.B. A mechanism for increased contractile strength of human pennate muscle in response to strength training: Changes in muscle architecture. J. Physiol. 2001, 534, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J. Appl. Physiol. 2012, 113, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, T.A., Jr.; Farrar, R.P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef]
- Wong, T.S.; Booth, F.W. Skeletal muscle enlargement with weight-lifting exercise by rats. J. Appl. Physiol. 1988, 65, 950–954. [Google Scholar] [CrossRef]
- Ogasawara, R.; Fujita, S.; Hornberger, T.A.; Kitaoka, Y.; Makanae, Y.; Nakazato, K.; Naokata, I. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci. Rep. 2016, 6, 31142. [Google Scholar] [CrossRef]
- Lowe, D.A.; Alway, S.E. Animal models for inducing muscle hypertrophy: Are they relevant for clinical applications in humans? J. Orthop. Sports Phys. Ther. 2002, 32, 36–43. [Google Scholar] [CrossRef]
- Goodman, C.A.; Frey, J.W.; Mabrey, D.M.; Jacobs, B.L.; Lincoln, H.C.; You, J.S.; Hornberger, T.A. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J. Physiol. 2011, 589, 5485–5501. [Google Scholar] [CrossRef]
- Holly, R.G.; Barnett, J.G.; Ashmore, C.R.; Taylor, R.G.; Mole, P.A. Stretch-induced growth in chicken wing muscles: A new model of stretch hypertrophy. Am. J. Physiol. 1980, 238, C62–C71. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Lieber, R.L. Skeletal muscle adaptability. I: Review of basic properties. Dev. Med. Child. Neurol. 1986, 28, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.A.; Gomez, C.G.; Novak, S.M.; Mi-Mi, L.; Gregorio, C.C. Overview of the Muscle Cytoskeleton. Comprehensive Physiol. 2017, 7, 891–944. [Google Scholar] [CrossRef]
- Narici, M.; Franchi, M.; Maganaris, C. Muscle structural assembly and functional consequences. J. Exp. Biol. 2016, 219, 276–284. [Google Scholar] [CrossRef]
- Betts, G.J.; Kelly, A.Y.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H.; Korol, O.; Johnson, J.E.; Womble, M.; DeSaix, P. Anatomy and Physiology; OpenStax: Houston, TX, USA, 2013. [Google Scholar]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef]
- Tedesco, F.S.; Moyle, L.A.; Perdiguero, E. Muscle Interstitial Cells: A Brief Field Guide to Non-satellite Cell Populations in Skeletal Muscle. Methods Mol. Biol. 2017, 1556, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.A.; Fry, C.S.; Kirby, T.J.; Jackson, J.R.; Lee, J.D.; White, S.H.; Dupont-Versteegden, E.E.; McCarthy, J.J.; Peterson, C.A. Starring or Supporting Role? Satellite Cells and Skeletal Muscle Fiber Size Regulation. Physiology 2018, 33, 26–38. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Sale, D.G.; Elder, G.C.; Sutton, J.R. Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 48, 117–126. [Google Scholar] [CrossRef]
- Seiden, D. Quantitative analysis of muscle cell changes in compensatory hypertrophy and work-induced hypertrophy. Am. J. Anat. 1976, 145, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Luthi, J.M.; Howald, H.; Claassen, H.; Rosler, K.; Vock, P.; Hoppeler, H. Structural changes in skeletal muscle tissue with heavy-resistance exercise. Int. J. Sports Med. 1986, 7, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, G.; Howells, K.F. Work-induced hypertrophy in exercised normal muscles of different ages and the reversibility of hypertrophy after cessation of exercise. J. Physiol. 1974, 239, 179–193. [Google Scholar] [CrossRef]
- Toth, M.J.; Miller, M.S.; VanBuren, P.; Bedrin, N.G.; LeWinter, M.M.; Ades, P.A.; Palmer, B.M. Resistance training alters skeletal muscle structure and function in human heart failure: Effects at the tissue, cellular and molecular levels. J. Physiol. 2012, 590, 1243–1259. [Google Scholar] [CrossRef]
- Lavorato, M.; Loro, E.; Debattisti, V.; Khurana, T.S.; Franzini-Armstrong, C. Elongated mitochondrial constrictions and fission in muscle fatigue. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, R.T. Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 389–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef]
- Billeter, R.; Hoppeler, H. Muscular Basis of Strength. In Strength and Power in Sport; Blackwell Science Ltd.: Oxford, UK, 2003. [Google Scholar]
- Edman, K.A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J. Physiol. 1979, 291, 143–159. [Google Scholar] [CrossRef]
- Gordon, A.M.; Huxley, A.F.; Julian, F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966, 184, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, T.J.; Lieber, R.L. Sarcomere length operating range of vertebrate muscles during movement. J. Exp. Biol. 2001, 204, 1529–1536. [Google Scholar]
- Fry, A.C. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004, 34, 663–679. [Google Scholar] [CrossRef]
- Scott, W.; Stevens, J.; Binder–Macleod, S.A. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001, 81, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Hilber, K.; Galler, S.; Gohlsch, B.; Pette, D. Kinetic properties of myosin heavy chain isoforms in single fibers from human skeletal muscle. FEBS Lett. 1999, 455, 267–270. [Google Scholar] [CrossRef]
- Smerdu, V.; Karsch-Mizrachi, I.; Campione, M.; Leinwand, L.; Schiaffino, S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am. J. Physiol. Cell Physiol. 1994, 267, C1723–C1728. [Google Scholar] [CrossRef]
- Ennion, S.; Sant’ana Pereira, J.; Sargeant, A.J.; Young, A.; Goldspink, G. Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. J. Muscle Res. Cell Motil. 1995, 16, 35–43. [Google Scholar] [CrossRef]
- Pette, D.; Peuker, H.; Staron, R.S. The impact of biochemical methods for single muscle fibre analysis. Acta Physiol. Scand. 1999, 166, 261–277. [Google Scholar] [CrossRef]
- Goldspink, G. Increase in Length of Skeletal Muscle during Normal Growth. Nature 1964, 204, 1095–1096. [Google Scholar] [CrossRef]
- Williams, P.E.; Goldspink, G. Longitudinal growth of striated muscle fibres. J. Cell Sci. 1971, 9, 751–767. [Google Scholar] [PubMed]
- Beaucage, K.L.; Pollmann, S.I.; Sims, S.M.; Dixon, S.J.; Holdsworth, D.W. Quantitative in vivo micro-computed tomography for assessment of age-dependent changes in murine whole-body composition. Bone Rep. 2016, 5, 70–80. [Google Scholar] [CrossRef]
- Zöllner, A.M.; Abilez, O.J.; Böl, M.; Kuhl, E. Stretching skeletal muscle: Chronic muscle lengthening through sarcomerogenesis. PLoS ONE 2012, 7, e45661. [Google Scholar] [CrossRef]
- Aoki, M.S.; Soares, A.G.; Miyabara, E.H.; Baptista, I.L.; Moriscot, A.S. Expression of genes related to myostatin signaling during rat skeletal muscle longitudinal growth. Muscle Nerve 2009, 40, 992–999. [Google Scholar] [CrossRef]
- Simpson, A.H.; Williams, P.E.; Kyberd, P.; Goldspink, G.; Kenwright, J. The response of muscle to leg lengthening. J. Bone Joint Surg. Br. 1995, 77, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Caiozzo, V.J.; Utkan, A.; Chou, R.; Khalafi, A.; Chandra, H.; Baker, M.; Rourke, B.; Adams, G.; Baldwin, K.; Green, S. Effects of distraction on muscle length: Mechanisms involved in sarcomerogenesis. Clin. Orthop. Relat. Res. 2002, S133–S145. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.R.; Edgerton, V.R. Response of mouse plantaris muscle to functional overload: Comparison with rat and cat. Comp. Biochem. Physiol. Physiol. 1995, 111, 569–575. [Google Scholar] [CrossRef]
- Terena, S.M.; Fernandes, K.P.; Bussadori, S.K.; Deana, A.M.; Mesquita-Ferrari, R.A. Systematic review of the synergist muscle ablation model for compensatory hypertrophy. Rev. Assoc. Med. Bras. (1992) 2017, 63, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Abe, T.; Kuno, S.Y.; Fukunaga, T. Training-induced changes in muscle architecture and specific tension. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 72, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.J.; Fleckenstein, J.; Stray-Gundersen, J.; Alway, S.E.; Peshock, R.; Gonyea, W.J. Adaptations in the elbow flexors of elderly males after heavy-resistance training. J. Appl. Physiol. (1985) 1993, 74, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Welle, S.; Totterman, S.; Thornton, C. Effect of age on muscle hypertrophy induced by resistance training. J. Gerontol. Biol. Sci. Med. Sci. 1996, 51, M270–M275. [Google Scholar] [CrossRef]
- Angleri, V.; Ugrinowitsch, C.; Libardi, C.A. Crescent pyramid and drop-set systems do not promote greater strength gains, muscle hypertrophy, and changes on muscle architecture compared with traditional resistance training in well-trained men. Eur. J. Appl. Physiol. 2017, 117, 359–369. [Google Scholar] [CrossRef]
- Damas, F.; Libardi, C.A.; Ugrinowitsch, C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Eur. J. Appl. Physiol. 2018, 118, 485–500. [Google Scholar] [CrossRef]
- Housh, D.J.; Housh, T.J.; Johnson, G.O.; Chu, W.K. Hypertrophic response to unilateral concentric isokinetic resistance training. J. Appl. Physiol. (1985) 1992, 73, 65–70. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G.E.; Morse, C.I.; Burden, A.; Winwood, K.; Onambele, G.L. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J. Strength Cond. Res. 2014, 28, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.M.; Joanisse, S.; Grubb, A.; Mitchell, C.J.; McKay, B.R.; Phillips, S.M.; Baker, S.; Parise, G. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS ONE 2014, 9, e109739. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.J.; Cannavan, D.; Coleman, D.R.; Horne, S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J. Appl. Physiol. (1985) 2007, 103, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Hoppeler, H.; Kayser, B.; Landoni, L.; Claassen, H.; Gavardi, C.; Conti, M.; Cerretelli, P. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol. Scand. 1996, 157, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A.; Katsuta, S. Are region-specific changes in fibre types attributable to nonuniform muscle hypertrophy by overloading? Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 499–504. [Google Scholar] [CrossRef]
- Gardiner, P.F.; Jasmin, B.J.; Corriveau, P. Rostrocaudal pattern of fiber-type changes in an overloaded rat ankle extensor. J. Appl. Physiol. (1985) 1991, 71, 558–564. [Google Scholar] [CrossRef]
- Antonio, J. Nonuniform Response of Skeletal Muscle to Heavy Resistance Training: Can Bodybuilders Induce Regional Muscle Hypertrophy? J. Strength Cond. Res. 2000, 14, 102–113. [Google Scholar] [CrossRef]
- Alway, S.E.; Winchester, P.K.; Davis, M.E.; Gonyea, W.J. Regionalized adaptations and muscle fiber proliferation in stretch-induced enlargement. J. Appl. Physiol. (1985) 1989, 66, 771–781. [Google Scholar] [CrossRef]
- Counts, B.R.; Buckner, S.L.; Mouser, J.G.; Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Loenneke, J.P. Muscle growth: To infinity and beyond? Muscle Nerve 2017, 56, 1022–1030. [Google Scholar] [CrossRef]
- Damas, F.; Phillips, S.M.; Lixandrao, M.E.; Vechin, F.C.; Libardi, C.A.; Roschel, H.; Tricoli, V.; Ugrinowitsch, C. Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur. J. Appl. Physiol. 2016, 116, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.C.; Faulkner, J.A.; Hyatt, G.J. Estimation of number of fibers in guinea pig skeletal muscles. J. Appl. Physiol. 1974, 37, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, K.W.; Hornberger, T.A. The Overlooked Role of Fiber Length in Mechanical Load-Induced Growth of Skeletal Muscle. Exerc. Sport Sci. Rev. 2019, 47, 258–259. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. Muscle-tendon structure and dimensions in adults and children. J. Anat. 2010, 216, 631–642. [Google Scholar] [CrossRef]
- Timmins, R.G.; Ruddy, J.D.; Presland, J.; Maniar, N.; Shield, A.J.; Williams, M.D.; Opar, D.A. Architectural Changes of the Biceps Femoris Long Head after Concentric or Eccentric Training. Med. Sci. Sports Exerc. 2016, 48, 499–508. [Google Scholar] [CrossRef]
- Sharifnezhad, A.; Marzilger, R.; Arampatzis, A. Effects of load magnitude, muscle length and velocity during eccentric chronic loading on the longitudinal growth of the vastus lateralis muscle. J. Exp. Biol. 2014, 217, 2726–2733. [Google Scholar] [CrossRef]
- Reeves, N.D.; Maganaris, C.N.; Longo, S.; Narici, M.V. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp. Physiol. 2009, 94, 825–833. [Google Scholar] [CrossRef]
- Baroni, B.M.; Geremia, J.M.; Rodrigues, R.; De Azevedo Franke, R.; Karamanidis, K.; Vaz, M.A. Muscle architecture adaptations to knee extensor eccentric training: Rectus femoris vs. vastus lateralis. Muscle Nerve 2013, 48, 498–506. [Google Scholar] [CrossRef]
- Ullrich, B.; Holzinger, S.; Soleimani, M.; Pelzer, T.; Stening, J.; Pfeiffer, M. Neuromuscular Responses to 14 Weeks of Traditional and Daily Undulating Resistance Training. Int. J. Sports Med. 2015, 36, 554–562. [Google Scholar] [CrossRef]
- Ema, R.; Wakahara, T.; Miyamoto, N.; Kanehisa, H.; Kawakami, Y. Inhomogeneous architectural changes of the quadriceps femoris induced by resistance training. Eur. J. Appl. Physiol. 2013, 113, 2691–2703. [Google Scholar] [CrossRef]
- Erskine, R.M.; Jones, D.A.; Williams, A.G.; Stewart, C.E.; Degens, H. Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur. J. Appl. Physiol. 2010, 110, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Alegre, L.M.; Ferri-Morales, A.; Rodriguez-Casares, R.; Aguado, X. Effects of isometric training on the knee extensor moment-angle relationship and vastus lateralis muscle architecture. Eur. J. Appl. Physiol. 2014, 114, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Ema, R.; Akagi, R.; Wakahara, T.; Kawakami, Y. Training-induced changes in architecture of human skeletal muscles: Current evidence and unresolved issues. J. Phys. Fitness Sports Med. 2016, 5, 37–46. [Google Scholar] [CrossRef]
- Franchi, M.V.; Atherton, P.J.; Maganaris, C.N.; Narici, M.V. Fascicle length does increase in response to longitudinal resistance training and in a contraction-mode specific manner. Springerplus 2016, 5, 94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Timmins, R.G.; Shield, A.J.; Williams, M.D.; Lorenzen, C.; Opar, D.A. Architectural adaptations of muscle to training and injury: A narrative review outlining the contributions by fascicle length, pennation angle and muscle thickness. Br. J. Sports Med. 2016, 50, 1467–1472. [Google Scholar] [CrossRef]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 2017, 8, 447. [Google Scholar] [CrossRef]
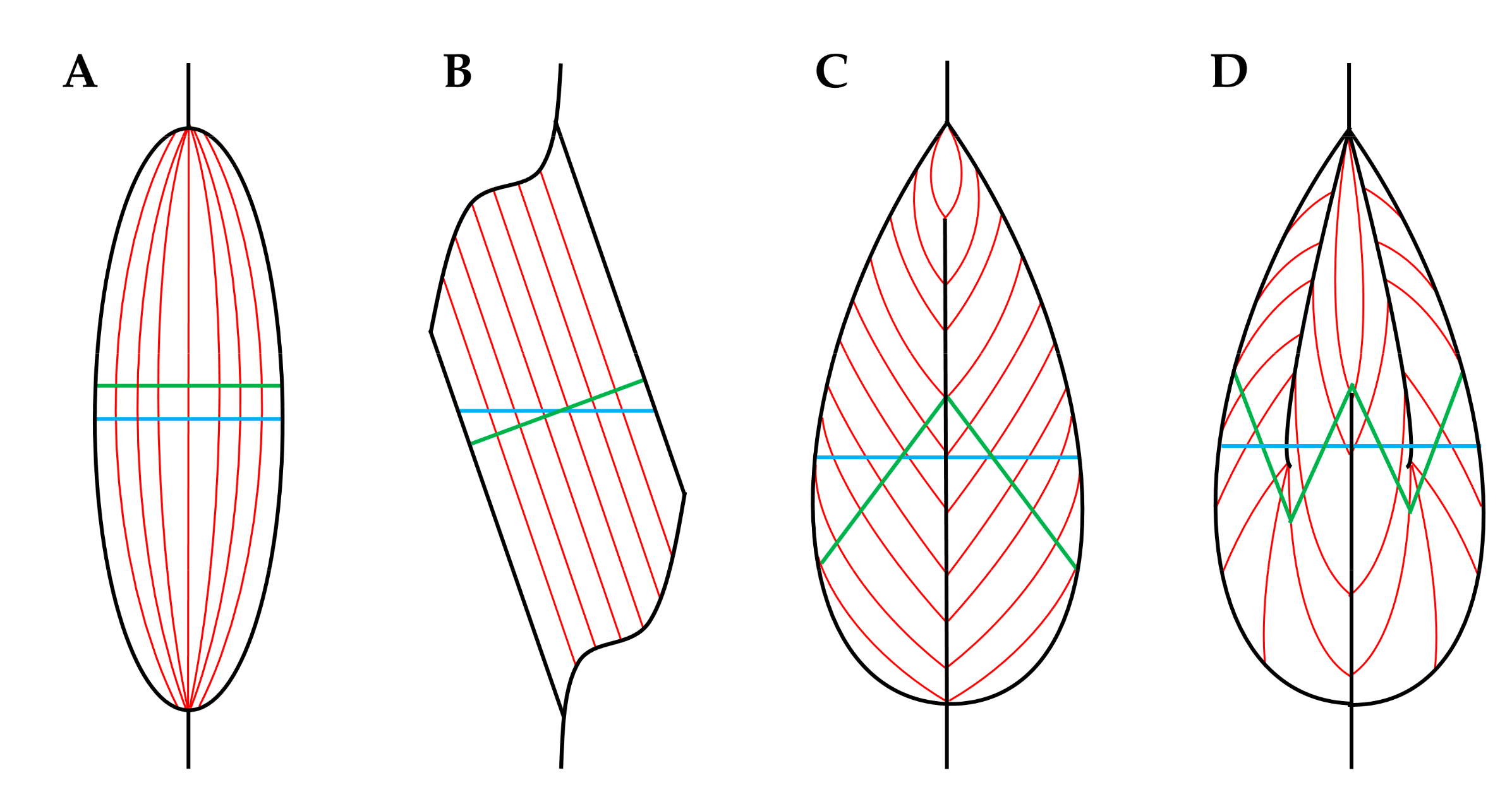
- Young, M.; Paul, A.; Rodda, J.; Duxson, M.; Sheard, P. Examination of intrafascicular muscle fiber terminations: Implications for tension delivery in series-fibered muscles. J. Morphol. 2000, 245, 130–145. [Google Scholar] [CrossRef]
- Paul, A.C.; Rosenthal, N. Different modes of hypertrophy in skeletal muscle fibers. J. Cell Biol. 2002, 156, 751–760. [Google Scholar] [CrossRef]
- Swatland, H.J.; Cassens, R.G. Muscle growth: The problem of muscle fibers with an intrafascicular termination. J. Anim. Sci. 1972, 35, 336–344. [Google Scholar] [CrossRef]
- Alway, S.E.; Gonyea, W.J.; Davis, M.E. Muscle fiber formation and fiber hypertrophy during the onset of stretch-overload. Am. J. Physiol. 1990, 259, C92–C102. [Google Scholar] [CrossRef]
- Roy, R.R.; Meadows, I.D.; Baldwin, K.M.; Edgerton, V.R. Functional significance of compensatory overloaded rat fast muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, C.A.; Makarov, M.R.; Shoemaker, S.; Birch, J.G.; Buschang, P.H.; Cherkashin, A.M.; Welch, R.D.; Samchukov, M.L. The effect of the amount of limb lengthening on skeletal muscle. Clin. Orthop. Relat. Res. 2002. [CrossRef] [PubMed]
- Butterfield, T.A.; Leonard, T.R.; Herzog, W. Differential serial sarcomere number adaptations in knee extensor muscles of rats is contraction type dependent. J. Appl. Physiol. (1985) 2005, 99, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Lynn, R.; Morgan, D.L. Decline running produces more sarcomeres in rat vastus intermedius muscle fibers than does incline running. J. Appl. Physiol. (1985) 1994, 77, 1439–1444. [Google Scholar] [CrossRef]
- You, J.S.; McNally, R.M.; Jacobs, B.L.; Privett, R.E.; Gundermann, D.M.; Lin, K.H.; Steinert, N.D.; Goodman, C.A.; Hornberger, T.A. The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J. 2019, 33, 4021–4034. [Google Scholar] [CrossRef]
- Goh, Q.; Song, T.; Petrany, M.J.; Cramer, A.A.; Sun, C.; Sadayappan, S.; Lee, S.J.; Millay, D.P. Myonuclear accretion is a determinant of exercise-induced remodeling in skeletal muscle. Elife 2019, 8. [Google Scholar] [CrossRef]
- Morpurgo, B. Eine experimentelle Studie. Arch. Pathol. Anat. Physiol. Klin. Med. 1897, 150, 522–554. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Roberts, B.M.; Vigotsky, A.D.; Schoenfeld, B.J.; Roberts, M.D. A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So Does the Measurement. Front. Physiol. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Gonyea, W.J.; Ericson, G.C. An experimental model for the study of exercise-induced skeletal muscle hypertrophy. J. Appl. Physiol. 1976, 40, 630–633. [Google Scholar] [CrossRef]
- Goldspink, G. The Combined Effects of Exercise and Reduced Food Intake on Skeletal Muscle Fibers. J. Cell Comp. Physiol 1964, 63, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Pierobon Bormioli, S.; Aloisi, M. Cell proliferation in rat skeletal muscle during early stages of compensatory hypertrophy. Virchows Archiv B 1972, 11, 268–273. [Google Scholar] [CrossRef]
- Sola, O.M.; Christensen, D.L.; Martin, A.W. Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp. Neurol. 1973, 41, 76–100. [Google Scholar] [CrossRef]
- Conceicao, M.S.; Vechin, F.C.; Lixandrao, M.; Damas, F.; Libardi, C.A.; Tricoli, V.; Roschel, H.; Camera, D.; Ugrinowitsch, C. Muscle Fiber Hypertrophy and Myonuclei Addition: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2018, 50, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Kosek, D.J.; Kim, J.S.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J. Appl. Physiol. (1985) 2006, 101, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.E.; Luecke, T.J.; Wendeln, H.K.; Toma, K.; Hagerman, F.C.; Murray, T.F.; Ragg, K.E.; Ratamess, N.A.; Kraemer, W.J.; Staron, R.S. Muscular adaptations in response to three different resistance-training regimens: Specificity of repetition maximum training zones. Eur. J. Appl. Physiol. 2002, 88, 50–60. [Google Scholar] [CrossRef]
- Antonio, J.; Gonyea, W.J. Progressive stretch overload of skeletal muscle results in hypertrophy before hyperplasia. J. Appl. Physiol. (1985) 1993, 75, 1263–1271. [Google Scholar] [CrossRef]
- Murach, K.A.; Dungan, C.M.; Peterson, C.A.; McCarthy, J.J. Muscle Fiber Splitting Is a Physiological Response to Extreme Loading in Animals. Exerc. Sport Sci. Rev. 2019, 47, 108–115. [Google Scholar] [CrossRef]
- Schwartz, M.S.; Sargeant, M.; Swash, M. Longitudinal fibre splitting in neurogenic muscular disorders--its relation to the pathogenesis of “myopathic” change. Brain 1976, 99, 617–636. [Google Scholar] [CrossRef]
- Pichavant, C.; Pavlath, G.K. Incidence and severity of myofiber branching with regeneration and aging. Skeletal Muscle 2014, 4, 9. [Google Scholar] [CrossRef]
- Antonio, J.; Gonyea, W.J. Muscle fiber splitting in stretch-enlarged avian muscle. Med. Sci. Sports Exerc. 1994, 26, 973–977. [Google Scholar] [CrossRef]
- Tamaki, T.; Akatsuka, A.; Tokunaga, M.; Uchiyama, S.; Shiraishi, T. Characteristics of compensatory hypertrophied muscle in the rat: I. Electron microscopic and immunohistochemical studies. Anat. Rec. 1996, 246, 325–334. [Google Scholar] [CrossRef]
- Eriksson, A.; Lindström, M.; Carlsson, L.; Thornell, L.-E. Hypertrophic muscle fibers with fissures in power-lifters; fiber splitting or defect regeneration? Histochem. Cell Biol. 2006, 126, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.W.; Roy, R.R.; Tweedle, C.D.; Heusner, W.W.; Van Huss, W.D.; Carrow, R.E. Skeletal muscle fiber splitting with weight-lifting exercise in rats. Am. J. Anat. 1980, 157, 433–440. [Google Scholar] [CrossRef]
- Gonyea, W.; Ericson, G.C.; Bonde-Petersen, F. Skeletal muscle fiber splitting induced by weight-lifting exercise in cats. Acta Physiol. Scand. 1977, 99, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, H.S.; Goldspink, G. Fibre number and fibre size in a surgically overloaded muscle. J. Anat. 1979, 129, 293–303. [Google Scholar] [PubMed]
- Gollnick, P.D.; Timson, B.F.; Moore, R.L.; Riedy, M. Muscular enlargement and number of fibers in skeletal muscles of rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 50, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, P.D.; Parsons, D.; Riedy, M.; Moore, R.L. Fiber number and size in overloaded chicken anterior latissimus dorsi muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 54, 1292–1297. [Google Scholar] [CrossRef]
- Timson, B.F.; Bowlin, B.K.; Dudenhoeffer, G.A.; George, J.B. Fiber number, area, and composition of mouse soleus muscle following enlargement. J. Appl. Physiol. 1985, 58, 619–624. [Google Scholar] [CrossRef]
- Gonyea, W.J.; Sale, D.G.; Gonyea, F.B.; Mikesky, A. Exercise induced increases in muscle fiber number. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 137–141. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Chen, Y.; Nie, Y.; Huang, H.; Chen, H.; Mo, D. Not all the number of skeletal muscle fibers is determined prenatally. BMC Dev. Biol. 2015, 15, 42. [Google Scholar] [CrossRef]
- Timson, B.F.; Dudenhoeffer, G.A. Skeletal muscle fibre number in the rat from youth to adulthood. J. Anat. 1990, 173, 33–36. [Google Scholar] [PubMed]
- Rehfeldt, C.; Stickland, N.C.; Fiedler, I.; Wegner, J. Environmental and Genetic Factors as Sources of Variation in Skeletal Muscle Fibre Number. Basic Appl. Myol. 1999, 9, 235–253. [Google Scholar]
- Kelley, G. Mechanical overload and skeletal muscle fiber hyperplasia: A meta-analysis. J. Appl. Physiol. 1996, 81, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.A.; Wilkinson, J.G. Exercise-induced skeletal muscle growth. Hypertrophy or hyperplasia? Sports Med. 1986, 3, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Gonyea, W.J. Role of exercise in inducing increases in skeletal muscle fiber number. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 421–426. [Google Scholar] [CrossRef]
- Alway, S.E. Stretch induces non-uniform isomyosin expression in the quail anterior latissimus dorsi muscle. Anat. Rec. 1993, 237, 1–7. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Esposito, F.; Marchionni, C.; di Tullio, S.; Belia, S.; Fulle, S.; Veicsteinas, A.; Marini, M. Moderate exercise training induces ROS-related adaptations to skeletal muscles. Int. J. Sports Med. 2013, 34, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E. Perpetuation of muscle fibers after removal of stretch in the Japanese quail. Am. J. Physiol. 1991, 260, C400–C408. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Gonyea, W.J. Skeletal muscle fiber hyperplasia. Med. Sci. Sports Exerc. 1993, 25, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Incledon, T. Skeletal Muscle Fiber Hyperplasia: Why It Can or Cannot Occur in Humans. Strength Cond. J. 2000, 22, 28. [Google Scholar] [CrossRef]
- Tamaki, T.; Uchiyama, S.; Nakano, S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med. Sci. Sports Exerc. 1992, 24, 881–886. [Google Scholar] [CrossRef]
- Williams, P.E.; Goldspink, G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J. Anat. 1973, 116, 45–55. [Google Scholar] [PubMed]
- Barnett, J.G.; Holly, R.G.; Ashmore, C.R. Stretch-induced growth in chicken wing muscles: Biochemical and morphological characterization. Am. J. Physiol. 1980, 239, C39–C46. [Google Scholar] [CrossRef]
- Koh, T.J. Do adaptations in serial sarcomere number occur with strength training? Hum. Mov. Sci. 1995, 14, 61–77. [Google Scholar] [CrossRef]
- Matano, T.; Tamai, K.; Kurokawa, T. Adaptation of skeletal muscle in limb lengthening: A light diffraction study on the sarcomere length in situ. J. Orthop. Res. 1994, 12, 193–196. [Google Scholar] [CrossRef]
- Kinney, M.C.; Dayanidhi, S.; Dykstra, P.B.; McCarthy, J.J.; Peterson, C.A.; Lieber, R.L. Reduced skeletal muscle satellite cell number alters muscle morphology after chronic stretch but allows limited serial sarcomere addition. Muscle Nerve 2017, 55, 384–392. [Google Scholar] [CrossRef]
- Goldspink, G. Alterations in myofibril size and structure during growth, exercise and change in environmental temperature. In Handbook of Physiology; American Physiological Society, Bethesda: Rockville, Maryland, USA, 1983; Volume 10, pp. 539–554. [Google Scholar]
- Griffin, G.E.; Williams, P.E.; Goldspink, G. Region of longitudinal growth in striated muscle fibres. Nat. New Biol. 1971, 232, 28–29. [Google Scholar] [CrossRef]
- Burkholder, T.J.; Fingado, B.; Baron, S.; Lieber, R.L. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J. Morphol. 1994, 221, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.S.; Charlton, M.P. Transverse sarcomere splitting. A possible means of longitudinal growth in crab muscles. J. Cell Biol. 1979, 80, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Crawford, G.N. An experimental study of muscle growth in the rabbit. J. Bone Jt. Surg. Br. 1954, 36-B, 294–303. [Google Scholar] [CrossRef]
- Mackay, B.; Harrop, T.J. An experimental study of the longitudinal growth of skeletal muscle in the rat. Acta Anat. 1969, 72, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Fernandez, H.; Lavidis, N.A. Development of the mature distribution of synapses on fibres in the frog sartorius muscle. J. Neurocytol. 1985, 14, 981–995. [Google Scholar] [CrossRef] [PubMed]
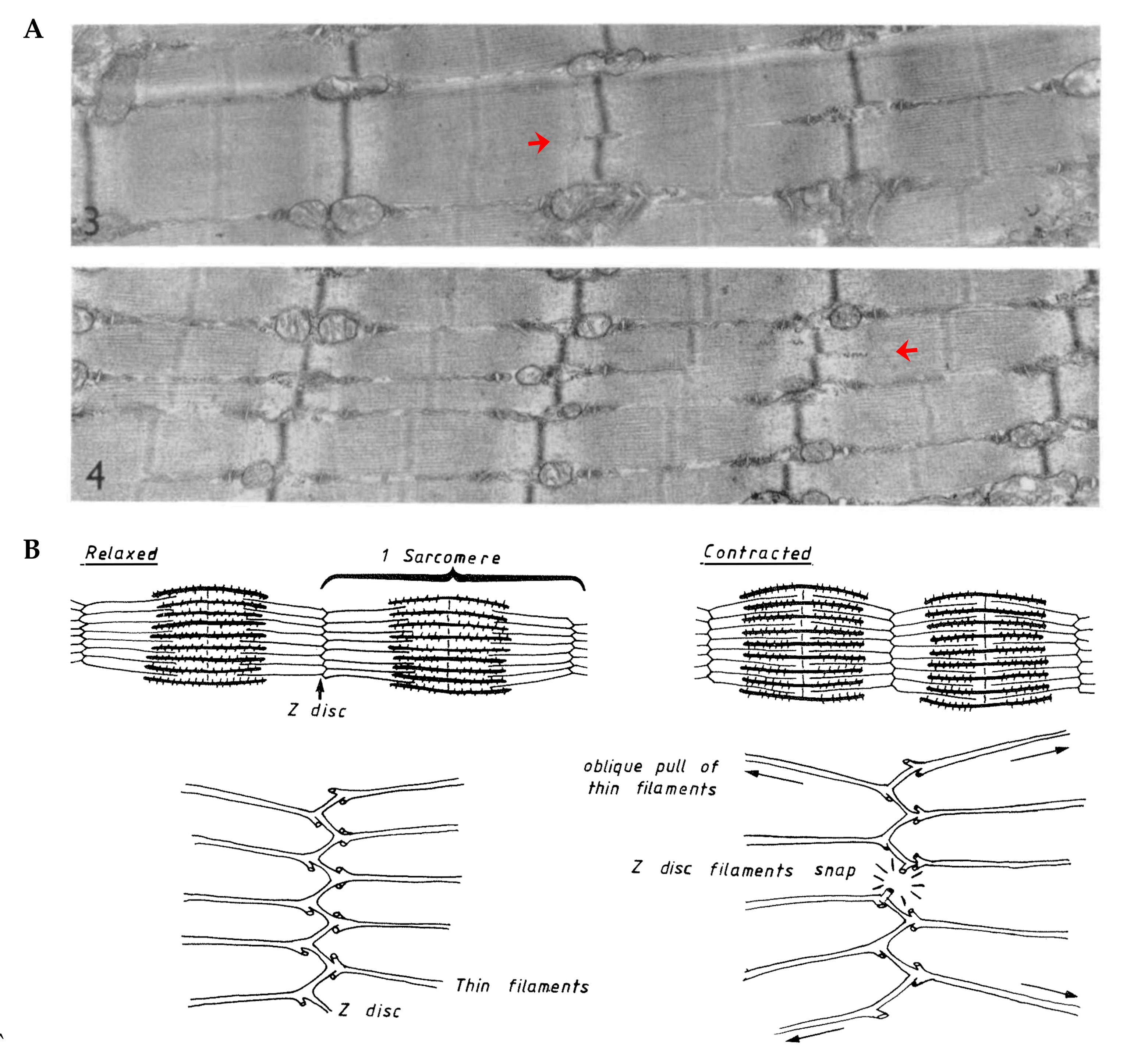
- Yu, J.G.; Carlsson, L.; Thornell, L.E. Evidence for myofibril remodeling as opposed to myofibril damage in human muscles with DOMS: An ultrastructural and immunoelectron microscopic study. Histochem. Cell Biol. 2004, 121, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Furst, D.O.; Thornell, L.E. The mode of myofibril remodelling in human skeletal muscle affected by DOMS induced by eccentric contractions. Histochem. Cell Biol. 2003, 119, 383–393. [Google Scholar] [CrossRef]
- Yu, J.G.; Thornell, L.E. Desmin and actin alterations in human muscles affected by delayed onset muscle soreness: A high resolution immunocytochemical study. Histochem. Cell Biol. 2002, 118, 171–179. [Google Scholar] [CrossRef]
- Carlsson, L.; Yu, J.G.; Moza, M.; Carpen, O.; Thornell, L.E. Myotilin: A prominent marker of myofibrillar remodelling. Neuromuscul. Disord. 2007, 17, 61–68. [Google Scholar] [CrossRef]
- Heidenhain, M. Uber die Noniusfelder der Muskelfaser. Anat. Hefte 1919, 56, 321–402. [Google Scholar]
- Friden, J. Changes in human skeletal muscle induced by long-term eccentric exercise. Cell Tissue Res. 1984, 236, 365–372. [Google Scholar] [CrossRef]
- Orfanos, Z.; Godderz, M.P.; Soroka, E.; Godderz, T.; Rumyantseva, A.; van der Ven, P.F.; Hawke, T.J.; Furst, D.O. Breaking sarcomeres by in vitro exercise. Sci. Rep. 2016, 6, 19614. [Google Scholar] [CrossRef]
- Friden, J.; Sjostrom, M.; Ekblom, B. Myofibrillar damage following intense eccentric exercise in man. Int. J. Sports Med. 1983, 4, 170–176. [Google Scholar] [CrossRef]
- Gibala, M.J.; MacDougall, J.D.; Tarnopolsky, M.A.; Stauber, W.T.; Elorriaga, A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J. Appl. Physiol. 1995, 78, 702–708. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Close, R.I. Dynamic properties of mammalian skeletal muscles. Physiol. Rev. 1972, 52, 129–197. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; McDonald, K.S.; Schluter, J.M. The determinants of skeletal muscle force and power: Their adaptability with changes in activity pattern. J. Biomech. 1991, 24 (Suppl. S1), 111–122. [Google Scholar] [CrossRef]
- Erskine, R.M.; Jones, D.A.; Maffulli, N.; Williams, A.G.; Stewart, C.E.; Degens, H. What causes in vivo muscle specific tension to increase following resistance training? Exp. Physiol. 2011, 96, 145–155. [Google Scholar] [CrossRef]
- Trappe, S.; Williamson, D.; Godard, M.; Porter, D.; Rowden, G.; Costill, D. Effect of resistance training on single muscle fiber contractile function in older men. J. Appl. Physiol. 2000, 89, 143–152. [Google Scholar] [CrossRef]
- Trappe, S.; Godard, M.; Gallagher, P.; Carroll, C.; Rowden, G.; Porter, D. Resistance training improves single muscle fiber contractile function in older women. Am. J. Physiol. Cell Physiol. 2001, 281, C398–C406. [Google Scholar] [CrossRef]
- Widrick, J.J.; Stelzer, J.E.; Shoepe, T.C.; Garner, D.P. Functional properties of human muscle fibers after short-term resistance exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R408–R416. [Google Scholar] [CrossRef]
- D’Antona, G.; Lanfranconi, F.; Pellegrino, M.A.; Brocca, L.; Adami, R.; Rossi, R.; Moro, G.; Miotti, D.; Canepari, M.; Bottinelli, R. Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J. Physiol. 2006, 570, 611–627. [Google Scholar] [CrossRef]
- Paoli, A.; Pacelli, Q.F.; Cancellara, P.; Toniolo, L.; Moro, T.; Canato, M.; Miotti, D.; Neri, M.; Morra, A.; Quadrelli, M.; et al. Protein Supplementation Does Not Further Increase Latissimus Dorsi Muscle Fiber Hypertrophy after Eight Weeks of Resistance Training in Novice Subjects, but Partially Counteracts the Fast-to-Slow Muscle Fiber Transition. Nutrients 2016, 8, 331. [Google Scholar] [CrossRef]
- Claflin, D.R.; Larkin, L.M.; Cederna, P.S.; Horowitz, J.F.; Alexander, N.B.; Cole, N.M.; Galecki, A.T.; Chen, S.; Nyquist, L.V.; Carlson, B.M.; et al. Effects of high- and low-velocity resistance training on the contractile properties of skeletal muscle fibers from young and older humans. J. Appl. Physiol. 2011, 111, 1021–1030. [Google Scholar] [CrossRef]
- Pansarasa, O.; Rinaldi, C.; Parente, V.; Miotti, D.; Capodaglio, P.; Bottinelli, R. Resistance training of long duration modulates force and unloaded shortening velocity of single muscle fibres of young women. J. Electromyogr. Kinesiol. 2009, 19, e290–e300. [Google Scholar] [CrossRef]
- Dankel, S.J.; Kang, M.; Abe, T.; Loenneke, J.P. Resistance training induced changes in strength and specific force at the fiber and whole muscle level: A meta-analysis. Eur. J. Appl. Physiol. 2019, 119, 265–278. [Google Scholar] [CrossRef]
- Mendias, C.L.; Schwartz, A.J.; Grekin, J.A.; Gumucio, J.P.; Sugg, K.B. Changes in muscle fiber contractility and extracellular matrix production during skeletal muscle hypertrophy. J. Appl. Physiol. 2017, 122, 571–579. [Google Scholar] [CrossRef]
- Vann, C.G.; Roberson, P.A.; Osburn, S.C.; Mumford, P.W.; Romero, M.A.; Fox, C.D.; Moore, J.H.; Haun, C.T.; Beck, D.T.; Moon, J.R.; et al. Skeletal Muscle Myofibrillar Protein Abundance Is Higher in Resistance-Trained Men, and Aging in the Absence of Training May Have an Opposite Effect. Sports 2020, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.; de Souza, R.W.; Aguiar, D.H.; Aguiar, R.C.; Vechetti, I.J., Jr.; Dal-Pai-Silva, M. Creatine does not promote hypertrophy in skeletal muscle in supplemented compared with nonsupplemented rats subjected to a similar workload. Nutr. Res. 2011, 31, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Penman, K.A. Ultrastructural changes in human striated muscle using three methods of training. Res. Q 1969, 40, 764–772. [Google Scholar] [CrossRef]
- Meijer, J.P.; Jaspers, R.T.; Rittweger, J.; Seynnes, O.R.; Kamandulis, S.; Brazaitis, M.; Skurvydas, A.; Pisot, R.; Simunic, B.; Narici, M.V.; et al. Single muscle fibre contractile properties differ between body-builders, power athletes and control subjects. Exp. Physiol. 2015, 100, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Haun, C.T.; Vann, C.G.; Osburn, S.C.; Mumford, P.W.; Roberson, P.A.; Romero, M.A.; Fox, C.D.; Johnson, C.A.; Parry, H.A.; Kavazis, A.N.; et al. Muscle fiber hypertrophy in response to 6 weeks of high-volume resistance training in trained young men is largely attributed to sarcoplasmic hypertrophy. PLoS ONE 2019, 14, e0215267. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Romero, M.A.; Mobley, C.B.; Mumford, P.W.; Roberson, P.A.; Haun, C.T.; Vann, C.G.; Osburn, S.C.; Holmes, H.H.; Greer, R.A.; et al. Skeletal muscle mitochondrial volume and myozenin-1 protein differences exist between high versus low anabolic responders to resistance training. PeerJ 2018, 6, e5338. [Google Scholar] [CrossRef] [PubMed]
- Kadi, F.; Eriksson, A.; Holmner, S.; Thornell, L.E. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med. Sci. Sports Exerc. 1999, 31, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Kadi, F.; Malm, C.; Thornell, L.E. Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem. Cell Biol. 2005, 124, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.S. Cross-sectional studies - what are they good for? Acta Obs. Gynecol. Scand. 2018, 97, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Setia, M.S. Methodology Series Module 3: Cross-sectional Studies. Indian J. Derm. 2016, 61, 261–264. [Google Scholar] [CrossRef]
- Mann, C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J. 2003, 20, 54–60. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Mobley, C.B.; Roberson, P.A.; Osburn, S.C.; Holmes, H.M.; Mumford, P.M.; Romero, M.A.; Young, K.C.; Moon, J.R.; et al. Effects of Graded Whey Supplementation During Extreme-Volume Resistance Training. Front. Nutr. 2018, 5, 84. [Google Scholar] [CrossRef]
- Huxley, H.E. Electron microscope studies of the organisation of the filaments in striated muscle. Biochim. Biophys. Acta 1953, 12, 387–394. [Google Scholar] [CrossRef]
- Millman, B.M. The filament lattice of striated muscle. Physiol. Rev. 1998, 78, 359–391. [Google Scholar] [CrossRef]
- Luther, P.K.; Squire, J.M. The intriguing dual lattices of the Myosin filaments in vertebrate striated muscles: Evolution and advantage. Biology 2014, 3, 846–865. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, C.R.; Summers, P.J. Stretch-induced growth in chicken wing muscles: Myofibrillar proliferation. Am. J. Physiol. 1981, 241, C93–C97. [Google Scholar] [CrossRef]
- Goldspink, G. The proliferation of myofibrils during muscle fibre growth. J. Cell Sci. 1970, 6, 593–603. [Google Scholar] [PubMed]
- Goldspink, G. Changes in striated muscle fibres during contraction and growth with particular reference to myofibril splitting. J. Cell Sci. 1971, 9, 123–137. [Google Scholar] [PubMed]
- Patterson, S.; Goldspink, G. Mechanism of myofibril growth and proliferation in fish muscle. J. Cell Sci. 1976, 22, 607–616. [Google Scholar] [PubMed]
- MacInstosh, B.; Gardiner, P.; McComas, A. Skeletal Muscle: Form and Function; Human Kinetics: Champaign, IL, USA, 2006. [Google Scholar]
- Komi, P.V. Strength and Power in Sport; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bourne, G.H. The Structure and Function of Muscle; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- McComas, A.J. Neuromuscular Function and Disorders; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
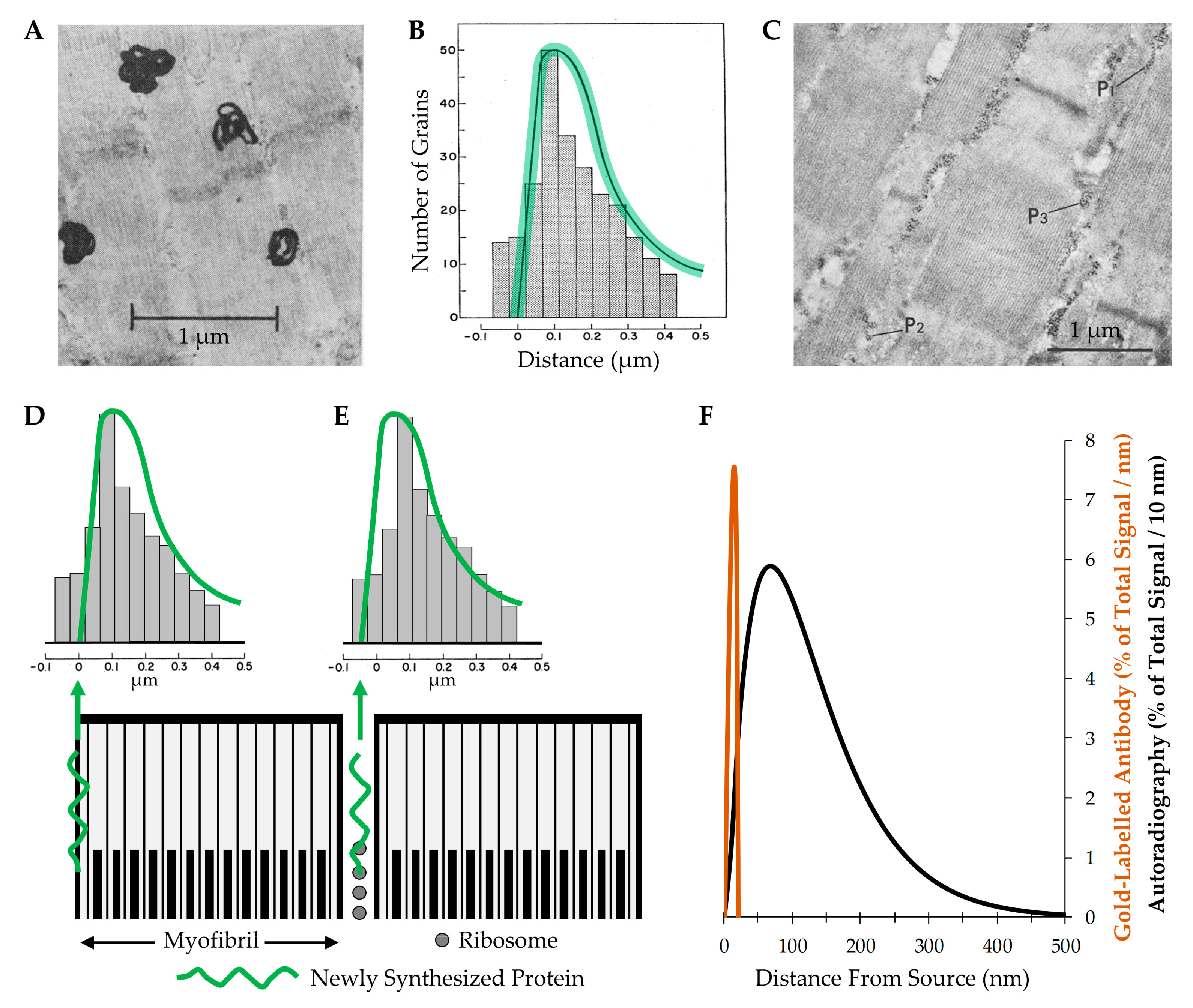
- Morkin, E. Postnatal muscle fiber assembly: Localization of newly synthesized myofibrillar proteins. Science 1970, 167, 1499–1501. [Google Scholar] [CrossRef]
- Galavazi, G.; Szirmai, J.A. The influence of age and testosterone on the ribosomal population in the m. levator ani and a thigh muscle of the rat. Z Zellforsch Mikrosk Anat. 1971, 121, 548–560. [Google Scholar] [CrossRef]
- Galavazi, G. Identification of helical polyribosomes in sections of mature skeletal muscle fibers. Z Zellforsch Mikrosk Anat. 1971, 121, 531–547. [Google Scholar] [CrossRef]
- Salpeter, M.M.; Bachmann, L.; Salpeter, E.E. Resolution in electron microscope radioautography. J. Cell Biol. 1969, 41, 1–32. [Google Scholar] [CrossRef]
- Caro, L.G. High-resolution autoradiogaphy. II. The problem of resolution. J. Cell Biol. 1962, 15, 189–199. [Google Scholar] [CrossRef]
- Goodman, C.A.; Mabrey, D.M.; Frey, J.W.; Miu, M.H.; Schmidt, E.K.; Pierre, P.; Hornberger, T.A. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011, 25, 1028–1039. [Google Scholar] [CrossRef]
- Tom Dieck, S.; Muller, A.; Nehring, A.; Hinz, F.I.; Bartnik, I.; Schuman, E.M.; Dieterich, D.C. Metabolic labeling with noncanonical amino acids and visualization by chemoselective fluorescent tagging. Curr. Protoc. Cell Biol. 2012, 56, 7.11.1–7.11.29. [Google Scholar] [CrossRef]
- Hermann, R.; Walther, P.; Muller, M. Immunogold labeling in scanning electron microscopy. Histochem. Cell Biol. 1996, 106, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Babcock, H.; Zhuang, X. Breaking the diffraction barrier: Super-resolution imaging of cells. Cell 2010, 143, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Raulf, A.; Spahn, C.K.; Zessin, P.J.; Finan, K.; Bernhardt, S.; Heckel, A.; Heilemann, M. Click chemistry facilitates direct labelling and super-resolution imaging of nucleic acids and proteinsdagger. Rsc Adv. 2014, 4, 30462–30466. [Google Scholar] [CrossRef] [PubMed]
- Mobius, W.; Posthuma, G. Sugar and ice: Immunoelectron microscopy using cryosections according to the Tokuyasu method. Tissue Cell 2019, 57, 90–102. [Google Scholar] [CrossRef]
- Klein, J.S.; Gnanapragasam, P.N.; Galimidi, R.P.; Foglesong, C.P.; West, A.P., Jr.; Bjorkman, P.J. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc. Natl. Acad. Sci. USA 2009, 106, 7385–7390. [Google Scholar] [CrossRef]
- Holmes, R.; Rasch, P.J. Effect of exercise on number of myofibrils per fiber in sartorius muscle of the rat. Am. J. Physiol. 1958, 195, 50–52. [Google Scholar] [CrossRef]
- Toffolo, R.L.; Ianuzzo, C.D. Myofibrillar adaptations during cardiac hypertrophy. Mol. Cell Biochem. 1994, 131, 141–149. [Google Scholar] [CrossRef]
- Richter, G.W.; Kellner, A. Hypertrophy of the human heart at the level of fine structure. An analysis and two postulates. J. Cell Biol. 1963, 18, 195–206. [Google Scholar] [CrossRef]
- Anversa, P.; Olivetti, G.; Melissari, M.; Loud, A.V. Stereological measurement of cellular and subcellular hypertrophy and hyperplasia in the papillary muscle of adult rat. J. Mol. Cell. Cardiol. 1980, 12, 781–795. [Google Scholar] [CrossRef]








| Adaptation | Evidence | Gaps in Knowledge |
|---|---|---|
| Longitudinal Growth of Fascicles | High | Does mechanical loading alter the number of fascicles? Can mechanical loading lead to the addition of new myofibers in-series? |
| Radial Growth of Fascicles | High | To what extent does myofiber hyperplasia, myofiber splitting, and the lengthening of myofibers with intrafascicular terminations contribute to the radial growth of fascicles? |
| Myofiber Splitting | Low | Do physiologically relevant models of mechanical loading induce myofiber splitting? |
| Myofiber Hyperplasia | Low & Controversial | To what extent, if any, does myofiber hyperplasia contribute to the radial growth of fascicles? |
| Longitudinal Growth of Myofibers | Mixed - Model Dependent | Do physiologically relevant forms of mechanical loading induce the longitudinal growth of myofibers? Where, and how, are new sarcomeres added during the longitudinal growth of myofibers? |
| Radial Growth of Myofibers | Extremely High | Is mechanical load-induced myofiber hypertrophy driven by myofibril hypertrophy and/or myofibril hyperplasia? |
| Sarcoplasmic Hypertrophy | Low & Controversial | Are there specific conditions during which sarcoplasmic hypertrophy might make substantive contribution to the mechanical load-induced growth of myofibers? |
| Myofibril Hypertrophy | Low | Does mechanical loading lead to myofibril hypertrophy? Where are new myofilaments deposited during myofibril hypertrophy? |
| Myofibril Hyperplasia | Very Low | Does mechanical loading lead to myofibril hyperplasia? Are new myofibrils generated via the process of myofibril splitting? |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorgenson, K.W.; Phillips, S.M.; Hornberger, T.A. Identifying the Structural Adaptations that Drive the Mechanical Load-Induced Growth of Skeletal Muscle: A Scoping Review. Cells 2020, 9, 1658. https://doi.org/10.3390/cells9071658
Jorgenson KW, Phillips SM, Hornberger TA. Identifying the Structural Adaptations that Drive the Mechanical Load-Induced Growth of Skeletal Muscle: A Scoping Review. Cells. 2020; 9(7):1658. https://doi.org/10.3390/cells9071658
Chicago/Turabian StyleJorgenson, Kent W., Stuart M. Phillips, and Troy A. Hornberger. 2020. "Identifying the Structural Adaptations that Drive the Mechanical Load-Induced Growth of Skeletal Muscle: A Scoping Review" Cells 9, no. 7: 1658. https://doi.org/10.3390/cells9071658
APA StyleJorgenson, K. W., Phillips, S. M., & Hornberger, T. A. (2020). Identifying the Structural Adaptations that Drive the Mechanical Load-Induced Growth of Skeletal Muscle: A Scoping Review. Cells, 9(7), 1658. https://doi.org/10.3390/cells9071658