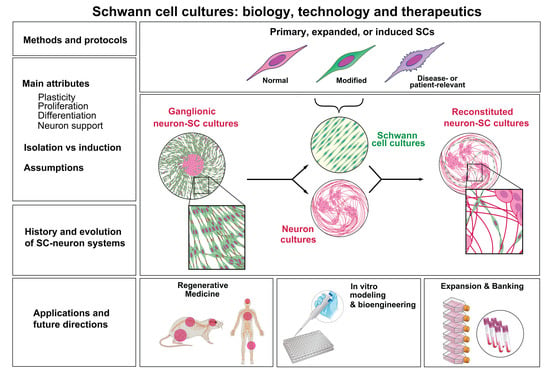
Schwann Cell Cultures: Biology, Technology and Therapeutics
Abstract
:1. Introduction
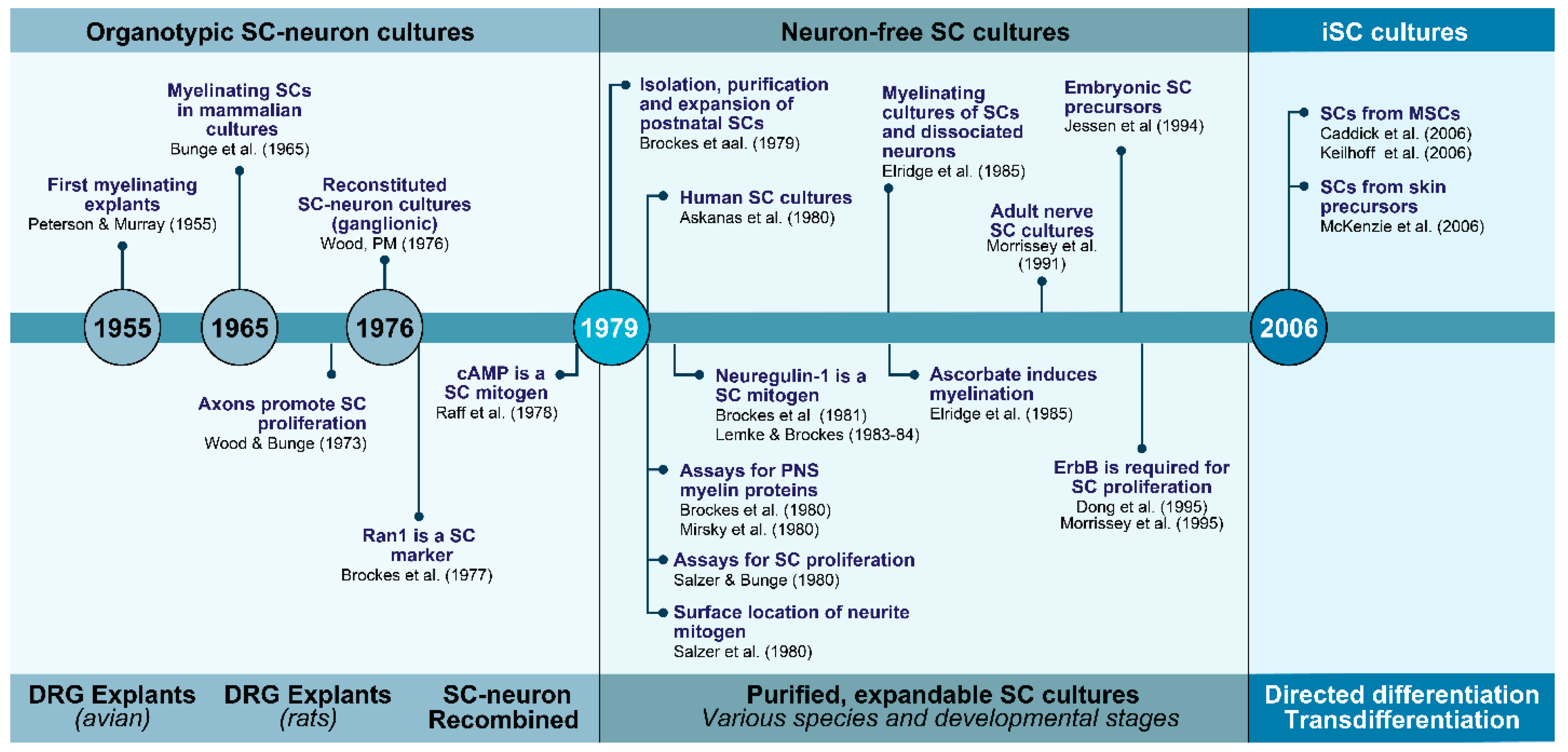
2. The Evolution of Schwann Cell Cultures: A Three-Tier Story at a Glance
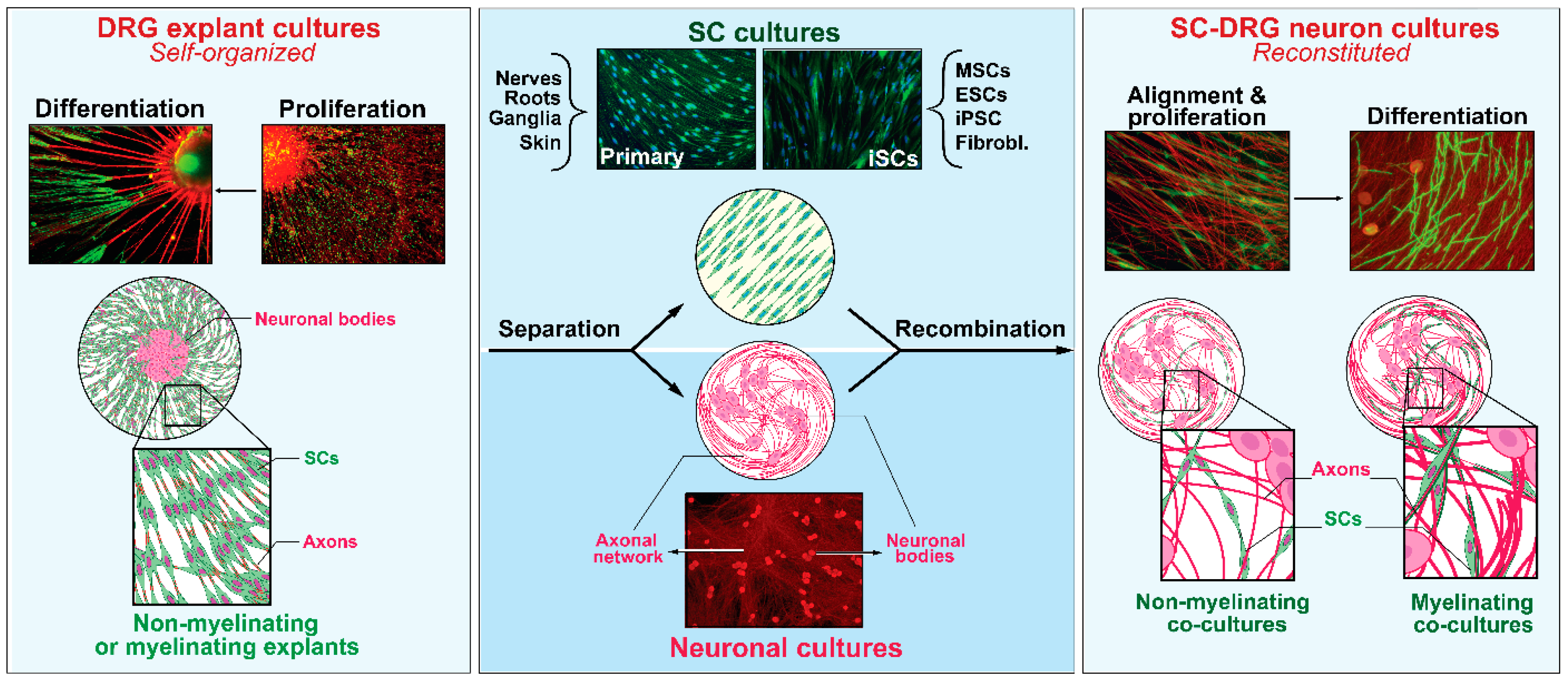
2.1. SCs with Neurons and the Dependency on Axon Contact for SC Expansion and Differentiation
2.2. SCs without Neurons and the Advent of Soluble Mitogenic Factors
2.3. iSCs and the Advent of Technologies to Recreate the SC Phenotype In Vitro
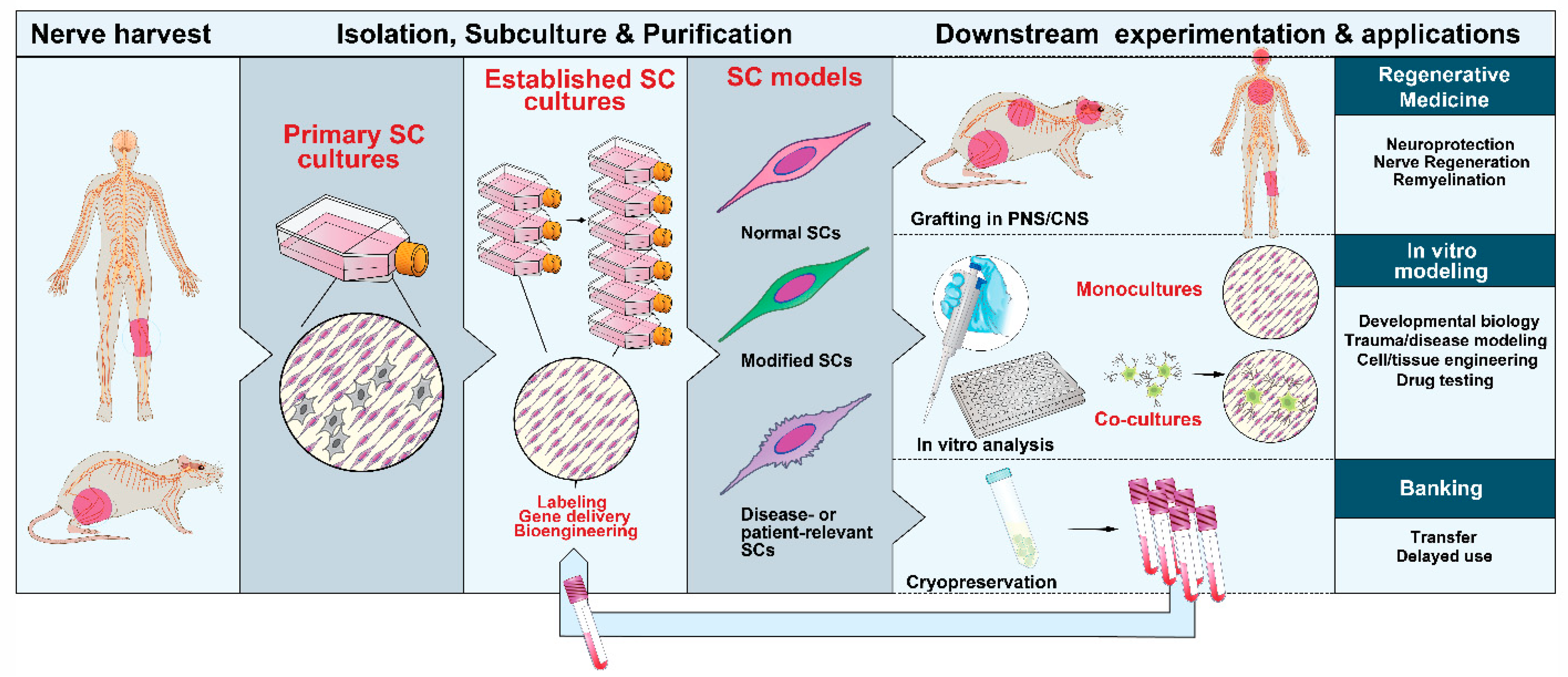
3. Methods and Protocols
3.1. Choice and Procurement of Source Tissues
3.2. Isolation and Management of the Initial Populations
3.3. Amplification and Subculture
3.4. Phenotypic Identification and Purification
3.5. Labeling, Tracing and Gene Delivery
3.6. Cell Banking
4. Attributes of Primary and Expanded SCs
4.1. Maintenance of Phenotype and Proliferation Controls
4.2. Potential for Differentiation
4.3. Promotion of Neuronal Health and Axon Growth
5. SC cultures in Translational Research
5.1. Cell Therapy
5.2. In Vitro Modeling
6. Long-Standing Assumptions in SC Culture and Their Implications
6.1. Cultured SCs Are Spindle-Shaped Cells That Express the Marker S100β
6.2. Cultured SCs Can Myelinate Axons
6.3. Soluble Mitogens Are Axon-Mimetics That Maintain the SC Phenotype In Vitro
6.4. Any Type of SC Can Give Rise to a Primary Culture
7. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Monk:, K.R.; Feltri, M.L.; Taveggia, C. New insights on Schwann cell development. Glia 2015, 63, 1376–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belin, S.; Zuloaga, K.L.; Poitelon, Y. Influence of Mechanical Stimuli on Schwann cell Biology. Front. Cell. Neurosci. 2017, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Aquino, J.B.; Sierra, R. Schwann cell precursors in health and disease. Glia 2018, 66, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. Negative regulation of myelination: Relevance for development, injury, and demyelinating disease. Glia 2008, 56, 1552–1565. [Google Scholar] [CrossRef]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef]
- Boyer, P.J.; Tuite, G.F.; Dauser, R.C.; Muraszko, K.M.; Tennekoon, G.I.; Rutkowski, J.L. Sources of human Schwann cells and the influence of donor age. Exp. Neurol. 1994, 130, 53–55. [Google Scholar] [CrossRef] [Green Version]
- Levi, A.D.; Evans, P.J.; Mackinnon, S.E.; Bunge, R.P. Cold storage of peripheral nerves: An in vitro assay of cell viability and function. Glia 1994, 10, 121–131. [Google Scholar] [CrossRef]
- El Seblani, N.; Welleford, A.S.; Quintero, J.E.; van Horne, C.G.; Gerhardt, G.A. Invited review: Utilizing peripheral nerve regenerative elements to repair damage in the CNS. J. Neurosci. Methods 2020, 335, 108623. [Google Scholar] [CrossRef]
- Guest, J.; Santamaria, A.J.; Benavides, F.D. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ. Transpl. 2013, 18, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Bunge, M.B.; Wood, P.M. Realizing the maximun potential of Schwann cells to promote recovery from spinal cord injury. Handb. Clin. Neurol. 2012, 109, 523–540. [Google Scholar]
- Monje, P.V. The properties of human Schwann cells: Lessons from in vitro culture and transplantation studies. Glia 2020, 68, 797–810. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Hoke, A. Use of engineered Schwann cells in peripheral neuropathy: Hopes and hazards. Brain Res. 2016, 1638, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.A. Isolation and Expansion of Schwann Cells from Transgenic Mouse Models. Methods Mol. Biol. 2018, 1739, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, B.; Baron-Van Evercooren, A. Schwann cells: Rescuers of central demyelination. Glia 2020. [Google Scholar] [CrossRef] [PubMed]
- Mosahebi, A.; Woodward, B.; Wiberg, M.; Martin, R.; Terenghi, G. Retroviral labeling of Schwann cells: In vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia 2001, 34, 8–17. [Google Scholar] [CrossRef]
- Han, G.H.; Peng, J.; Liu, P.; Ding, X.; Wei, S.; Lu, S.; Wang, Y. Therapeutic strategies for peripheral nerve injury: Decellularized nerve conduits and Schwann cell transplantation. Neural Regen. Res. 2019, 14, 1343–1351. [Google Scholar] [CrossRef]
- Bunge, M.; Monje, P.V.; Khan, A.; Wood, P.M. From transplanting Schwann cells in experimental rat spinal cord injury to their transplantation into human injured spinal cord in clinical trials. Handb. Clin. Neurol. 2017, 231, 107–133. [Google Scholar]
- Bunge, R.P. Changing uses of nerve tissue culture 1950–1975. In The Nervous System; Tower, D.B., Ed.; Raven Press: New York, NY, USA, 1975; Volume 1, p. 31. [Google Scholar]
- Peterson, E.R.; Murray, M.R. Myelin sheath formation in cultures of avian spinal ganglia. Am. J. Anat. 1955, 96, 319–355. [Google Scholar] [CrossRef]
- Bunge, M.B.; Bunge, R.P.; Peterson, E.R.; Murray, M.R. A light and electron microscope study of long-term organized cultures of rat dorsal root ganglia. J. Cell Biol. 1967, 32, 439–466. [Google Scholar] [CrossRef]
- Wood, P.M. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976, 115, 361–375. [Google Scholar] [CrossRef]
- Wood, P.M.; Bunge, R.P. Evidence that sensory axons are mitogenic for Schwann cells. Nature 1975, 256, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L.; Williams, A.K.; Glaser, L.; Bunge, R.P. Studies of Schwann cell proliferation. II. Characterization of the stimulation and specificity of the response to a neurite membrane fraction. J. Cell Biol. 1980, 84, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L.; Bunge, R.P.; Glaser, L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J. Cell Biol. 1980, 84, 767–778. [Google Scholar] [CrossRef] [Green Version]
- Salzer, J.L.; Bunge, R.P. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J. Cell Biol. 1980, 84, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.B.; Williams, A.K.; Wood, P.M.; Uitto, J.; Jeffrey, J.J. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J. Cell Biol. 1980, 84, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Brockes, J.P.; Fields, K.L.; Raff, M.C. A surface antigenic marker for rat Schwann cells. Nature 1977, 266, 364–366. [Google Scholar] [CrossRef]
- Raff, M.C.; Fields, K.L.; Hakomori, S.I.; Mirsky, R.; Pruss, R.M.; Winter, J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979, 174, 283–308. [Google Scholar] [CrossRef]
- Brockes, J.P.; Fryxell, K.J.; Lemke, G.E. Studies on cultured Schwann cells: The induction of myelin synthesis, and the control of their proliferation by a new growth factor. J. Exp. Biol. 1981, 95, 215–230. [Google Scholar]
- Brockes, J.P.; Raff, M.C.; Nishiguchi, D.J.; Winter, J. Studies on cultured rat Schwann cells. III. Assays for peripheral myelin proteins. J. Neurocytol. 1980, 9, 67–77. [Google Scholar] [CrossRef]
- Mirsky, R.; Winter, J.; Abney, E.R.; Pruss, R.M.; Gavrilovic, J.; Raff, M.C. Myelin-specific proteins and glycolipids in rat Schwann cells and oligodendrocytes in culture. J. Cell Biol. 1980, 84, 483–494. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Lisak, R.P.; Raff, M.C. Cell type-specific markers for human glial and neuronal cells in culture. Lab. Invest. 1980, 43, 342–351. [Google Scholar] [PubMed]
- Lemke, G.E.; Brockes, J.P. Glial growth factor: A mitogenic polypeptide of the brain and pituitary. Fed. Proc. 1983, 42, 2627–2629. [Google Scholar] [PubMed]
- Lemke, G.E.; Brockes, J.P. Identification and purification of glial growth factor. J. Neurosci. 1984, 4, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, T.K.; Levi, A.D.; Nuijens, A.; Sliwkowski, M.X.; Bunge, R.P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl. Acad. Sci. USA 1995, 92, 1431–1435. [Google Scholar] [CrossRef] [Green Version]
- Raff, M.C.; Hornby-Smith, A.; Brockes, J.P. Cyclic AMP as a mitogenic signal for cultured rat Schwann cells. Nature 1978, 273, 672–673. [Google Scholar] [CrossRef]
- Raff, M.C.; Abney, E.; Brockes, J.P.; Hornby-Smith, A. Schwann cell growth factors. Cell 1978, 15, 813–822. [Google Scholar] [CrossRef]
- Stewart, H.J.; Eccleston, P.A.; Jessen, K.R.; Mirsky, R. Interaction between cAMP elevation, identified growth factors, and serum components in regulating Schwann cell growth. J. Neurosci. Res. 1991, 30, 346–352. [Google Scholar] [CrossRef]
- Brockes, J.P.; Lemke, G.E.; Balzer, D.R., Jr. Purification and preliminary characterization of a glial growth factor from the bovine pituitary. J. Biol. Chem. 1980, 255, 8374–8377. [Google Scholar]
- Brockes, J.P.; Raff, M.C. Studies on cultured rat Schwann cells. II. Comparison with a rat Schwann cell line. Vitro 1979, 15, 772–778. [Google Scholar] [CrossRef]
- Bunge, R.P.; Bunge, M.B. Evidence that contact with connective tissue matrix is required for normal interaction between Schwann cells and nerve fibers. J. Cell Biol. 1978, 78, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Moya, F.; Bunge, M.B.; Bunge, R.P. Schwann cells proliferate but fail to differentiate in defined medium. Proc. Natl. Acad. Sci. USA 1980, 77, 6902–6906. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, C.F.; Bunge, M.B.; Bunge, R.P.; Wood, P.M. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 1987, 105, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Bunge, R.P.; Bunge, M.B.; Eldridge, C.F. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu. Rev. Neurosci. 1986, 9, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Ratner, N.; Glaser, L.; Bunge, R.P. PC12 cells as a source of neurite-derived cell surface mitogen, which stimulates Schwann cell division. J. Cell Biol. 1984, 98, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R.; Morgan, L. Role of cyclic AMP and proliferation controls in Schwann cell differentiation. Ann. N. Y. Acad. Sci. 1991, 633, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Monje, P.V. Axon contact-driven Schwann cell dedifferentiation. Glia 2017, 65, 864–882. [Google Scholar] [CrossRef]
- Bacallao, K.; Monje, P.V. Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination. PLoS ONE 2015, 10, e0116948. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee-Clavin, B.; Mi, R.; Kern, B.; Choi, I.Y.; Lim, H.; Oh, Y.; Lannon, B.; Kim, K.J.; Bell, S.; Hur, J.K.; et al. Comparison of three congruent patient-specific cell types for the modelling of a human genetic Schwann-cell disorder. Nat. Biomed. Eng. 2019, 3, 571–582. [Google Scholar] [CrossRef]
- Brockes, J.P.; Fields, K.L.; Raff, M.C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979, 165, 105–118. [Google Scholar] [CrossRef]
- Jessen, K.R.; Morgan, L.; Stewart, H.J.; Mirsky, R. Three markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: Development and regulation by neuron-Schwann cell interactions. Development 1990, 109, 91–103. [Google Scholar]
- Askanas, V.; Engel, W.K.; Dalakas, M.C.; Lawrence, J.V.; Carter, L.S. Human schwann cells in tissue culture: Histochemical and ultrastructural studies. Arch. Neurol. 1980, 37, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.L.; Tennekoon, G.I.; McGillicuddy, J.E. Selective culture of mitotically active human Schwann cells from adult sural nerves. Ann. Neurol. 1992, 31, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Brennan, A.; Liu, N.; Yarden, Y.; Lefkowitz, G.; Mirsky, R.; Jessen, K.R. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron 1995, 15, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Levi, A.D.; Bunge, R.P.; Lofgren, J.A.; Meima, L.; Hefti, F.; Nikolics, K.; Sliwkowski, M.X. The influence of heregulins on human Schwann cell proliferation. J. Neurosci. 1995, 15, 1329–1340. [Google Scholar] [CrossRef] [Green Version]
- Keilhoff, G.; Goihl, A.; Stang, F.; Wolf, G.; Fansa, H. Peripheral nerve tissue engineering: Autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue Eng. 2006, 12, 1451–1465. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006, 26, 6651–6660. [Google Scholar] [CrossRef]
- Thoma, E.C.; Merkl, C.; Heckel, T.; Haab, R.; Knoflach, F.; Nowaczyk, C.; Flint, N.; Jagasia, R.; Jensen Zoffmann, S.; Truong, H.H.; et al. Chemical conversion of human fibroblasts into functional Schwann cells. Stem Cell Rep. 2014, 3, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Lee, J.; Lee, D.Y.; Kim, Y.D.; Kim, J.Y.; Lim, H.J.; Lim, S.; Cho, Y.S. Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Rep. 2017, 8, 1714–1726. [Google Scholar] [CrossRef] [Green Version]
- Caddick, J.; Kingham, P.J.; Gardiner, N.J.; Wiberg, M.; Terenghi, G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 2006, 54, 840–849. [Google Scholar] [CrossRef]
- Mantovani, C.; Terenghi, G.; Shawcross, S.G. Isolation of adult stem cells and their differentiation to schwann cells. Methods Mol. Biol. 2012, 916, 47–57. [Google Scholar]
- Cai, S.; Tsui, Y.P.; Tam, K.W.; Shea, G.K.; Chang, R.S.; Ao, Q.; Shum, D.K.; Chan, Y.S. Directed Differentiation of Human Bone Marrow Stromal Cells to Fate-Committed Schwann Cells. Stem Cell Rep. 2017, 9, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Dworski, S.; Feinberg, K.; Jones, K.; Johnston, A.P.; Paul, S.; Paris, M.; Peles, E.; Bagli, D.; Forrest, C.R.; et al. Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Rep. 2014, 3, 85–100. [Google Scholar] [CrossRef]
- Mazzara, P.G.; Massimino, L.; Pellegatta, M.; Ronchi, G.; Ricca, A.; Iannielli, A.; Giannelli, S.G.; Cursi, M.; Cancellieri, C.; Sessa, A.; et al. Two factor-based reprogramming of rodent and human fibroblasts into Schwann cells. Nat. Commun. 2017, 8, 14088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, E.; Li, X.; Brunschwig, J.P.; Olson, L.; Levi, A.D. Assessment of the malignant potential of mitogen stimulated human Schwann cells. J. Peripher. Nerv. Syst. 1999, 4, 107–116. [Google Scholar] [PubMed]
- Weiss, T.; Taschner-Mandl, S.; Bileck, A.; Slany, A.; Kromp, F.; Rifatbegovic, F.; Frech, C.; Windhager, R.; Kitzinger, H.; Tzou, C.H.; et al. Proteomics and transcriptomics of peripheral nerve tissue and cells unravel new aspects of the human Schwann cell repair phenotype. Glia 2016, 64, 2133–2153. [Google Scholar] [CrossRef]
- Mathon, N.F.; Malcolm, D.S.; Harrisingh, M.C.; Cheng, L.; Lloyd, A.C. Lack of replicative senescence in normal rodent glia. Science 2001, 291, 872–875. [Google Scholar] [CrossRef]
- Wewetzer, K.; Radtke, C.; Kocsis, J.; Baumgartner, W. Species-specific control of cellular proliferation and the impact of large animal models for the use of olfactory ensheathing cells and Schwann cells in spinal cord repair. Exp. Neurol. 2011, 229, 80–87. [Google Scholar] [CrossRef]
- Casella, G.T.; Bunge, R.P.; Wood, P.M. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia 1996, 17, 327–338. [Google Scholar] [CrossRef]
- Mirsky, R.; Jessen, K.R. Isolation of Schwann Cell Precursors from Rodents. Methods Mol. Biol. 2018, 1739, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Whitlon, D.S.; Tieu, D.; Grover, M. Purification and transfection of cochlear Schwann cells. Neuroscience 2010, 171, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.A.; Kumar, R.; Sinha, S.; Shah, P.; Stykel, M.; Shapira, Y.; Midha, R.; Biernaskie, J. Purification and Characterization of Schwann Cells from Adult Human Skin and Nerve. eNeuro 2017, 4, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etxaniz, U.; Perez-San Vicente, A.; Gago-Lopez, N.; Garcia-Dominguez, M.; Iribar, H.; Aduriz, A.; Perez-Lopez, V.; Burgoa, I.; Irizar, H.; Munoz-Culla, M.; et al. Neural-competent cells of adult human dermis belong to the Schwann lineage. Stem Cell Rep. 2014, 3, 774–788. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R. Why do Schwann cells survive in the absence of axons? Ann. N. Y. Acad. Sci. 1999, 883, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Sinanan, A.; Parkinson, D.; Parmantier, E.; Mirsky, R.; Jessen, K.R. Schwann cell development in embryonic mouse nerves. J. Neurosci. Res. 1999, 56, 334–348. [Google Scholar] [CrossRef]
- Meier, C.; Parmantier, E.; Brennan, A.; Mirsky, R.; Jessen, K.R. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J. Neurosci. 1999, 19, 3847–3859. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, C.; Karyala, S.; Marchionni, M.A.; Kim, H.A.; Krasnoselsky, A.L.; Happel, B.; Isaacs, I.; Brackenbury, R.; Ratner, N. Schwann cells express NDF and SMDF/n-ARIA mRNAs, secrete neuregulin, and show constitutive activation of erbB3 receptors: Evidence for a neuregulin autocrine loop. Exp. Neurol. 1997, 148, 604–615. [Google Scholar] [CrossRef]
- Morrissey, T.K.; Kleitman, N.; Bunge, R.P. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J. Neurosci. 1991, 11, 2433–2442. [Google Scholar] [CrossRef] [Green Version]
- Andersen, N.D.; Srinivas, S.; Pinero, G.; Monje, P.V. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci. Rep. 2016, 6, 31781. [Google Scholar] [CrossRef]
- Gu, Y.; Ji, Y.; Zhao, Y.; Liu, Y.; Ding, F.; Gu, X.; Yang, Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012, 33, 6672–6681. [Google Scholar] [CrossRef]
- Urbanski, M.M.; Kingsbury, L.; Moussouros, D.; Kassim, I.; Mehjabeen, S.; Paknejad, N.; Melendez-Vasquez, C.V. Myelinating glia differentiation is regulated by extracellular matrix elasticity. Sci. Rep. 2016, 6, 33751. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, M.M.; Melendez-Vasquez, C.V. Preparation of Matrices of Variable Stiffness for the Study of Mechanotransduction in Schwann Cell Development. Methods Mol. Biol. 2018, 1739, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, J.; Athauda, G.; De La Cruz, G.; Chan, W.M.; Golshani, R.; Berrocal, Y.; Henao, M.; Lalwani, A.; Mannoji, C.; Assi, M.; et al. Human Schwann cells exhibit long-term cell survival, are not tumorigenic and promote repair when transplanted into the contused spinal cord. Glia 2017, 65, 1278–1301. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.J.; Rougon, G.; Dong, Z.; Dean, C.; Jessen, K.R.; Mirsky, R. TGF-betas upregulate NCAM and L1 expression in cultured Schwann cells, suppress cyclic AMP-induced expression of O4 and galactocerebroside, and are widely expressed in cells of the Schwann cell lineage in vivo. Glia 1995, 15, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Krasnoselsky, A.; Massay, M.J.; DeFrances, M.C.; Michalopoulos, G.; Zarnegar, R.; Ratner, N. Hepatocyte growth factor is a mitogen for Schwann cells and is present in neurofibromas. J. Neurosci. 1994, 14, 7284–7290. [Google Scholar] [CrossRef]
- Cheng, L.; Khan, M.; Mudge, A.W. Calcitonin gene-related peptide promotes Schwann cell proliferation. J. Cell Biol. 1995, 129, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, J.; Hammonds, G.; Phillips, H.; Armanini, M.; Wood, P.; Bunge, R.; Godowski, P.J.; Sliwkowski, M.X.; Mather, J.P. Identification of Gas6 as a growth factor for human Schwann cells. J. Neurosci. 1996, 16, 2012–2019. [Google Scholar] [CrossRef] [Green Version]
- Stevens, B.; Ishibashi, T.; Chen, J.F.; Fields, R.D. Adenosine: An activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 2004, 1, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Meintanis, S.; Thomaidou, D.; Jessen, K.R.; Mirsky, R.; Matsas, R. The neuron-glia signal beta-neuregulin promotes Schwann cell motility via the MAPK pathway. Glia 2001, 34, 39–51. [Google Scholar] [CrossRef]
- Monje, P.V.; Athauda, G.; Wood, P.M. Protein kinase A-mediated gating of neuregulin-dependent ErbB2-ErbB3 activation underlies the synergistic action of cAMP on Schwann cell proliferation. J. Biol. Chem. 2008, 283, 34087–34100. [Google Scholar] [CrossRef] [Green Version]
- Monje, P.V.; Rendon, S.; Athauda, G.; Bates, M.; Wood, P.M.; Bunge, M.B. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia 2009, 57, 947–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, K.L.; Raine, C.S. Ultrastructure and immunocytochemistry of rat Schwann cells and fibroblasts in vitro. J. Neuroimmunol. 1982, 2, 155–166. [Google Scholar] [CrossRef]
- Fields, K.L.; Brockes, J.P.; Mirsky, R.; Wendon, L.M. Cell surface markers for distinguishing different types of rat dorsal root ganglion cells in culture. Cell 1978, 14, 43–51. [Google Scholar] [CrossRef]
- Cheng, L.; Mudge, A.W. Cultured Schwann cells constitutively express the myelin protein P0. Neuron 1996, 16, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Fryxell, K.J.; Balzer, D.R., Jr.; Brockes, J.P. Development and applications of a solid-phase radioimmunoassay for the PO protein of peripheral myelin. J. Neurochem. 1983, 40, 538–546. [Google Scholar] [CrossRef]
- Kaewkhaw, R.; Scutt, A.M.; Haycock, J.W. Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nat. Protoc. 2012, 7, 1996–2004. [Google Scholar] [CrossRef]
- Jirsova, K.; Sodaar, P.; Mandys, V.; Bar, P.R. Cold jet: A method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J. Neurosci. Methods 1997, 78, 133–137. [Google Scholar] [CrossRef]
- Haastert, K.; Mauritz, C.; Chaturvedi, S.; Grothe, C. Human and rat adult Schwann cell cultures: Fast and efficient enrichment and highly effective non-viral transfection protocol. Nat. Protoc. 2007, 2, 99–104. [Google Scholar] [CrossRef]
- Haastert, K.; Seef, P.; Stein, V.M.; Tipold, A.; Grothe, C. A new cell culture protocol for enrichment and genetic modification of adult canine Schwann cells suitable for peripheral nerve tissue engineering. Res. Vet. Sci. 2009, 87, 140–142. [Google Scholar] [CrossRef]
- Morrissey, T.K.; Kleitman, N.; Bunge, R.P. Human Schwann cells in vitro. II. Myelination of sensory axons following extensive purification and heregulin-induced expansion. J. Neurobiol. 1995, 28, 190–201. [Google Scholar] [CrossRef]
- Kanno, H.; Pressman, Y.; Moody, A.; Berg, R.; Muir, E.M.; Rogers, J.H.; Ozawa, H.; Itoi, E.; Pearse, D.D.; Bunge, M.B. Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J. Neurosci. 2014, 34, 1838–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Ashizawa, A.T.; Kim, K.S.; Falk, D.J.; Notterpek, L. Liposomes to target peripheral neurons and Schwann cells. PLoS ONE 2013, 8, e78724. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Gander, B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 2010, 15, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lavdas, A.A.; Efrose, R.; Douris, V.; Gaitanou, M.; Papastefanaki, F.; Swevers, L.; Thomaidou, D.; Iatrou, K.; Matsas, R. Soluble forms of the cell adhesion molecule L1 produced by insect and baculovirus-transduced mammalian cells enhance Schwann cell motility. J. Neurochem. 2010, 115, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Valle, C.; Wood, P.M.; Bunge, M.B. Localization of focal adhesion kinase in differentiating Schwann cell/neuron cultures. Microsc. Res. Tech. 1998, 41, 416–430. [Google Scholar] [CrossRef]
- Casella, G.T.; Wieser, R.; Bunge, R.P.; Margitich, I.S.; Katz, J.; Olson, L.; Wood, P.M. Density dependent regulation of human Schwann cell proliferation. Glia 2000, 30, 165–177. [Google Scholar] [CrossRef]
- Ridley, A.J.; Paterson, H.F.; Noble, M.; Land, H. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. Embo. J. 1988, 7, 1635–1645. [Google Scholar] [CrossRef]
- Eccleston, P.A.; Mirsky, R.; Jessen, K.R. Spontaneous immortalisation of Schwann cells in culture: Short-term cultured Schwann cells secrete growth inhibitory activity. Development 1991, 112, 33–42. [Google Scholar]
- Funk, D.; Fricke, C.; Schlosshauer, B. Aging Schwann cells in vitro. Eur. J. Cell Biol. 2007, 86, 207–219. [Google Scholar] [CrossRef]
- Langford, L.A.; Porter, S.; Bunge, R.P. Immortalized rat Schwann cells produce tumours in vivo. J. Neurocytol. 1988, 17, 521–529. [Google Scholar] [CrossRef]
- Morgan, L.; Jessen, K.R.; Mirsky, R. The effects of cAMP on differentiation of cultured Schwann cells: Progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J. Cell Biol. 1991, 112, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, P.V.; Soto, J.; Bacallao, K.; Wood, P.M. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: Role of cAMP and JNK in the maintenance of the differentiated state. J. Biol. Chem. 2010, 285, 31024–31036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monuki, E.S.; Weinmaster, G.; Kuhn, R.; Lemke, G. SCIP: A glial POU domain gene regulated by cyclic AMP. Neuron 1989, 3, 783–793. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Dickinson, S.; Bhaskaran, A.; Kinsella, M.T.; Brophy, P.J.; Sherman, D.L.; Sharghi-Namini, S.; Duran Alonso, M.B.; Mirsky, R.; Jessen, K.R. Regulation of the myelin gene periaxin provides evidence for Krox-20-independent myelin-related signalling in Schwann cells. Mol. Cell Neurosci. 2003, 23, 13–27. [Google Scholar] [CrossRef]
- Sobue, G.; Shuman, S.; Pleasure, D. Schwann cell responses to cyclic AMP: Proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986, 362, 23–32. [Google Scholar] [CrossRef]
- Varon, S.S.; Bunge, R.P. Trophic mechanisms in the peripheral nervous system. Annu. Rev. Neurosci. 1978, 1, 327–361. [Google Scholar] [CrossRef]
- Xu, X.M.; Guenard, V.; Kleitman, N.; Bunge, M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 1995, 351, 145–160. [Google Scholar] [CrossRef]
- Lopez-Leal, R.; Diaz-Viraque, F.; Catalan, R.J.; Saquel, C.; Enright, A.; Iraola, G.; Court, F.A. Schwann cell reprogramming into repair cells increases exosome-loaded miRNA-21 promoting axonal growth. J. Cell Sci. 2020. [Google Scholar] [CrossRef]
- Hood, B.; Levene, H.B.; Levi, A.D. Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects. Neurosurg. Focus 2009, 26, E4. [Google Scholar] [CrossRef]
- Muhammad, A.; Kim, K.; Epifantseva, I.; Aghamaleky-Sarvestany, A.; Simpkinson, M.E.; Carmona, S.; Landeros, J.; Bell, S.; Svaren, J.; Baloh, R.H. Cell transplantation strategies for acquired and inherited disorders of peripheral myelin. Ann. Clin. Transl. Neurol. 2018, 5, 186–200. [Google Scholar] [CrossRef] [Green Version]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askanas, V.; Engel, W.K.; Berginer, V.M.; Odenwald, W.F.; Galdi, A. Lysosomal abnormalities in cultured schwann cells from a patient with peripheral neuropathy and continuous muscle fiber activity. Ann. Neurol. 1981, 10, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Hoke, A. Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neurol. Disord. Drug Targets 2010, 9, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Lloyd, A.C. Neurofibroma development in NF1-insights into tumour initiation. Trends Cell Biol. 2009, 19, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Staff, N.P.; Hoke, A. Modeling chemotherapy induced peripheral neuropathy (CIPN) in vitro: Prospects and limitations. Exp. Neurol. 2020, 326, 113140. [Google Scholar] [CrossRef]
- Rambukkana, A.; Yamada, H.; Zanazzi, G.; Mathus, T.; Salzer, J.L.; Yurchenco, P.D.; Campbell, K.P.; Fischetti, V.A. Role of alpha-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science 1998, 282, 2076–2079. [Google Scholar] [CrossRef]
- Guertin, A.D.; Zhang, D.P.; Mak, K.S.; Alberta, J.A.; Kim, H.A. Microanatomy of axon/glial signaling during Wallerian degeneration. J. Neurosci. 2005, 25, 3478–3487. [Google Scholar] [CrossRef] [Green Version]
- Grimpe, B.; Pressman, Y.; Lupa, M.D.; Horn, K.P.; Bunge, M.B.; Silver, J. The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol. Cell Neurosci. 2005, 28, 18–29. [Google Scholar] [CrossRef]
- Echave, P.; Conlon, I.J.; Lloyd, A.C. Cell size regulation in mammalian cells. Cell Cycle 2007, 6, 218–224. [Google Scholar] [CrossRef]
- Ulrich, R.; Imbschweiler, I.; Kalkuhl, A.; Lehmbecker, A.; Ziege, S.; Kegler, K.; Becker, K.; Deschl, U.; Wewetzer, K.; Baumgartner, W. Transcriptional profiling predicts overwhelming homology of Schwann cells, olfactory ensheathing cells, and Schwann cell-like glia. Glia 2014, 62, 1559–1581. [Google Scholar] [CrossRef]
- Monje, P.V.; Sant, D.; Wang, G. Phenotypic and Functional Characteristics of Human Schwann Cells as Revealed by Cell-Based Assays and RNA-SEQ. Mol. Neurobiol. 2018, 55, 6637–6660. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Hoke, A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Chong, S.Y.; Tuck, S.J.; Corey, J.M.; Chan, J.R. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. Protoc. 2013, 8, 771–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Valle, C.; Fregien, N.; Wood, P.M.; Bunge, M.B. Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development 1993, 119, 867–880. [Google Scholar] [PubMed]
- Chan, J.R.; Watkins, T.A.; Cosgaya, J.M.; Zhang, C.; Chen, L.; Reichardt, L.F.; Shooter, E.M.; Barres, B.A. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 2004, 43, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; Xu, X.; Esper, R.M.; Loeb, J.A.; Shrager, P.; et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, T.K.; Bunge, R.P.; Kleitman, N. Human Schwann cells in vitro. I. Failure to differentiate and support neuronal health under co-culture conditions that promote full function of rodent cells. J. Neurobiol. 1995, 28, 171–189. [Google Scholar] [CrossRef]
- Mithen, F.A.; Cochran, M.; Cornbrooks, C.J.; Bunge, R.P. Expression of the trembler mouse mutation in organotypic cultures of dorsal root ganglia. Brain Res. 1982, 256, 407–415. [Google Scholar] [CrossRef]
- Clark, A.J.; Kaller, M.S.; Galino, J.; Willison, H.J.; Rinaldi, S.; Bennett, D.L.H. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain 2017, 140, 898–913. [Google Scholar] [CrossRef] [Green Version]
- Hoke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [Green Version]
- Obremski, V.J.; Johnson, M.I.; Bunge, M.B. Fibroblasts are required for Schwann cell basal lamina deposition and ensheathment of unmyelinated sympathetic neurites in culture. J. Neurocytol. 1993, 22, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, L.; Khakbiz, M.; Moosazadeh Moghaddam, M.; Bonakdar, S. A biomaterials approach to Schwann cell development in neural tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 2425–2446. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.; Clark, M.B.; Glaser, L.; Bunge, R.P. Schwann cells stimulated to proliferate in the absence of neurons retain full functional capability. J. Neurosci. 1986, 6, 3070–3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monje, P.V. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells 2020, 9, 1848. https://doi.org/10.3390/cells9081848
Monje PV. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells. 2020; 9(8):1848. https://doi.org/10.3390/cells9081848
Chicago/Turabian StyleMonje, Paula V. 2020. "Schwann Cell Cultures: Biology, Technology and Therapeutics" Cells 9, no. 8: 1848. https://doi.org/10.3390/cells9081848
APA StyleMonje, P. V. (2020). Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells, 9(8), 1848. https://doi.org/10.3390/cells9081848