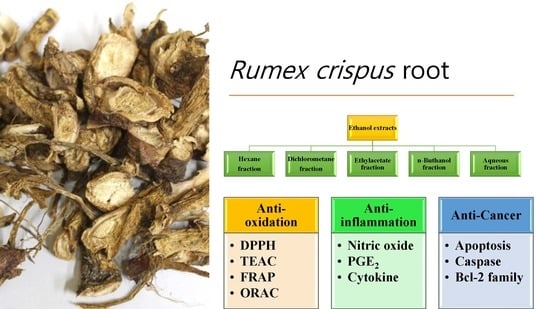
In Vitro Antioxidant, Antiinflammation, and Anticancer Activities and Anthraquinone Content from Rumex crispus Root Extract and Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Extracts and Fractions
2.3. Determination of Total Phenol and Flavonoid Contents
2.4. HPLC Analysis of Anthraquinone Derivative
2.5. DPPH Radical Scavenging Activity
2.6. Hydroxyl Radical Scavenging Activity
2.7. Superoxide Radical Scavenging Activity
2.8. TEAC Assay
2.9. ORAC Assay
2.10. FRAP Assay
2.11. Cell Culture and Cell Viability Assays
2.12. NO Production
2.13. Cytokine Analysis
2.14. Annexin V-FITC/PI Analysis
2.15. Western Blot
2.16. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Polyphenol, Flavonoid, and Anthraquinone Contents
3.2. Radical Scavenging Activities of R. crispus Extracts and Fractions
3.3. Antioxidant Capacities of R. crispus Extracts and Fractions
3.4. Anti-Inflammation Activities of R. crispus Extracts and Fractions
3.5. Anticancer Activities of R. crispus Extracts and Fractions
3.6. Modulation of Apoptotic Regulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Vasaa, A.; Orban-Gyapai, O.; Hohmann, J. The Geneus Rumex: Review of traditional uses, phytochemistry and pharmacoly. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef]
- Bello, O.M.; Fasinu, P.S.; Bello, O.E.; Ogbesejana, A.B.; Adetunji, C.O.; Dada, A.O.; Ibitoye, O.S.; Aloko, S.; Oguntoye, O.S. Wild vegetable Rumex acetosa Linn.: Its ethonobotany, pharmacology and phytochemistry—A review. S. Afr. J. Bot. 2019, 125, 149–160. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, Z.Z.; Mao, X.J.; Xu, Q.L.; Lin, R.C.; Dai, Z. Determination of free and total anthraquinones in 3 kinds of Rumex by HPLC: A comparative study. Chin. J. Pharm. Anal. 2017, 37, 615–623. [Google Scholar]
- Jang, S.J.; Kuk, Y.I. Effects of different fractions of Rheum palmatum root extract and anthraquinone compounds on fungicidal, insecticidal, and herbicidal activities. J. Plant. Dis Protect. 2018, 125, 451–460. [Google Scholar] [CrossRef]
- Prateeksha; Yusuf, M.A.; Singh, B.N.; Sudheer, S.; Kharwar, R.N.; Siddiqui, S.; Abdel-Azeem, A.M.; Fraceto, L.F.; Dashora, K.; Gupta, V.K. Chrysophanol: A natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Pekal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Method 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Eom, T.K.; Senevirathne, M.; Kim, S.K. Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ. Toxicol. Pharm. 2012, 34, 519–527. [Google Scholar] [CrossRef]
- Lebel, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Ooi, V.E.C.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frigola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 1999, 299, 15–27. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Tech. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007, 100, 1409–1418. [Google Scholar] [CrossRef]
- Malik, E.M.; Muller, C.E. Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Park, Y.S.; Hong, M.W.; Kim, D.K. Qunatotative analysis of anthraquinones from the roots of Korean natural Rumex spcies plant. Kor. J. Pharm. 2011, 42, 297–301. [Google Scholar]
- Wegiera, M.; Smolarz, H.D.; Wianowska, D.; Dawidowicz, A.L. Anthracene derivatives in some species of Rumex, L. genus. Acta Soc. Bot. Pol. 2007, 76, 103–108. [Google Scholar]
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crop. Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
- Lee, N.J.; Choi, J.H.; Koo, B.S.; Ryu, S.Y.; Han, Y.H.; Lee, S.I.; Lee, D.U. Antimutagenicity and cytotoxicity of the constituents from the aerial parts of Rumex acetosa. Biol. Pharm. Bull. 2005, 28, 2158–2161. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Antioxidant and antibacterial activities of Rumex japonicus HOUTT. Aerial parts. Biol. Pharm. Bull. 2005, 28, 2225–2230. [Google Scholar] [CrossRef] [Green Version]
- Ansuya, N.; Gomathi, R.; Manian, S.; Sivaram, V.; Menon, A. Evalution of Basella rubra L., Rumex nepalensis spreng. and Commelina benghalensis L. for antioxidant activity. Int. J. Pharm. Pharm. Sci. 2012, 4, 714–720. [Google Scholar]
- RiceEvans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. leaves. J. Med. Plants Res. 2011, 5, 2755–2765. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Im, N.K.; Jung, Y.S.; Choi, J.H.; Yu, M.H.; Jeong, G.S. Inhibitoy effect of leaves of Rumex crispus L. on LPS-induced nitric oxide production and the expression of iNOS and COX-2 in macrophage. Nat. Prod. Sci. 2014, 20, 51–57. [Google Scholar]
- Ng, S.; Galipeau, J. Concise review: Engineering the fusion of Cytokines for the modulation of immune cellular responses in cancer and autoimmune disorders. Stem Cell Transl. Med. 2015, 4, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth F R 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Xie, Q.C.; Yang, Y.P. Anti-proliferative of physcion 8-O-beta-glucopyranoside isolated from Rumex japonicus Houtt. on A549 cell lines via inducing apoptosis and cell cycle arrest. BMC Complement. Altern. Med. 2014, 14, 377. [Google Scholar] [CrossRef] [Green Version]
- Borner, C. The Bcl-2 protein family: Sensors and checkpoints for life-or-death decisions. Mol. Immunol. 2003, 39, 615–647. [Google Scholar] [CrossRef]
- Casciolarosen, L.A.; Miller, D.K.; Anhalt, G.J.; Rosen, A. Specific cleavage of the 70-Kda protein-component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell-death. J. Biol. Chem. 1994, 269, 30757–30760. [Google Scholar]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X.D. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Issaeva, N. p53 signaling in cancers. Cancers 2019, 11, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipuk, J.E.; Green, D.R. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006, 13, 994–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Samples 2 | Total Phenols (mg GAE/g) | Total Flavonoids (mg QE/g) |
|---|---|---|
| EE | 21.84 ± 1.15 c | 14.58 ± 0.61 d |
| HF | 10.68 ± 0.06 e | 24.15 ± 0.47 b |
| DCMF | 28.16 ± 1.42 b | 30.67 ± 0.97 a |
| EAF | 83.26 ± 2.49 a | 21.31 ± 0.33 c |
| BF | 19.03 ± 1.04 c,d | 11.28 ± 0.40 e |
| AF | 19.79 ± 0.32 c,d | 11.52 ± 0.70 e |
| Samples 2 | Concentration (mg/g) | |||||
|---|---|---|---|---|---|---|
| Aloeemodin | Chrysophanol | Emodin | Physcion | Rhein | Total | |
| EE | 0.141 ± 0.002 c | 9.714 ± 0.02 c | 8.779 ± 0.011 d | 4.282 ± 0.006 c | 0.057 ± 0.002 d | 22.97 ± 0.026 c |
| HF | 0.048 ± 0.006 e | 48.644 ±0.171 b | 14.64 ± 0.037 b | 15.433 ± 0.058 b | 0.106 ± 0.001 c | 79.095 ± 0.259 b |
| DCMF | 0.595 ± 0.003 a | 66.964 ± 0.244 a | 160.434 ± 0.651 a | 34.896 ± 0.109 a | 0.466 ± 0.002 a | 263.356 ± 0.666 a |
| EAF | 0.218 ± 0.001 b | 3.154 ± 0.009 d | 13.627 ± 0.053 c | 1.722 ± 0.006 d | 0.151 ± 0.002 b | 18.923 ± 0.062 d |
| BF | 0.181 ± 0.007 d | 0.083 ± 0.002 e | 0.639 ± 0.004 e | 0.054 ± 0.002 e | 0.001> | 0.856 ± 0.006 e |
| AF | - | - | - | - | - | - |
| Samples 2 | EC503 | ||
|---|---|---|---|
| DPPH Radical | Hydroxyl Radical | Superoxide Radical | |
| EE | 46.5 ± 2.6 b | 19.65 ± 0.64 c | 51.72 ± 2.00 c |
| HF | 126.2 ± 1.3 e | 62.47 ± 2.44 f | >200 f |
| DCMF | 65.6 ± 1.2 d | 0.54 ± 0.13 a | 45.83 ± 2.00 b |
| EAF | 11.9 ± 2.5 a | 0.65 ± 0.06 a | 4.45 ± 0.42 a |
| BF | 55.1 ± 1.5 c | 3.84 ± 0.35 d | 61.00 ± 2.81 d |
| AF | 44.2 ± 3.4 b | 43.12 ± 0.00 e | 71.29 ± 2.39 e |
| Samples 2 | FRAP (mM FeSO4/g) | TEAC (mM TE/g) | ORAC (mM TE/g) |
|---|---|---|---|
| EE | 48.14 ± 0.47 b | 2.46 ± 0.11 c | 1396 ± 204 b,c |
| HF | 13.66 ± 0.15 e | 0.43 ± 0.06 f | 983 ± 88 c,d |
| DCMF | 37.74 ± 0.77 c,d | 3.56 ± 0.11 b | 1790 ± 246 b |
| EAF | 135.58 ± 3.62 a | 5.65 ± 0.00 a | 4817 ± 331 a |
| BF | 33.06 ± 0.80 d | 1.97 ± 0.13 d | 909 ± 121 d |
| AF | 40.83 ± 0.16 c | 2.30 ± 0.13 c | 945 ± 87 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, T.; Kim, E.; Kim, J.-S. In Vitro Antioxidant, Antiinflammation, and Anticancer Activities and Anthraquinone Content from Rumex crispus Root Extract and Fractions. Antioxidants 2020, 9, 726. https://doi.org/10.3390/antiox9080726
Eom T, Kim E, Kim J-S. In Vitro Antioxidant, Antiinflammation, and Anticancer Activities and Anthraquinone Content from Rumex crispus Root Extract and Fractions. Antioxidants. 2020; 9(8):726. https://doi.org/10.3390/antiox9080726
Chicago/Turabian StyleEom, Taekil, Ekyune Kim, and Ju-Sung Kim. 2020. "In Vitro Antioxidant, Antiinflammation, and Anticancer Activities and Anthraquinone Content from Rumex crispus Root Extract and Fractions" Antioxidants 9, no. 8: 726. https://doi.org/10.3390/antiox9080726
APA StyleEom, T., Kim, E., & Kim, J.-S. (2020). In Vitro Antioxidant, Antiinflammation, and Anticancer Activities and Anthraquinone Content from Rumex crispus Root Extract and Fractions. Antioxidants, 9(8), 726. https://doi.org/10.3390/antiox9080726