The Resistance of Drosophila melanogaster to Oxidative, Genotoxic, Proteotoxic, Osmotic Stress, Infection, and Starvation Depends on Age According to the Stress Factor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila Rearing
2.2. Analysis of Stress Resistance
2.3. Analysis of Experimental Data
2.4. Age Estimation Algorithm for the Survival Data of Flies with an Unknown Age
3. Results
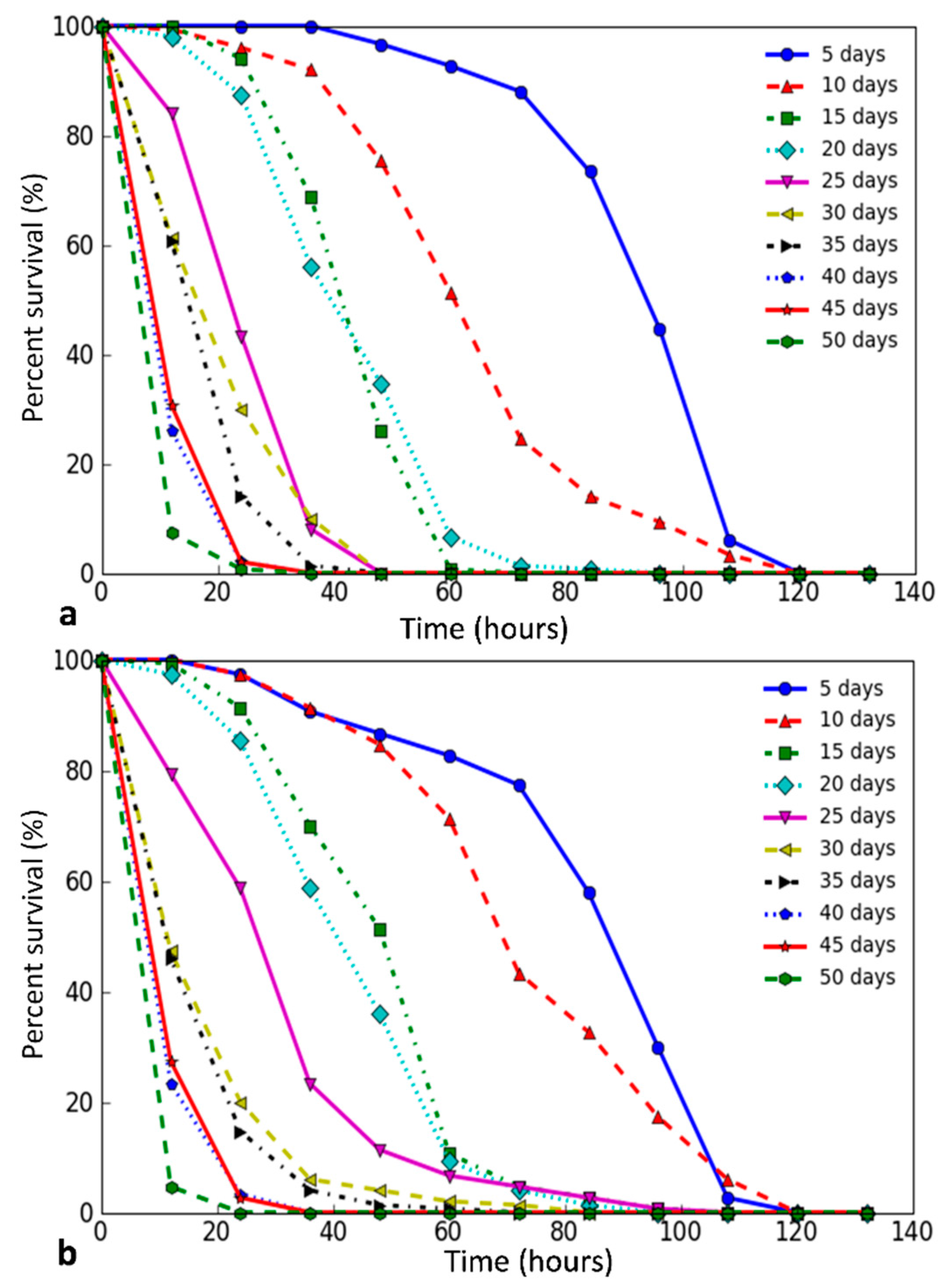
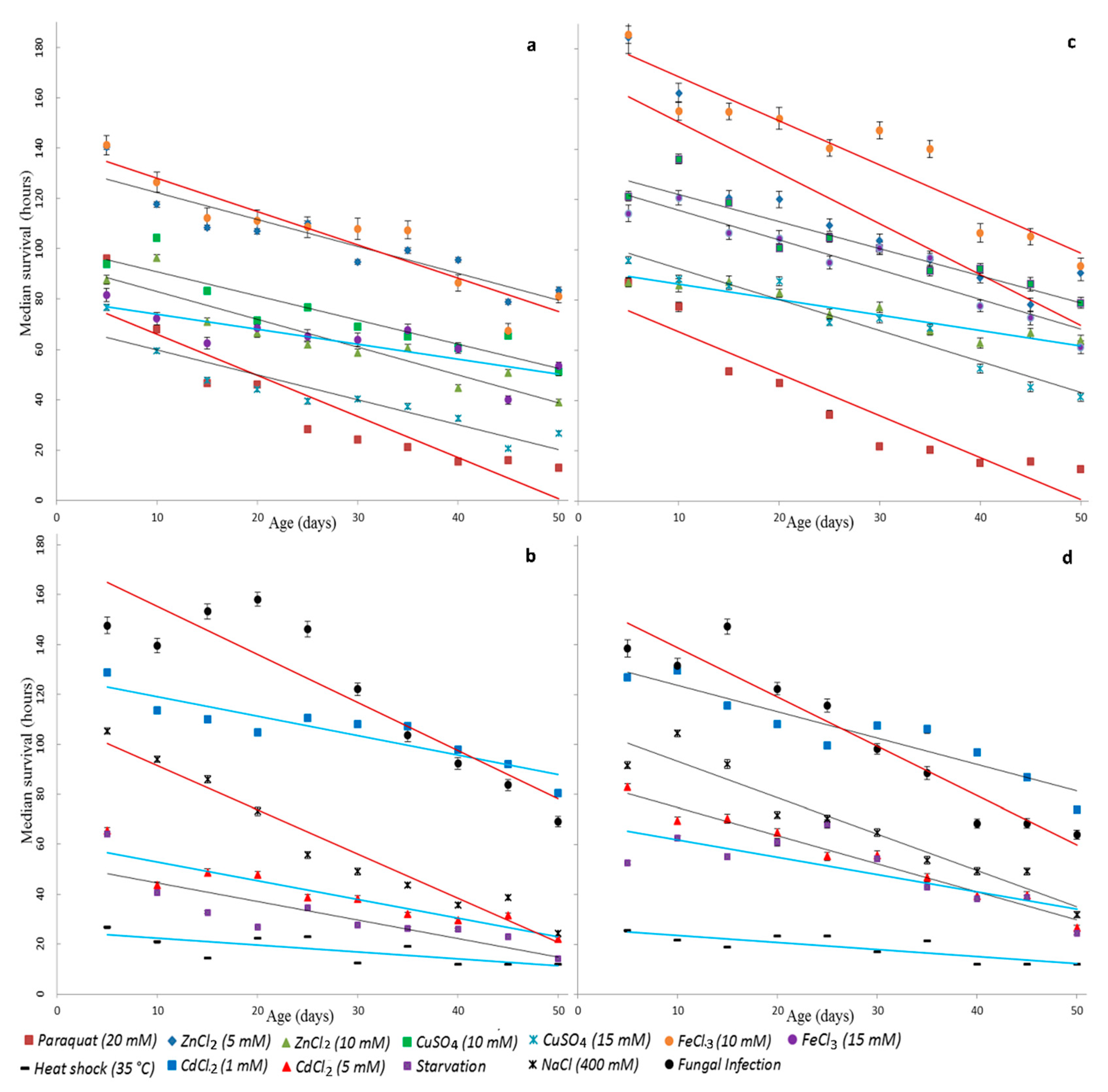
3.1. Age Dynamics of Stress Resistance
3.2. Prediction of the Biological Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Coppede, F.; Migliore, L. DNA repair in premature aging disorders and neurodegeneration. Curr. Aging Sci. 2010, 3, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Proshkina, E.N.; Shaposhnikov, M.V. Gadd45 Proteins in Aging and Longevity of Mammals and Drosophila. In Life Extension; Springer: Berlin/Heidelberg, Germany, 2015; pp. 39–65. [Google Scholar]
- Tower, J. Heat shock proteins and Drosophila aging. Exp. Gerontol. 2011, 46, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, V.; Scapagnini, G.; Ravagna, A.; Colombrita, C.; Spadaro, F.; Butterfield, D.; Stella, A.G. Increased expression of heat shock proteins in rat brain during aging: Relationship with mitochondrial function and glutathione redox state. Mech. Ageing Dev. 2004, 125, 325–335. [Google Scholar] [CrossRef]
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Wong, S.; Carney, C.; Shen, B.; Tower, J.; Davies, K.J.A. The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging 2017, 9, 1153–1185. [Google Scholar] [CrossRef] [Green Version]
- Tomaru, U.; Takahashi, S.; Ishizu, A.; Miyatake, Y.; Gohda, A.; Suzuki, S.; Ono, A.; Ohara, J.; Baba, T.; Murata, S. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am. J. Pathol. 2012, 180, 963–972. [Google Scholar] [CrossRef]
- Aravinthan, A. Cellular senescence: A hitchhiker’s guide. Hum. Cell 2015, 28, 51–64. [Google Scholar] [CrossRef]
- Kültz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef]
- Dues, D.J.; Andrews, E.K.; Senchuk, M.M.; Van Raamsdonk, J.M. Resistance to Stress Can Be Experimentally Dissociated from Longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 1206–1214. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, T.B.; Austad, S.N. Why do we age? Nature 2000, 408, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L.; Kenyon, C. Genetic pathways that regulate ageing in model organisms. Nature 2000, 408, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Deepashree, S.; Niveditha, S.; Shivanandappa, T.; Ramesh, S.R. Oxidative stress resistance as a factor in aging: Evidence from an extended longevity phenotype of Drosophila melanogaster. Biogerontology 2019, 20, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Fujii, M.; Hartman, P.S.; Tsuda, M.; Yasuda, K.; Senoo-Matsuda, N.; Yanase, S.; Ayusawa, D.; Suzuki, K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature 1998, 394, 694–697. [Google Scholar] [CrossRef]
- Ikeyama, S.; Kokkonen, G.; Shack, S.; Wang, X.T.; Holbrook, N.J. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J. 2002, 16, 114–116. [Google Scholar] [CrossRef] [Green Version]
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Soreide, K. Molecular and biological hallmarks of ageing. Br. J. Surg. 2016, 103, e29–e46. [Google Scholar] [CrossRef] [Green Version]
- Baker, G.T., III; Sprott, R.L. Biomarkers of aging. Exp. Gerontol. 1988, 23, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Qian, W.; Wu, G.; Chen, W.; Xian, B.; Chen, X.; Cao, Y.; Green, C.D.; Zhao, F.; Tang, K. Three-dimensional human facial morphologies as robust aging markers. Cell Res. 2015, 25, 574–587. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Cohen, H.J.; Muss, H.B. Incorporating biomarkers into cancer and aging research. J. Clin. Oncol. 2014, 32, 2611–2616. [Google Scholar] [CrossRef] [Green Version]
- Pincus, Z.; Smith-Vikos, T.; Slack, F.J. MicroRNA predictors of longevity in Caenorhabditis elegans. PLoS Genet. 2011, 7, e1002306. [Google Scholar] [CrossRef] [Green Version]
- Herndon, L.A.; Schmeissner, P.J.; Dudaronek, J.M.; Brown, P.A.; Listner, K.M.; Sakano, Y.; Paupard, M.C.; Hall, D.H.; Driscoll, M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002, 419, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Feng, Z.; Hsieh, M.Y.; Xu, X.Z. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol. Aging 2009, 30, 1498–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.K.; Bundy, J.G.; Leroi, A.M. Metabolic Youth in Middle Age: Predicting Aging in Caenorhabditis elegans Using Metabolomics. J. Proteome Res. 2015, 14, 4603–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, H.-G.; Carey, J.R.; Wu, D.; Liedo, P.; Vaupel, J.W. Reproductive potential predicts longevity of female Mediterranean fruitflies. Proc. R. Soc. Lond. B Biol. Sci. 2001, 268, 445–450. [Google Scholar] [CrossRef]
- Mueller, L.D.; Shahrestani, P.; Rauser, C.L.; Rose, M.R. The death spiral: Predicting death in Drosophila cohorts. Biogerontology 2016, 17, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Wax, T.M.; Goodrick, C.L. Nearness to death and wheelrunning behavior in mice. Exp. Gerontol. 1978, 13, 233–236. [Google Scholar] [CrossRef]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring biological aging in humans: A quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Schiffer, E.; Song, Z.; Wang, J.; Zurbig, P.; Thedieck, K.; Moes, S.; Bantel, H.; Saal, N.; Jantos, J.; et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc. Natl. Acad. Sci. USA 2008, 105, 11299–11304. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef] [Green Version]
- Dhahbi, J.M. Circulating small noncoding RNAs as biomarkers of aging. Ageing Res. Rev. 2014, 17, 86–98. [Google Scholar] [CrossRef]
- Olivieri, F.; Capri, M.; Bonafe, M.; Morsiani, C.; Jung, H.J.; Spazzafumo, L.; Vina, J.; Suh, Y. Circulating miRNAs and miRNA shuttles as biomarkers: Perspective trajectories of healthy and unhealthy aging. Mech. Ageing Dev. 2017, 165, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Hong, R.; Chen, W.; Xu, M.; Wang, L. The role of long noncoding RNA in major human disease. Bioorg. Chem. 2019, 92, 103214. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.; Yang, J.; Ford, D.; Tavaré, S.; Tower, J. Simultaneous tracking of movement and gene expression in multiple Drosophila melanogaster flies using GFP and DsRED fluorescent reporter transgenes. BMC Res. Notes 2009, 2, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, G.N.; Abdueva, D.; Skvortsov, D.; Yang, J.; Rabin, B.E.; Carrick, J.; Tavare, S.; Tower, J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2004, 101, 7663–7668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, G.; Shen, J.; Tower, J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging 2012, 4, 768–789. [Google Scholar] [CrossRef] [Green Version]
- De Magalhães, J.P.; Curado, J.; Church, G.M. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 2009, 25, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef] [Green Version]
- Salmon, A.B.; Richardson, A.; Pérez, V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 2010, 48, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Holmstrom, K.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Aliper, A.; Belikov, A.V.; Garazha, A.; Jellen, L.; Artemov, A.; Suntsova, M.; Ivanova, A.; Venkova, L.; Borisov, N.; Buzdin, A.; et al. In search for geroprotectors: In silico screening and in vitro validation of signalome-level mimetics of young healthy state. Aging 2016, 8, 2127–2152. [Google Scholar] [CrossRef] [Green Version]
- Geissmann, Q.; Beckwith, E.J.; Gilestro, G.F. Most sleep does not serve a vital function: Evidence from Drosophila melanogaster. Sci. Adv. 2019, 5, eaau9253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leech, T.; Sait, S.M.; Bretman, A. Sex-specific effects of social isolation on ageing in Drosophila melanogaster. J. Insect Physiol. 2017, 102, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaposhnikov, M.; Proshkina, E.; Shilova, L.; Zhavoronkov, A.; Moskalev, A. Lifespan and Stress Resistance in Drosophila with Overexpressed DNA Repair Genes. Sci. Rep. 2015, 5, 15299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-S.; Nam, H.-J.; Seo, M.; Han, S.K.; Choi, Y.; Nam, H.G.; Lee, S.-J.; Kim, S. OASIS: Online Application for the Survival Analysis of Lifespan Assays Performed in Aging Research. PLoS ONE 2011, 6, e23525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Core, T.R. General-Purpose Optimization. Available online: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/optim.html (accessed on 20 November 2020).
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Kourtis, N.; Tavernarakis, N. Cellular stress response pathways and ageing: Intricate molecular relationships. EMBO J. 2011, 30, 2520–2531. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kaarniranta, K. ER stress and hormetic regulation of the aging process. Ageing Res. Rev. 2010, 9, 211–217. [Google Scholar] [CrossRef]
- Pletcher, S.D.; Macdonald, S.J.; Marguerie, R.; Certa, U.; Stearns, S.C.; Goldstein, D.B.; Partridge, L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol. 2002, 12, 712–723. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, J.G.; Nielsen, M.M.; Kruhøffer, M.; Justesen, J.; Loeschcke, V. Full genome gene expression analysis of the heat stress response in Drosophila melanogaster. Cell Stress Chaperones 2005, 10, 312–328. [Google Scholar] [CrossRef]
- Pall, M.L.; Levine, S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Acta Physiol. Sin. 2015, 67, 1–18. [Google Scholar]
- Shao, X.; Fu, Y.; Ma, J.; Li, X.; Lu, C.; Zhang, R. Functional alterations and transcriptomic changes during zebrafish cardiac aging. Biogerontology 2020, 21, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Sanders, V.M.; Snyder, J.M.; Uhr, J.W.; Vitetta, E.S. Characterization of the physical interaction between antigen-specific B and T cells. J. Immunol. 1986, 137, 2395–2404. [Google Scholar] [PubMed]
- Niedernhofer, L.J.; Gurkar, A.U.; Wang, Y.; Vijg, J.; Hoeijmakers, J.H.J.; Robbins, P.D. Nuclear Genomic Instability and Aging. Annu. Rev. Biochem. 2018, 87, 295–322. [Google Scholar] [CrossRef]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef] [Green Version]
- Sas, K.; Szabo, E.; Vecsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef] [Green Version]
- Kregel, K.C. Invited review: Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef] [Green Version]
- Tower, J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch. Biochem. Biophys. 2015, 576, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ahn, Y.J.; Asmis, R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020, 31, 101410. [Google Scholar] [CrossRef]
- Niveditha, S.; Deepashree, S.; Ramesh, S.R.; Shivanandappa, T. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J. Comp. Physiol. B 2017, 187, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Adeogun, A.O.; Ibor, O.R.; Omiwole, R.; Chukwuka, A.V.; Adewale, A.H.; Kumuyi, O.; Arukwe, A. Sex-differences in physiological and oxidative stress responses and heavy metals burden in the black jaw tilapia, Sarotherodon melanotheron from a tropical freshwater dam (Nigeria). Comp. Biochem. Physiol. C Toxicol. Pharm. 2020, 229, 108676. [Google Scholar] [CrossRef] [PubMed]
- Barcena de Arellano, M.L.; Pozdniakova, S.; Kuhl, A.A.; Baczko, I.; Ladilov, Y.; Regitz-Zagrosek, V. Sex differences in the aging human heart: Decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging 2019, 11, 1918–1933. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Nugent, B.M. Epigenetic Contributions to Hormonally-Mediated Sexual Differentiation of the Brain. J. Neuroendocrinol. 2013, 25, 1133–1140. [Google Scholar] [CrossRef] [Green Version]
- Nugent, B.M.; Wright, C.L.; Shetty, A.C.; Hodes, G.E.; Lenz, K.M.; Mahurkar, A.; Russo, S.J.; Devine, S.E.; McCarthy, M.M. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 2015, 18, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Fano, G.; Mecocci, P.; Vecchiet, J.; Belia, S.; Fulle, S.; Polidori, M.C.; Felzani, G.; Senin, U.; Vecchiet, L.; Beal, M.F. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J. Muscle Res. Cell Motil. 2001, 22, 345–351. [Google Scholar] [CrossRef]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- LeMaoult, J.; Szabo, P.; Weksler, M.E. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol. Rev. 1997, 160, 115–126. [Google Scholar] [CrossRef]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef]
- De Benedictis, G.; Franceschi, C. The unusual genetics of human longevity. SAGE KE 2006, 2006, pe20. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, D.K.; Bussière, L.F.; Tinsley, M.C. Senescence of the cellular immune response in Drosophila melanogaster. Exp. Gerontol. 2011, 46, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Nam, H.J.; Chung, H.Y.; Kim, N.D.; Ryu, J.H.; Lee, W.J.; Arking, R.; Yoo, M.A. Role of xanthine dehydrogenase and aging on the innate immune response of Drosophila. J. Am. Aging Assoc. 2001, 24, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Karpac, J.; Tran, S.L.; Jasper, H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell 2014, 156, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Chippindale, A.K.; Chu, T.J.; Rose, M.R. Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 1996, 50, 753–766. [Google Scholar] [CrossRef]
- Harshman, L.; Hoffmann, A.; Clark, A. Selection for starvation resistance in Drosophila melanogaster: Physiological correlates, enzyme activities and multiple stress responses. J. Evol. Biol. 1999, 12, 370–379. [Google Scholar] [CrossRef]
- Service, P.M. Physiological mechanisms of increased stress resistance in Drosophila melanogaster selected for postponed senescence. Physiol. Zool. 1987, 60, 321–326. [Google Scholar] [CrossRef]
- Harbison, S.T.; Chang, S.; Kamdar, K.P.; Mackay, T.F. Quantitative genomics of starvation stress resistance in Drosophila. Genome Biol. 2005, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.; Hallas, R.; Anderson, A.; Telonis-Scott, M. Evidence for a robust sex-specific trade-off between cold resistance and starvation resistance in Drosophila melanogaster. J. Evol. Biol. 2005, 18, 804–810. [Google Scholar] [CrossRef]
- Colinet, H.; Chertemps, T.; Boulogne, I.; Siaussat, D. Age-related Decline of Abiotic Stress Tolerance in Young Drosophila melanogaster Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 71, 1574–1580. [Google Scholar] [CrossRef]
- Sano, Y.; Akimaru, H.; Okamura, T.; Nagao, T.; Okada, M.; Ishii, S. Drosophila activating transcription factor-2 is involved in stress response via activation by p38, but not c-Jun NH2-terminal kinase. Mol. Biol. Cell 2005, 16, 2934–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, H.; Tateno, M.; Fujimura-Kamada, K.; Takaesu, G.; Adachi-Yamada, T.; Ninomiya-Tsuji, J.; Irie, K.; Nishida, Y.; Matsumoto, K. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J. 2001, 20, 5421–5430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Huang, Y.; Chinnappan, R.; Bocchini, C.; Gustin, M.C.; Stern, M. The Drosophila inebriated-encoded neurotransmitter/osmolyte transporter: Dual roles in the control of neuronal excitability and the osmotic stress response. Genetics 2002, 160, 561–569. [Google Scholar] [PubMed]
- Gibbs, A.G.; Louie, A.K.; Ayala, J.A. Effects of temperature on cuticular lipids and water balance in a desert Drosophila: Is thermal acclimation beneficial? J. Exp. Biol. 1998, 201, 71–80. [Google Scholar] [PubMed]
- Chippindale, A.K.; Gibbs, A.G.; Sheik, M.; Yee, K.J.; Djawdan, M.; Bradley, T.J.; Rose, M.R. Resource acquisition and the evolution of stress resistance in Drosophila melanogaster. Evolution 1998, 52, 1342–1352. [Google Scholar] [CrossRef]
- Yankner, B.A.; Lu, T.; Loerch, P. The aging brain. Annu. Rev. Pathmechdis. Mech. Dis. 2008, 3, 41–66. [Google Scholar] [CrossRef]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Moskalev, A.; Aliper, A.; Smit-McBride, Z.; Buzdin, A.; Zhavoronkov, A. Genetics and epigenetics of aging and longevity. Cell Cycle 2014, 13, 1063–1077. [Google Scholar] [CrossRef] [Green Version]
- Mitnitski, A.; Howlett, S.E.; Rockwood, K. Heterogeneity of human aging and its assessment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Bachi, K.; Sierra, S.; Volkow, N.D.; Goldstein, R.Z.; Alia-Klein, N. Is biological aging accelerated in drug addiction? Curr. Opin. Behav. Sci. 2017, 13, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, M.J.; Joehanes, R.; Pilling, L.C.; Schurmann, C.; Conneely, K.N.; Powell, J.; Reinmaa, E.; Sutphin, G.L.; Zhernakova, A.; Schramm, K.; et al. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 2015, 6, 8570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Kawai, K.; Takeshita, Y.; Honda, M.; Takamura, T.; Kaneko, S.; Matoba, R.; Matsubara, K. Identification of blood biomarkers of aging by transcript profiling of whole blood. Biochem. Biophys. Res. Commun. 2012, 418, 313–318. [Google Scholar] [CrossRef]
- Menni, C.; Kastenmuller, G.; Petersen, A.K.; Bell, J.T.; Psatha, M.; Tsai, P.C.; Gieger, C.; Schulz, H.; Erte, I.; John, S.; et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int. J. Epidemiol. 2013, 42, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Krištić, J.; Vučković, F.; Menni, C.; Klarić, L.; Keser, T.; Beceheli, I.; Pučić-Baković, M.; Novokmet, M.; Mangino, M.; Thaqi, K. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Aliper, A.M.; Csoka, A.B.; Buzdin, A.; Jetka, T.; Roumiantsev, S.; Moskalev, A.; Zhavoronkov, A. Signaling pathway activation drift during aging: Hutchinson-Gilford Progeria Syndrome fibroblasts are comparable to normal middle-age and old-age cells. Aging 2015, 7, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.C.; Bohmann, D.; Jasper, H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev. Cell 2003, 5, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. In Methods in Enzymolology; Elsevier: Amsterdam, The Netherlands, 1990; pp. 465–478. [Google Scholar]
- Demontis, F.; Perrimon, N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 2010, 143, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, J.; Lambert, A.J.; Portero-Otín, M.; Pamplona, R.; Magwere, T.; Miwa, S.; Driege, Y.; Brand, M.D.; Partridge, L. Biomarkers of aging in Drosophila. Aging Cell 2010, 9, 466–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Mutcherson, R.; Helfand, S.L. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell 2005, 4, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Karpac, J.; Supoyo, S.; DeGennaro, M.; Lehmann, R.; Jasper, H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010, 6, e1001159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef] [Green Version]
- Rera, M.; Azizi, M.J.; Walker, D.W. Organ-specific mediation of lifespan extension: More than a gut feeling? Ageing Res. Rev. 2013, 12, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Kaji, K.; Ohta, T.; Horie, N.; Naru, E.; Hasegawa, M.; Kanda, N. Donor age reflects the replicative lifespan of human fibroblasts in culture. Hum. Cell 2009, 22, 38–42. [Google Scholar] [CrossRef]
- Putin, E.; Mamoshina, P.; Aliper, A.; Korzinkin, M.; Moskalev, A.; Kolosov, A.; Ostrovskiy, A.; Cantor, C.; Vijg, J.; Zhavoronkov, A. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging 2016, 8, 1021. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Kritchevsky, S.B.; Newman, A.B.; Goodpaster, B.H.; Tylavsky, F.A.; Nevitt, M.C.; Harris, T.B. Lower serum albumin concentration and change in muscle mass: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 531–537. [Google Scholar]
- WHO. Health in 2015: From MDGs, Millennium Development Goals to SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Cohen, A.A.; Milot, E.; Li, Q.; Bergeron, P.; Poirier, R.; Dusseault-Bélanger, F.; Fülöp, T.; Leroux, M.; Legault, V.; Metter, E.J. Detection of a novel, integrative aging process suggests complex physiological integration. PLoS ONE 2015, 10, e0116489. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Cho, B.; Kwon, H.; Lee, C. Developing a biological age assessment equation using principal component analysis and clinical biomarkers of aging in Korean men. Arch. Gerontol. Geriatr. 2009, 49, 7–12. [Google Scholar] [CrossRef]

| Stressor | Sex | Intermediate Ages (days) 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7.5 | 12.5 | 17.5 | 22.5 | 27.5 | 32.5 | 37.5 | 42.5 | 47.5 | ||
| Paraquat | male | 0.021 | 1.458 | 0.242 | 0.574 | 0.41 | 0.008 | 1.785 | 0.554 | 0.426 |
| Paraquat | female | 0.026 | 0.689 | 0.436 | 0.17 | 0.372 | 0.701 | 0.662 | 0.914 | 1.909 |
| Starvation | male | 1.044 | 0.894 | 5.964 | 2.321 | 3.24 | 0.522 | 1.423 | 0.273 | 1.856 |
| Starvation | female | 0.149 | 1.627 | 0.971 | 0.835 | 10.282 | 0.546 | 1.421 | 0.046 | 0.377 |
| Heat shock | male | 11.005 | 1.842 | 3.169 | 2.293 | 12.721 | 17.619 | 11.005 | 1.842 | 3.169 |
| Heat shock | female | 0.714 | 10.676 | 5.475 | 0.714 | 10.676 | 5.475 | 0.714 | 10.676 | 5.475 |
| Fungal infection | male | 5.567 | 0.364 | 0.976 | 3.381 | 0.596 | 0.101 | 0.251 | 0.346 | 0.169 |
| Fungal infection | female | 1.253 | 0.472 | 4.116 | 0.026 | 0.413 | 0.392 | 0.356 | 0.71 | 1.476 |
| NaCl (400 mM) | male | 0.777 | 0.156 | 0.284 | 0.255 | 0.29 | 0.529 | 3.531 | 0.274 | 0.06 |
| NaCl (400 mM) | female | 1.477 | 0.999 | 0.569 | 0.127 | 0.03 | 0.193 | 1.634 | 0.389 | 0.085 |
| ZnCl2 (5 mM) | male | 0.166 | 5.121 | 2.37 | 2.181 | 0.856 | 0.927 | 1.232 | 0.112 | 0.764 |
| ZnCl2 (5 mM) | female | 0.008 | 0.196 | 1.088 | 0.445 | 0.17 | 0.2 | 0.25 | 0.579 | 0.315 |
| ZnCl2 (10 mM) | male | 0.401 | 0.465 | 0.28 | 0.312 | 2.067 | 1.102 | 4.551 | 1.875 | 0.075 |
| ZnCl2 (10 mM) | female | 3.388 | 0.378 | 0.745 | 0.445 | 0.188 | 0.207 | 3.247 | 1.969 | 5.283 |
| CuSO4 (10 mM) | male | 0.77 | 0.684 | 1.575 | 0.206 | 2.014 | 0.509 | 3.152 | 0.849 | 0.286 |
| CuSO4 (10 mM) | female | 1.651 | 2.315 | 1.201 | 1.078 | 3.545 | 0.384 | 0.385 | 1.568 | 0.667 |
| CuSO4 (15 mM) | male | 0.405 | 0.226 | 0.257 | 2.355 | 0.847 | 1.77 | 0.096 | 0.611 | 2.619 |
| CuSO4 (15 mM) | female | 0.256 | 2.042 | 2.519 | 0.365 | 1.039 | 0.608 | 0.21 | 0.252 | 0.393 |
| CdCl2 (1 mM) | male | 0.248 | 2.211 | 9.968 | 4.063 | 2.996 | 5.207 | 0.478 | 0.541 | 0.623 |
| CdCl2 (1 mM) | female | 0.067 | 0.144 | 0.25 | 10.195 | 2.957 | 1.883 | 2.348 | 0.297 | 0.404 |
| CdCl2 (5 mM) | male | 7.518 | 0.085 | 2.084 | 4.069 | 0.419 | 0.628 | 2.112 | 0.166 | 0.308 |
| CdCl2 (5 mM) | female | 0.131 | 1.119 | 0.076 | 0.507 | 0.079 | 0.042 | 0.036 | 0.556 | 0.314 |
| FeCl3 (10 mM) | male | 0.184 | 0.097 | 3.583 | 0.114 | 1.98 | 0.168 | 0.069 | 0.186 | 0.806 |
| FeCl3 (10 mM) | female | 0.385 | 1.201 | 1.514 | 2.423 | 1.443 | 3.772 | 0.001 | 0.144 | 0.025 |
| FeCl3 (15 mM) | male | 0.203 | 11.538 | 6.478 | 0.295 | 3.773 | 7.816 | 0.507 | 0.184 | 2.977 |
| FeCl3 (15 mM) | female | 0.691 | 0.037 | 0.867 | 5.516 | 1.732 | 2.774 | 0.28 | 0.001 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belyi, A.A.; Alekseev, A.A.; Fedintsev, A.Y.; Balybin, S.N.; Proshkina, E.N.; Shaposhnikov, M.V.; Moskalev, A.A. The Resistance of Drosophila melanogaster to Oxidative, Genotoxic, Proteotoxic, Osmotic Stress, Infection, and Starvation Depends on Age According to the Stress Factor. Antioxidants 2020, 9, 1239. https://doi.org/10.3390/antiox9121239
Belyi AA, Alekseev AA, Fedintsev AY, Balybin SN, Proshkina EN, Shaposhnikov MV, Moskalev AA. The Resistance of Drosophila melanogaster to Oxidative, Genotoxic, Proteotoxic, Osmotic Stress, Infection, and Starvation Depends on Age According to the Stress Factor. Antioxidants. 2020; 9(12):1239. https://doi.org/10.3390/antiox9121239
Chicago/Turabian StyleBelyi, Alexei A., Alexey A. Alekseev, Alexander Y. Fedintsev, Stepan N. Balybin, Ekaterina N. Proshkina, Mikhail V. Shaposhnikov, and Alexey A. Moskalev. 2020. "The Resistance of Drosophila melanogaster to Oxidative, Genotoxic, Proteotoxic, Osmotic Stress, Infection, and Starvation Depends on Age According to the Stress Factor" Antioxidants 9, no. 12: 1239. https://doi.org/10.3390/antiox9121239
APA StyleBelyi, A. A., Alekseev, A. A., Fedintsev, A. Y., Balybin, S. N., Proshkina, E. N., Shaposhnikov, M. V., & Moskalev, A. A. (2020). The Resistance of Drosophila melanogaster to Oxidative, Genotoxic, Proteotoxic, Osmotic Stress, Infection, and Starvation Depends on Age According to the Stress Factor. Antioxidants, 9(12), 1239. https://doi.org/10.3390/antiox9121239







