Developmental Origins of Kidney Disease: Why Oxidative Stress Matters?
Abstract
:1. Introduction
2. Oxidative Stress and Developmental Programming
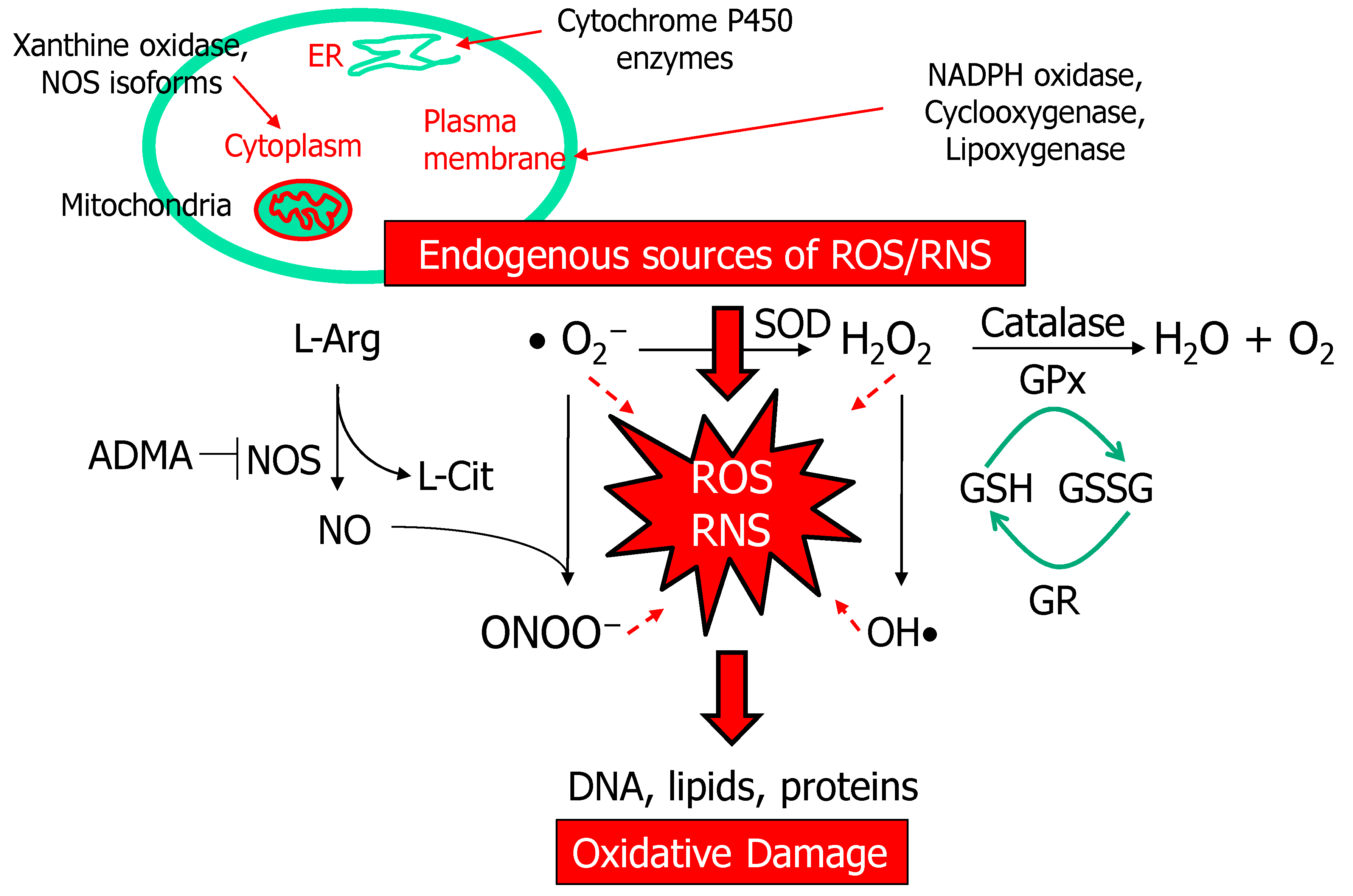
2.1. ROS and NO Signal
2.2. Redox State During Pregnancy
2.3. Oxidative Stress in Fetal Programming
3. Developmental Origins of Kidney Disease
3.1. Kidney Development
3.2. Oxidative Stress-Related Renal Programming in Animal Models
3.3. Nephron Number and Oxidative Stress
3.4. Reported Mechanisms of Oxidative Stress in Renal Programming
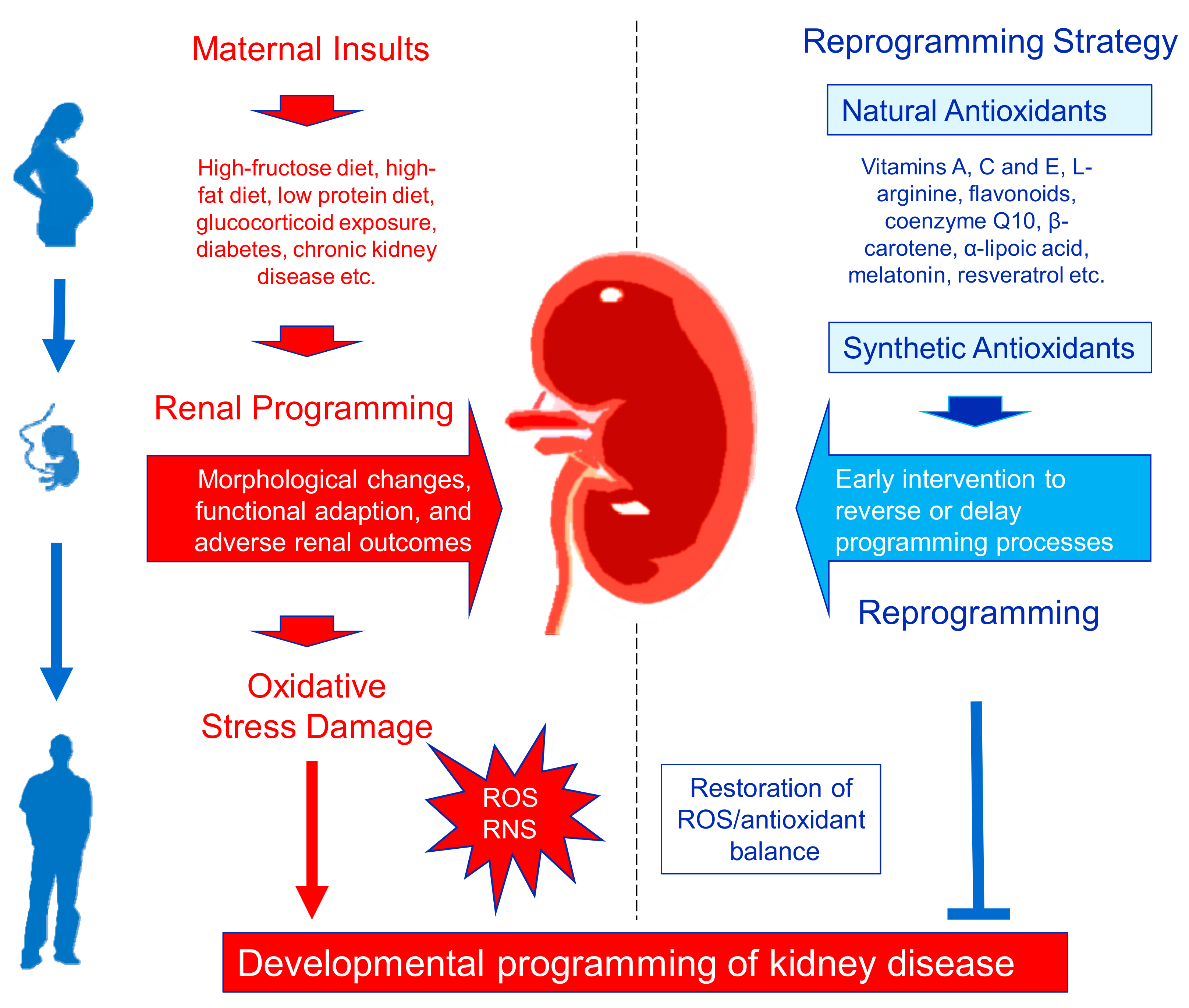
4. Targeting Oxidative Stress by Antioxidants as a Reprogramming Strategy
4.1. Antioxidants
4.2. Antioxidant Therapy as a Reprogramming Strategy
4.3. Vitamins
4.4. Amino Acids
4.5. Melatonin
4.6. Resveratrol
4.7. Synthetic Antioxidants
4.8. N-Acetylcysteine
5. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Developmental Origins of Chronic Kidney Disease: Should We Focus on Early Life? Int. J. Mol. Sci. 2017, 18, 381. [Google Scholar] [CrossRef] [Green Version]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Ingelfinger, J.R.; Kalantar-Zadeh, K.; Schaefer, F.; World Kidney Day Steering Committee. World Kidney Day 2016: Averting the legacy of kidney disease-focus on childhood. Pediatr. Nephrol. 2016, 31, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Nüsken, E.; Dötsch, J.; Weber, L.T.; Nüsken, K.D. Developmental programming of renal function and re-programming approaches. Front. Pediatr. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyckx, V.A.; Brenner, B.M. Birth weight, malnutrition and kidney-associated outcomes—A global concern. Nat. Rev. Nephrol. 2015, 11, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2016, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Krata, N.; Zagożdżon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Ther. Exp. 2018, 66, 211–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef] [PubMed]
- Zullino, S.; Buzzella, F.; Simoncini, T. Nitric oxide and the biology of pregnancy. Vascul. Pharmacol. 2018, 110, 71–74. [Google Scholar] [CrossRef]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef]
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Tain, Y.L. Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int. J. Mol. Sci. 2019, 20, 681. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens. 2004, 13, 93–99. [Google Scholar] [CrossRef]
- Jun, M.; Venkataraman, V.; Razavian, M.; Cooper, B.; Zoungas, S.; Ninomiya, T.; Webster, A.C.; Perkovic, V. Antioxidants for chronic kidney disease. Cochrane Database Syst. Rev. 2012, 10, CD008176. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Reactive oxygen species: Roles in blood pressure and kidney function. Curr. Hypertens. Rep. 2002, 4, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Wilson, R.; Roberts, J.; Miller, H.; McKillop, J.H.; Walker, J.J. Antioxidants: Their role in pregnancy and miscarriage. Antioxid. Redox Signal. 2000, 2, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Early-life programming and reprogramming of adult kidney disease and hypertension: The interplay between maternal nutrition and oxidative stress. Int. J. Mol. Sci. 2020, 21, 3572. [Google Scholar] [CrossRef] [PubMed]
- Kone, B.C. Nitric oxide synthesis in the kidney: Isoforms, biosynthesis, and functions in health. Semin. Nephrol. 2004, 24, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Carter, A.M. Placental oxygen consumption. Part I. In vivo studies—A review. Placenta 2000, 21, S31–S37. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell Longev. 2014, 2014, 283180. [Google Scholar] [CrossRef]
- Cambonie, G.; Comte, B.; Yzydorczyk, C.; Ntimbane, T.; Germain, N.; Lê, N.L.; Pladys, P.; Gauthier, C.; Lahaie, I.; Abran, D.; et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1236–R1245. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Lee, C.T.; Lin, Y.J.; Tsai, C.C. N-Acetylcysteine prevents programmed hypertension in male rat offspring born to suramin-treated mothers. Biol. Reprod. 2016, 95, 8. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-l-arginine-methyl ester (l-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Huang, L.T.; Tain, Y.L. Early Postweaning Treatment with Dimethyl Fumarate Prevents Prenatal Dexamethasone- and Postnatal High-Fat Diet-Induced Programmed Hypertension in Male Rat Offspring. Oxid. Med. Cell Longev. 2018, 2018, 5343462. [Google Scholar] [CrossRef] [Green Version]
- Stangenberg, S.; Nguyen, L.T.; Chen, H.; Al-Odat, I.; Killingsworth, M.C.; Gosnell, M.E.; Anwer, A.G.; Goldys, E.M.; Pollock, C.A.; Saad, S. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. Int. J. Biochem. Cell Biol. 2015, 64, 81–90. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Huang, L.T.; Lau, Y.T.; Lin, C.Y.; Tain, Y.L. The combined ratios of l-arginine and asymmetric and symmetric dimethylarginine as biomarkers in spontaneously hypertensive rats. Transl. Res. 2012, 159, 90–98. [Google Scholar] [CrossRef]
- Chabrashvili, T.; Tojo, A.; Onozato, M.L.; Kitiyakara, C.; Quinn, M.T.; Fujita, T.; Welch, W.J.; Wilcox, C.S. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 2002, 39, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, A.; Hedner, T.; Milsom, I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet. Gynecol. Scand. 1998, 77, 808–813. [Google Scholar] [PubMed]
- Arya, S.; Ye, C.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B.; Retnakaran, R. Asymmetric dimethylarginine and arginine metabolites in women with and without a history of gestational diabetes. J. Diabetes Complicat. 2017, 31, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Maruta, E.; Wang, J.; Kotani, T.; Tsuda, H.; Nakano, T.; Imai, K.; Sumigama, S.; Niwa, Y.; Mitsui, T.; Yoshida, S.; et al. Association of serum asymmetric dimethylarginine, homocysteine, and l-arginine concentrations during early pregnancy with hypertensive disorders of pregnancy. Clin. Chim. Acta 2017, 475, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Bollenbach, A.; Savvidou, M.D. Inverse correlation between maternal plasma asymmetric dimethylarginine (ADMA) and birthweight percentile in women with impaired placental perfusion: Circulating ADMA as an NO-independent indicator of fetal growth restriction? Amino Acids 2018, 50, 341–351. [Google Scholar] [CrossRef]
- Mittermayer, F.; Prusa, A.R.; Pollak, A.; Wolzt, M. Umbilical vein plasma concentrations of asymmetrical dimethylarginine are increased in male but not female neonates delivered preterm: A pilot study. Early Hum. Dev. 2006, 82, 421–424. [Google Scholar] [CrossRef]
- Little, M.H.; McMahon, A.P. Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 2012, 4, a008300. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Brenner, B.M. The clinical importance of nephron mass. J. Am. Soc. Nephrol. 2010, 21, 898–910. [Google Scholar] [CrossRef] [Green Version]
- Hartman, H.A.; Lai, H.L.; Patterson, L.T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007, 310, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Garrett, M.R. Nephron number, hypertension, and CKD: Physiological and genetic insight from humans and animal models. Physiol Genom. 2017, 49, 180–192. [Google Scholar] [CrossRef]
- Murugapoopathy, V.; Gupta, I.R. A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT). Clin. J. Am. Soc. Nephrol. 2020, 15, 723–731. [Google Scholar] [CrossRef]
- Nenov, V.D.; Taal, M.W.; Sakharova, O.V.; Brenner, B.M. Multi-hit nature of chronic renal disease. Curr. Opin. Nephrol. Hypertens. 2000, 9, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Yamamoto, K.T.; Henry, R.K.; de Roos, A.J.; Flynn, J.T. Prenatal risk factors for childhood CKD. J. Am. Soc. Nephrol. 2014, 25, 2105–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, S.L.; Perkovic, V.; Cass, A.; Chang, C.L.; Poulter, N.R.; Spector, T.; Haysom, L.; Craig, J.C.; Salmi, I.A.; Chadban, S.J.; et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am. J. Kidney Dis. 2009, 54, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Luh, H.; Lin, C.Y.; Hsu, C.N. Incidence and risks of congenital anomalies of kidney and urinary tract in newborns: A population-based case-control study in Taiwan. Medicine 2016, 95, e2659. [Google Scholar] [CrossRef]
- Bertram, J.F.; Douglas-Denton, R.N.; Diouf, B.; Hughson, M.D.; Hoy, W.E. Human nephron number: Implications for health and disease. Pediatr. Nephrol. 2011, 26, 1529–1533. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016, 38, 86–92. [Google Scholar] [CrossRef]
- Jeje, S.O.; Akindele, O.O.; Ushie, G.; Rajil, Y. Changes in kidney function and oxidative stress biomarkers in offspring from dams treated with dexamethasone during lactation in Wistar rats. Afr. J. Med. Med. Sci. 2016, 45, 237–242. [Google Scholar]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 30, e1800066. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018, 19, 2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Lin, Y.J.; Tain, Y.L. Maternal exposure to bisphenol A combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda, N.B.; Hennington, B.S.; Williamson, D.T.; Hill, M.L.; Betson, N.E.; Sartori-Valinotti, J.C.; Reckelhoff, J.F.; Royals, T.P.; Alexander, B.T. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 2012, 60, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.L.; Hsu, C.N.; Tain, Y.L. Whether AICAR in Pregnancy or Lactation Prevents Hypertension Programmed by High Saturated Fat Diet: A Pilot Study. Nutrients 2020, 12, 448. [Google Scholar] [CrossRef] [Green Version]
- Svitok, P.; Okuliarova, M.; Varga, I.; Zeman, M. Renal impairment induced by prenatal exposure to angiotensin II in male rat offspring. Exp. Biol. Med. (Maywood) 2019, 244, 923–931. [Google Scholar] [CrossRef]
- Vieira, L.D.; Farias, J.S.; de Queiroz, D.B.; Cabral, E.V.; Lima-Filho, M.M.; Sant’Helena, B.R.M.; Aires, R.S.; Ribeiro, V.S.; Santos-Rocha, J.; Xavier, F.E.; et al. Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway: Prevention by early treatment with α-tocopherol. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3577–3587. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Chen, L.; Wang, X.J.; Jiang, Q.H.; Bei, X.Y.; Sun, W.L.; Xia, S.J.; Jiang, J.T. Maternal exposure to di-n-butyl phthalate (DBP) induces renal fibrosis in adult rat offspring. Oncotarget 2017, 8, 31101–31111. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Mak, C.H.; Chen, H.; Zaky, A.A.; Wong, M.G.; Pollock, C.A.; Saad, S. SIRT1 Attenuates Kidney Disorders in Male Offspring Due to Maternal High-Fat Diet. Nutrients 2019, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Sukjamnong, S.; Chan, Y.L.; Zakarya, R.; Nguyen, L.T.; Anwer, A.G.; Zaky, A.A.; Santiyanont, R.; Oliver, B.G.; Goldys, E.; Pollock, C.A.; et al. MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci. Rep. 2018, 8, 6631. [Google Scholar] [CrossRef]
- Gwathmey, T.M.; Shaltout, H.A.; Rose, J.C.; Diz, D.I.; Chappell, M.C. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 2011, 57, 620–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Gray, S.P.; Denton, K.M.; Cullen-McEwen, L.; Bertram, J.F.; Moritz, K.M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 2010, 21, 1891–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, L.A.; Quan, A.; Weinberg, A.; Baum, M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001, 59, 1663–1669. [Google Scholar] [CrossRef] [Green Version]
- Boubred, F.; Buffat, C.; Feuerstein, J.M.; Daniel, L.; Tsimaratos, M.; Oliver, C.; Lelièvre-Pégorier, M.; Simeoni, U. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am. J. Physiol. Ren. Physiol. 2007, 293, F1944–F1949. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Targeting on asymmetric dimethylarginine related nitric oxide-reactive oxygen species imbalance to reprogram the development of hypertension. Int. J. Mol. Sci. 2016, 17, 2020. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Nimse, S.B.; Palb, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC. Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 2012, 17, 311–321. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxid. Med. Cell Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yin, N.; Deng, Y.; Wei, Y.; Huang, Y.; Pu, X.; Li, L.; Zheng, Y.; Guo, J.; Yu, J.; et al. Ascorbic Acid Protects against Hypertension through Downregulation of ACE1 Gene Expression Mediated by Histone Deacetylation in Prenatal Inflammation-Induced Offspring. Sci. Rep. 2016, 6, 39469. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; Racasan, S.; Koomans, H.A.; Joles, J.A.; Braam, B. Nitric oxide, superoxide and renal blood flow autoregulation in SHR after perinatal l-arginine and antioxidants. Acta Physiol. 2007, 190, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Racasan, S.; Braam, B.; van der Giezen, D.M.; Goldschmeding, R.; Boer, P.; Koomans, H.A.; Joles, J.A. Perinatal l-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension 2004, 44, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Koeners, M.P.; Braam, B.; van der Giezen, D.M.; Goldschmeding, R.; Joles, J.A. Perinatal micronutrient supplements ameliorate hypertension and proteinuria in adult fawn-hooded hypertensive rats. Am. J. Hypertens. 2010, 23, 802–808. [Google Scholar] [CrossRef]
- Koeners, M.P.; van Faassen, E.E.; Wesseling, S.; Sain-van der Velden, M.; Koomans, H.A.; Braam, B.; Joles, J.A. Maternal supplementation with citrulline increases renal nitric oxide in young spontaneously hypertensive rats and has long-term antihypertensive effects. Hypertension 2007, 50, 1077–1084. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Yura, S.; Tatsumi, K.; Kondoh, E.; Mogami, H.; Fujita, K.; Kakui, K.; Aoe, S.; Itoh, H.; Sagawa, N.; et al. Branched-chain amino acid supplemented diet during maternal food restriction prevents developmental hypertension in adult rat offspring. J. Dev. Orig. Health Dis. 2011, 2, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Lin, Y.J.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal melatonin or agomelatine therapy prevents programmed hypertension in male offspring of mother exposed to continuous light. Biol. Reprod. 2017, 97, 636–643. [Google Scholar] [CrossRef]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef]
- Tain, Y.L.; Leu, S.; Lee, W.C.; Wu, K.L.H.; Chan, J.Y.H. Maternal Melatonin Therapy Attenuated Maternal High-Fructose Combined with Post-Weaning High-Salt Diets-Induced Hypertension in Adult Male Rat Offspring. Molecules 2018, 23, 886. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Chen, C.C.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Melatonin attenuates prenatal dexamethasone-induced blood pressure increase in a rat model. J. Am. Soc. Hypertens. 2014, 8, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Yu, H.R.; Chen, C.C.; Tiao, M.M.; Hsu, C.N.; Lin, Y.J.; Kuo, K.C.; Huang, L.T. Maternal Melatonin Therapy Rescues Prenatal Dexamethasone and Postnatal High-Fat Diet Induced Programmed Hypertension in Male Rat Offspring. Front. Physiol. 2015, 6, 377. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Sirajudeen, K.N.; Sundaram, A.; Zakaria, R.; Singh, H.J. Effects of antenatal, postpartum and post-weaning melatonin supplementation on blood pressure and renal antioxidant enzyme activities in spontaneously hypertensive rats. J. Physiol. Biochem. 2011, 67, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.E.; Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Resveratrol prevents combined prenatal NG-nitro-l-arginine-methyl ester (l-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Yu, H.R.; Lin, I.C.; Sheen, J.M.; Huang, L.T.; Tain, Y.L. Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy. Int. J. Mol. Sci. 2019, 20, 3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racasan, S.; Braam, B.; Koomans, H.A.; Joles, J.A. Programming blood pressure in adult SHR by shifting perinatal balance of NO and reactive oxygen species toward NO: The inverted barker phenomenon. Am. J. Physiol. Ren. Physiol. 2005, 288, F626–F636. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal N-Acetylcysteine therapy prevents hypertension in spontaneously hypertensive rat offspring: Implications of hydrogen sulfide-generating pathway and gut microbiota. Antioxidants (Basel) 2020, 9, 856. [Google Scholar] [CrossRef]
- Azzi, A.; Ricciarelli, R.; Zingg, J.M. Non-antioxidant molecular functions of alpha-tocopherol (vitamin E). FEBS Lett. 2002, 519, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Boucknooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef]
- Trachtman, H.; Sturman, J.A. Taurine and experimental kidney disease. Adv. Exp. Med. Biol. 1994, 359, 149–157. [Google Scholar]
- Han, X.; Chesney, R.W. The role of taurine in renal disorders. Amino Acids 2012, 43, 2249–2263. [Google Scholar] [CrossRef] [PubMed]
- Cherla, G.; Jaimes, E.A. Role of l-arginine in the pathogenesis and treatment of renal disease. J. Nutr. 2004, 134, 2801S–2806S. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamics properties of oral l-citrulline and l-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Krause, J.; Krause, M.; Rocha, I.M.G.D.; Umpierre, D.; Fayh, A.P.T. Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. The potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y. Transcriptional regulation of programmed hypertension by melatonin: An epigenetic perspective. Int. J. Mol. Sci. 2014, 15, 18484–18495. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Ohashi, N.; Ishigaki, S.; Isobe, S. The pivotal role of melatonin in ameliorating chronic kidney disease by suppression of the renin-angiotensin system in the kidney. Hypertens. Res. 2019, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Hrenak, J.; Paulis, L.; Repova, K.; Aziriova, S.; Nagtegaal, E.J.; Reiter, R.J.; Simko, F. Melatonin and renal protection: Novel perspectives from animal experiments and human studies (review). Curr. Pharm. Des. 2015, 21, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017, 18, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Huang, L.T.; Tain, Y.L. Perinatal Use of Melatonin for Offspring Health: Focus on Cardiovascular and Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 5681. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Hsu, C.N. Developmental Programming of the Metabolic Syndrome: Can We Reprogram with Resveratrol? Int. J. Mol. Sci. 2018, 19, 2584. [Google Scholar] [CrossRef] [Green Version]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [Green Version]
- Den Hartogh, D.J.; Tsiani, E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients 2019, 11, 1624. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.H.; Leonard, S.; Shi, X.; Goins, M.R.; Vallyathan, V. Antioxidant activity of lazaroid (U-75412E) and its protective effects against crystalline silica-induced cytotoxicity. Free Radic. Biol. Med. 1998, 24, 529–536. [Google Scholar] [CrossRef]
- James, A.M.; Smith, R.A.; Murphy, M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch. Biochem. Biophys. 2004, 423, 47–56. [Google Scholar] [CrossRef]
- Tintoré, M.; Sastre-Garriga, J. Multiple sclerosis: Dimethyl fumarate is coming of age. Nat. Rev. Neurol. 2016, 12, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Drug antioxidant effects. A basis for drug selection? Drugs 1991, 42, 569–605. [Google Scholar] [CrossRef] [PubMed]
- Šalamon, Š.; Kramar, B.; Marolt, T.P.; Poljšak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva, C.; Kross, R.D. Antioxidant-induced stress. Int. J. Mol Sci. 2012, 13, 2091–2109. [Google Scholar] [CrossRef] [Green Version]
| Animal Models | Species/Gender | Age at Evaluation | Mechanisms of Oxidative Stress | Morphological Changes | Renal Phenotype | Ref. |
|---|---|---|---|---|---|---|
| Maternal caloric restriction diet, 50% | SD rat/M | 12 weeks | ↑ ADMA, ↓ NO, ↑ Renal 8-OHdG expression | ↓ NN, glomerular hypertrophy, ↑ tubulointerstitial injury | ↔ GFR, hypertension | [29,30] |
| Maternal low protein diet, 9% | Wistar rat/M | 12 weeks | ↑ F2-isoprostane, ↓ glutathione | Hypertension | [31] | |
| Streptozotocin-induced diabetes | SD rat/M | 12 weeks | ↑ ADMA, ↓ NO | ↓ NN, ↑ tuburo-interstitial injury | ↔ GFR, hypertension | [32] |
| Maternal suramin administration | SD rat/M | 12 weeks | ↑ ADMA, ↓ NO | Hypertension | [33] | |
| Maternal l-NAME administration | SD rat/M | 12 weeks | ↑ Renal F2-isoprostane level | Hypertension | [34] | |
| Maternal high-fructose diet, 60% | SD rat/M | 12 weeks | ↑ Renal 8-OHdG expression, ↓ NO | Hypertension | [56] | |
| Dexamethasone administration in lactation | Wistar rat/M and F | 12 weeks | ↑ Renal MDA level, ↓ SOD and catalase activity | ↑ Tubular necrosis | ↑ Cr level | [57] |
| Maternal plus post-weaning high-fructose diet, 60% | SD rat/M | 12 weeks | ↑ Renal 8-OHdG expression | Hypertension | [58] | |
| Maternal methyl-deficient diet | SD rat/M | 12 weeks | ↑ Renal 8-OHdG expression | ↔ Cr level, hypertension | [59] | |
| Maternal high methyl-donor diet | SD rat/M | 12 weeks | ↑ Renal 8-OHdG expression | ↔ Cr level, hypertension | [59] | |
| Maternal adenine-induced CKD | SD rat/M | 12 weeks | ↑ ADMA, ↓ NO | Renal hypertrophy | ↔ Cr level, hypertension | [60] |
| Prenatal dexamethasone at gestational day 15 and 16. | SD rat/M | 16 weeks | ↓ Renal NO | Hypertension | [35] | |
| Prenatal dexamethasone exposure plus postnatal high-fat intake | SD rat/M | 16 weeks | ↑ Renal 8-OHdG expression, ↓ NO | Hypertension | [61] | |
| Prenatal dexamethasone plus TCDD exposure | SD rat/M | 16 weeks | ↑ Renal 8-OHdG expression, ↑ ADMA | Hypertension | [62] | |
| Prenatal bisphenol A exposure plus high-fat diet | SD rat/M | 16 weeks | ↑ Renal 8-OHdG expression, ↑ ADMA, ↓ NO | Hypertension | [63] | |
| Reduced uterine perfusion | SD rat/M | 16 weeks | ↑ Urinary F2-isoprostane level and renal NADPH-oxidase dependent superoxide | Hypertension | [64] | |
| Maternal plus post-weaning high-fat diet, 58% | SD rat/M | 16 weeks | ↑ Renal 8-OHdG expression | Hypertension | [65] | |
| Maternal angiotensin II administration | Wistar rat/M | 18 weeks | ↑ Renal ROS | ↔ NN, ↑ tuburo-interstitial injury | [66] | |
| Prenatal LPS Exposure | Wistar rat/M | 28 weeks | ↑ Renal MDA level | Hypertension | [67] | |
| Maternal di-n-butyl phthalate exposure | SD rat/M and F | 18 months | ↑ Renal ROS | Renal dysplasia, ↑ tuburo-interstitial injury | [68] | |
| Maternal high-fat diet | C57BL/6 mice/M | 9 weeks | ↑ Renal 8-OHdG expression | Renal hypertrophy | Albuminuria | [69] |
| Maternal smoking exposure | Balb/c mice/M | 13 weeks | ↑ Renal ROS | ↓ NN | Albuminuria | [70] |
| Prenatal betamethasone exposure at gestational day 80 and 81 | Sheep/M and F | 18 months | ↑ ROS, ↓ NO | Hypertension | [71] |
| Antioxidant Intervention | Animal Models | Species/Gender | Age at Evaluation (Week) | Reprogramming Effects | Ref. |
|---|---|---|---|---|---|
| Natural antioxidants | |||||
| Vitamin C 350 mg/kg/day i.p. daily at gestational day 8 to 14 | Prenatal LPS Exposure | SD rat/M | 12 | ↓ BP | [82] |
| α-tocopherol 350 mg/kg/day via gavage at gestational day 13 to 20 | Prenatal LPS Exposure | Wistar rat/M | 28 | ↓ BP | [67] |
| l-arginine, l-taurine, Vitamins C and E 2 weeks before until 8 weeks after birth | Genetic hypertension | SHR/M and F | 9 | ↓ BP | [83] |
| l-arginine, l-taurine, Vitamins C and E 2 weeks before until 8 weeks after birth | Genetic hypertension | SHR/M and F | 50 | ↓ BP, ↓ proteinuria | [84] |
| l-arginine, l-taurine, Vitamins C and E 2 weeks before until 4 weeks after birth | Genetic hypertension | FHH rat/M and F | 36 | ↓ BP, ↓ proteinuria, ↓ glomerulosclerosis | [85] |
| 0.25% l-citrulline in drinking water in pregnancy and lactation | Maternal caloric restriction diet, 50% | SD rat/M | 12 | ↓ kidney injury, ↑ nephron number | [29] |
| 0.25% l-citrulline in drinking water in pregnancy and lactation | Maternal streptozotocin -induced diabetes | SD rat/M | 12 | ↓ BP, ↓ kidney injury | [32] |
| 0.25% l-citrulline in drinking water in pregnancy and lactation | Prenatal dexamethasone exposure | SD rat/M | 12 | ↓ BP | [35] |
| 0.25% l-citrulline in drinking water 2 weeks before until 6 weeks after birth | Genetic hypertension | SHR/M and F | 50 | ↓ BP | [86] |
| l-tryptophan 200 mg/kg BW/day via oral gavage in pregnancy | Maternal adenosine-induced CKD | SD rat/M | 12 | ↓ BP, ↓ Cr level | [60] |
| BCAA-supplemented diet in pregnancy | Maternal caloric Restriction, 70% | SD rat/M | 16 | ↓ BP | [87] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Maternal caloric restriction | SD rat/M | 12 | ↓ BP | [30] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Maternal l-NAME exposure | SD rat/M | 12 | ↓ BP | [34] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Maternal methyl-donor diet | SD rat/M | 12 | ↓ BP | [59] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Maternal constant light exposure | SD rat/M | 12 | ↓ BP | [88] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Maternal high-fructose diet, 60% | SD rat/M | 12 | ↓ BP | [89] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Maternal high-fructose diet plus post-weaning high-salt diet | SD rat/M | 12 | ↓ BP | [90] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Prenatal dexamethasone exposure | SD rat/M | 16 | ↓ BP | [91] |
| 0.01% melatonin in drinking water in pregnancy and lactation | Prenatal dexamethasone exposure plus post-weaning high-fat diet | SD rat/M | 16 | ↓ BP | [92] |
| Melatonin 10 mg/kg/day in drinking water in pregnancy | Genetic hypertension model | SHR/M | 16 | ↓ BP | [93] |
| 0.05% resveratrol in drinking water in pregnancy and lactation | Maternal TCDD and dexamethasone exposures | SD rat/M | 16 | ↓ BP | [62] |
| 50 mg/L resveratrol in drinking water in pregnancy and lactation | Maternal bisphenol A exposure and high-fat diet | SD rat/M | 16 | ↓ BP | [63] |
| 50 mg/L resveratrol in drinking water in pregnancy and lactation | Maternal plus post-weaning high-fructose diet | SD rat/M | 12 | ↓ BP | [58] |
| 50 mg/L resveratrol in drinking water in pregnancy and lactation | Maternal l-NAME plus postnatal high-fat diet | SD rat/M | 16 | ↓ BP | [94] |
| Synthetic antioxidants | |||||
| Lazaroid 10 mg/kg/day via gavage in pregnancy | Maternal low protein diet, 9% | Wistar rat/M | 12 | ↓ BP | [31] |
| MitoQ 500 µM in drinking water in pregnancy and lactation | Maternal smoking exposure | Balb/c mice/M | 13 | ↓ BP, ↓ kidney injury, ↑ nephron number | [70] |
| Dimethyl fumarate 50 mg/kg/day via gavage in pregnancy | Prenatal dexamethasone and postnatal high-fat diet | SD rat/M | 16 | ↓ BP | [95] |
| Tempol 172 mg/L in drinking water 2 weeks before until 8 weeks after birth | Genetic hypertension | SHR/Mand F | 50 | ↓ BP, ↓ proteinuria | [96] |
| 1% NAC in drinking water in pregnancy and lactation | Suramin administration | SD rat/M | 12 | ↓ BP | [33] |
| 1% NAC in drinking water in pregnancy and lactation | Maternal l-NAME exposure | SD rat/M | 12 | ↓ BP | [34] |
| 1% NAC in drinking water in pregnancy and lactation | Prenatal dexamethasone and postnatal high-fat diet | SD rat/M | 12 | ↓ BP | [36] |
| 1% NAC in drinking water in pregnancy and lactation | Genetic hypertension model | SHR rat/M | 12 | ↓ BP | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Tain, Y.-L. Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants 2021, 10, 33. https://doi.org/10.3390/antiox10010033
Hsu C-N, Tain Y-L. Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants. 2021; 10(1):33. https://doi.org/10.3390/antiox10010033
Chicago/Turabian StyleHsu, Chien-Ning, and You-Lin Tain. 2021. "Developmental Origins of Kidney Disease: Why Oxidative Stress Matters?" Antioxidants 10, no. 1: 33. https://doi.org/10.3390/antiox10010033
APA StyleHsu, C.-N., & Tain, Y.-L. (2021). Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants, 10(1), 33. https://doi.org/10.3390/antiox10010033






