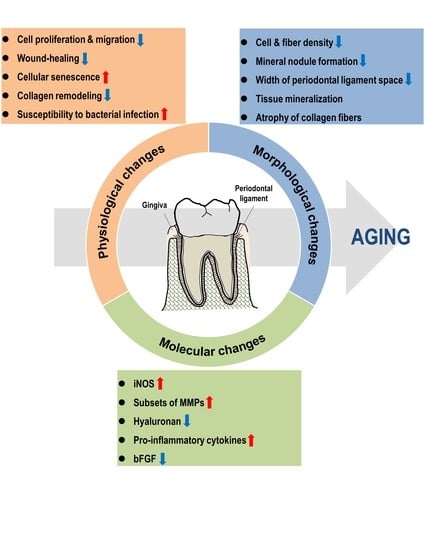
Effect of Aging on Homeostasis in the Soft Tissue of the Periodontium: A Narrative Review
Abstract
:1. Introduction
2. Anatomical and Physiological Homeostasis in the Gingiva
3. Anatomical and Physiological Homeostasis in the Periodontal Ligament
4. Molecular Profile Changes with Age in the Periodontium
4.1. Inducible Nitric Oxide Synthase
4.2. Matrix Metalloproteinase (MMP)
4.3. Others
5. Physiological Changes with Age in the Periodontium
5.1. Responsiveness to Pathogens with Age
5.2. Drug-Influenced Alterations in the Periodontium
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flatt, T. A new definition of aging? Front. Genet. 2012, 3, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesniewski, L.A.; Seals, D.R.; Walker, A.E.; Henson, G.D.; Blimline, M.W.; Trott, D.W.; Bosshardt, G.C.; LaRocca, T.J.; Lawson, B.R.; Zigler, M.C.; et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 2017, 16, 17–26. [Google Scholar] [CrossRef]
- An, J.Y.; Kerns, K.A.; Ouellette, A.; Robinson, L.; Morris, H.D.; Kaczorowski, C.; Park, S.I.; Mekvanich, T.; Kang, A.; McLean, J.S.; et al. Rapamycin rejuvenates oral health in aging mice. Elife 2020, 9. [Google Scholar] [CrossRef]
- Majumder, S.; Caccamo, A.; Medina, D.X.; Benavides, A.D.; Javors, M.A.; Kraig, E.; Strong, R.; Richardson, A.; Oddo, S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell 2012, 11, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.L.; Lawrence, I.; Hoffman, M.; Elgindi, D.; Nadhan, K.; Potnis, M.; Jin, A.; Sershon, C.; Binnebose, R.; Lorenzini, A.; et al. Topical rapamycin reduces markers of senescence and aging in human skin: An exploratory, prospective, randomized trial. Geroscience 2019, 41, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, E.; Graber, S.; Szego, E.M.; Moisoi, N.; Martins, L.M.; Outeiro, T.F.; Kermer, P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS ONE 2011, 6, e28855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Nunez, O.; Pallas, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women. Nutrients 2020, 12, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabreiro, F.; Au, C.; Leung, K.Y.; Vergara-Irigaray, N.; Cocheme, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Jiang, G.; Zhang, J.; Guo, J.; Li, Z.; Hao, K.; Liu, L.; Cheng, Z.; Tong, X.; Dai, F. Metformin prolongs lifespan through remodeling the energy distribution strategy in silkworm, Bombyx mori. Aging 2019, 11, 240–248. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Lin, H.; Li, Q.; Ye, Z.; Lu, Q.; Chen, L.; Tu, Z.; Tian, G. Improved Human Age Prediction by Using Gene Expression Profiles From Multiple Tissues. Front. Genet. 2020, 11, 1025. [Google Scholar] [CrossRef]
- Malamon, J.S.; Kriete, A. Erosion of Gene Co-expression Networks Reveal Deregulation of Immune System Processes in Late-Onset Alzheimer’s Disease. Front. Neurosci. 2020, 14, 228. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef]
- Karin, O.; Agrawal, A.; Porat, Z.; Krizhanovsky, V.; Alon, U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat. Commun. 2019, 10, 5495. [Google Scholar] [CrossRef] [Green Version]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrei, M.; Dinischiotu, A.; Didilescu, A.C.; Ionita, D.; Demetrescu, I. Periodontal materials and cell biology for guided tissue and bone regeneration. Ann. Anat. 2018, 216, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Ito, Y.; Holliday, S.; Walker, C.; Daniel, J.; Galang, T.M.; Fukui, T.; Yamane, A.; Begole, E.; Evans, C.; et al. Extracellular matrix-mediated tissue remodeling following axial movement of teeth. J. Histochem. Cytochem. 2007, 55, 127–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribble, G.D.; Lamont, R.J. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontology 2000 2010, 52, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontology 2000 2000, 24, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Hormia, M.; Owaribe, K.; Virtanen, I. The dento-epithelial junction: Cell adhesion by type I hemidesmosomes in the absence of a true basal lamina. J. Periodontol. 2001, 72, 788–797. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsunaga, T.; Yamamoto, G.; Nishii, K.; Usui, M.; Yamamoto, M.; Tachikawa, T. Comprehensive analysis of gene expression in the junctional epithelium by laser microdissection and microarray analysis. J. Periodontal Res. 2010, 45, 618–625. [Google Scholar] [CrossRef]
- Nishii, K.; Usui, M.; Yamamoto, G.; Yajima, S.; Tsukamoto, Y.; Tanaka, J.; Tachikawa, T.; Yamamoto, M. The distribution and expression of S100A8 and S100A9 in gingival epithelium of mice. J. Periodontal Res. 2013, 48, 235–242. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Usui, M.; Yamamoto, G.; Takagi, Y.; Tachikawa, T.; Yamamoto, M.; Nakamura, M. Role of the junctional epithelium in periodontal innate defense and homeostasis. J. Periodontal Res. 2012, 47, 750–757. [Google Scholar] [CrossRef]
- Nakamura, M. Histological and immunological characteristics of the junctional epithelium. Jpn. Dent. Sci. Rev. 2018, 54, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Battegay, E.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F.; Ross, R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 1990, 63, 515–524. [Google Scholar] [CrossRef]
- Schroeder, H.E.; Page, R. Lymphocyte-fibroblast interaction in the pathogenesis of inflammatory gingival disease. Experientia 1972, 28, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Talari, A. The increase with age of the width of attached gingiva. J. Periodontal Res. 1976, 11, 182–188. [Google Scholar] [CrossRef]
- Bhatia, G.; Kumar, A.; Khatri, M.; Bansal, M.; Saxena, S. Assessment of the width of attached gingiva using different methods in various age groups: A clinical study. J. Indian Soc. Periodontol. 2015, 19, 199–202. [Google Scholar] [CrossRef]
- Schatzle, M.; Loe, H.; Burgin, W.; Anerud, A.; Boysen, H.; Lang, N.P. Clinical course of chronic periodontitis. I. Role of gingivitis. J. Clin. Periodontol. 2003, 30, 887–901. [Google Scholar] [CrossRef]
- Caceres, M.; Oyarzun, A.; Smith, P.C. Defective Wound-healing in Aging Gingival Tissue. J. Dent. Res. 2014, 93, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, O.A.; Stromberg, A.J.; Huggins, P.M.; Gonzalez-Martinez, J.; Novak, M.J.; Ebersole, J.L. Apoptotic genes are differentially expressed in aged gingival tissue. J. Dent. Res. 2011, 90, 880–886. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Songhee Song, K.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2016, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.M.; Wang, W.X.; Takao, Y.; Chen, Z.X. Effects of cementum-dentine junction and cementum on the mechanical response of tooth supporting structure. J. Dent. 2010, 38, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Naveh, G.R.; Lev-Tov Chattah, N.; Zaslansky, P.; Shahar, R.; Weiner, S. Tooth-PDL-bone complex: Response to compressive loads encountered during mastication—A review. Arch. Oral Biol. 2012, 57, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Beertsen, W.; Everts, V. Junctions between fibroblasts in mouse periodontal ligament. J. Periodontal Res. 1980, 15, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Chiba, M.; Mitani, H. The induction of c-fos mRNA expression by mechanical stress in human periodontal ligament cells. Arch. Oral Biol. 2002, 47, 465–471. [Google Scholar] [CrossRef]
- Kook, S.H.; Hwang, J.M.; Park, J.S.; Kim, E.M.; Heo, J.S.; Jeon, Y.M.; Lee, J.C. Mechanical force induces type I collagen expression in human periodontal ligament fibroblasts through activation of ERK/JNK and AP-1. J. Cell Biochem. 2009, 106, 1060–1067. [Google Scholar] [CrossRef]
- Wattanaroonwong, N.; Schoenmaker, T.; de Vries, T.J.; Everts, V. Oestrogen inhibits osteoclast formation induced by periodontal ligament fibroblasts. Arch. Oral Biol. 2011, 56, 212–219. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Athanassiou-Papaefthymiou, M.; Papagerakis, P.; Papagerakis, S. Isolation and Characterization of Human Adult Epithelial Stem Cells from the Periodontal Ligament. J. Dent. Res. 2015, 94, 1591–1600. [Google Scholar] [CrossRef]
- Van der Velden, U. Effect of age on the periodontium. J. Clin. Periodontol. 1984, 11, 281–294. [Google Scholar] [CrossRef]
- Benatti, B.B.; Silverio, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Influence of aging on biological properties of periodontal ligament cells. Connect. Tissue Res. 2008, 49, 401–408. [Google Scholar] [CrossRef]
- Nishimura, F.; Terranova, V.P.; Braithwaite, M.; Orman, R.; Ohyama, H.; Mineshiba, J.; Chou, H.H.; Takashiba, S.; Murayama, Y. Comparison of in vitro proliferative capacity of human periodontal ligament cells in juvenile and aged donors. Oral Dis. 1997, 3, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Benatti, B.B.; Silverio, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Inflammatory and bone-related genes are modulated by aging in human periodontal ligament cells. Cytokine 2009, 46, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod. Craniofac. Res. 2009, 12, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Liu, B.; Mah, S.J.; Chen, S.; Helms, J.A. The molecular and cellular effects of ageing on the periodontal ligament. J. Clin. Periodontol. 2014, 41, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, Y.; Gao, L.N.; Zhang, Y.J.; Jin, Y.; Chen, F.M. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 2012, 33, 6974–6986. [Google Scholar] [CrossRef]
- Lossdorfer, S.; Kraus, D.; Jager, A. Aging affects the phenotypic characteristics of human periodontal ligament cells and the cellular response to hormonal stimulation in vitro. J. Periodontal Res. 2010, 45, 764–771. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Kong, Q.; Lin, C.L. Oxidative damage to RNA: Mechanisms, consequences, and diseases. Cell Mol. Life Sci. 2010, 67, 1817–1829. [Google Scholar] [CrossRef] [Green Version]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [Green Version]
- Bredt, D.S. Nitric oxide signaling specificity--the heart of the problem. J. Cell Sci. 2003, 116, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.R.; Brock, T.A.; Chang, L.Y.; Crapo, J.; Briscoe, P.; Ku, D.; Bradley, W.A.; Gianturco, S.H.; Gore, J.; Freeman, B.A.; et al. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. USA 1994, 91, 1044–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulligan, M.S.; Hevel, J.M.; Marletta, M.A.; Ward, P.A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc. Natl. Acad. Sci. USA 1991, 88, 6338–6342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paxinou, E.; Chen, Q.; Weisse, M.; Giasson, B.I.; Norris, E.H.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.; Ischiropoulos, H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J. Neurosci. 2001, 21, 8053–8061. [Google Scholar] [CrossRef] [PubMed]
- Laurent, M.; Lepoivre, M.; Tenu, J.P. Kinetic modelling of the nitric oxide gradient generated in vitro by adherent cells expressing inducible nitric oxide synthase. Biochem. J. 1996, 314, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, Y.; Kim, T.J.; Lee, J.M.; Kim, D.Y. SOD2 is upregulated in periodontitis to reduce further inflammation progression. Oral Dis. 2018, 24, 1572–1580. [Google Scholar] [CrossRef]
- Tanaka, T.; Basisty, N.; Fantoni, G.; Candia, J.; Moore, A.Z.; Bioancotto, A.; Schilling, B.; Bandinelli, S.; Ferrucci, L. Plasma proteomic biomarker signature of age predicts health and life span. Elife 2020, 9. [Google Scholar] [CrossRef]
- Amargant, F.; Manuel, S.L.; Tu, Q.; Parkes, W.S.; Rivas, F.; Zhou, L.T.; Rowley, J.E.; Villanueva, C.E.; Hornick, J.E.; Shekhawat, G.S.; et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020, 19, e13259. [Google Scholar] [CrossRef]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef]
- Feinberg, R.N.; Beebe, D.C. Hyaluronate in vasculogenesis. Science 1983, 220, 1177–1179. [Google Scholar] [CrossRef]
- Yildirim, S.; Ozener, H.O.; Dogan, B.; Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J. Periodontol. 2018, 89, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Diniz, I.M.; Chen, C.; Sarrion, P.; Tamayol, A.; Wu, B.M.; Moshaverinia, A. Human Periodontal Ligament- and Gingiva-derived Mesenchymal Stem Cells Promote Nerve Regeneration When Encapsulated in Alginate/Hyaluronic Acid 3D Scaffold. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Atkuru, S.; Muniraj, G.; Sudhaharan, T.; Chiam, K.H.; Wright, G.D.; Sriram, G. Cellular ageing of oral fibroblasts differentially modulates extracellular matrix organization. J. Periodontal Res. 2020. [Google Scholar] [CrossRef]
- Marin, S.; Popovic-Pejicic, S.; Radosevic-Caric, B.; Trtic, N.; Tatic, Z.; Selakovic, S. Hyaluronic acid treatment outcome on the post-extraction wound healing in patients with poorly controlled type 2 diabetes: A randomized controlled split-mouth study. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e154–e160. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Socransky, S.; Nyman, S.; Westfelt, E.; Haffajee, A. Effect of age on healing following periodontal therapy. J. Clin. Periodontol. 1985, 12, 774–787. [Google Scholar] [CrossRef]
- Holm-Pedersen, P.; Loe, H. Wound healing in the gingiva of young and old individuals. Scand. J. Dent. Res. 1971, 79, 40–53. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, S.H.; Lee, J.S.; Song, J.E.; Cho, S.H.; Jung, S.; Kim, S.K.; Kim, S.H.; Lee, K.P.; Kwon, K.S.; et al. Differential Matrix Metalloprotease (MMP) Expression Profiles Found in Aged Gingiva. PLoS ONE 2016, 11, e0158777. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- The Gene Ontology, C. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimand, J.; Kull, M.; Peterson, H.; Hansen, J.; Vilo, J. g:Profiler--a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 2007, 35, W193–W200. [Google Scholar] [CrossRef] [PubMed]
- Beklen, A.; Tuter, G.; Sorsa, T.; Hanemaaijer, R.; Virtanen, I.; Tervahartiala, T.; Konttinen, Y.T. Gingival tissue and crevicular fluid co-operation in adult periodontitis. J. Dent. Res. 2006, 85, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Kinney, J.S.; Herr, A.E.; Braun, T.; Sugai, J.V.; Shelburne, C.A.; Rayburn, L.A.; Tran, H.M.; Singh, A.K.; Giannobile, W.V. Identification of pathogen and host-response markers correlated with periodontal disease. J. Periodontol. 2009, 80, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, U.K.; Kononen, E.; Pradhan-Palikhe, P.; Tervahartiala, T.; Pussinen, P.J.; Suominen-Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Valenzuela, M.A.; Lopez-Otin, C.; Alvarez, J.; Lopez, J.M.; Vernal, R.; Gamonal, J. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J. Periodontol. 2006, 77, 1863–1870. [Google Scholar] [CrossRef]
- Hernandez Rios, M.; Sorsa, T.; Obregon, F.; Tervahartiala, T.; Valenzuela, M.A.; Pozo, P.; Dutzan, N.; Lesaffre, E.; Molas, M.; Gamonal, J. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: Initial evidence for MMP-13/MMP-9 activation cascade. J. Clin. Periodontol. 2009, 36, 1011–1017. [Google Scholar] [CrossRef]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [Green Version]
- Kuula, H.; Salo, T.; Pirila, E.; Tuomainen, A.M.; Jauhiainen, M.; Uitto, V.J.; Tjaderhane, L.; Pussinen, P.J.; Sorsa, T. Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis. Infect. Immun. 2009, 77, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, M.; Gamonal, J.; Salo, T.; Tervahartiala, T.; Hukkanen, M.; Tjaderhane, L.; Sorsa, T. Reduced expression of lipopolysaccharide-induced CXC chemokine in Porphyromonas gingivalis-induced experimental periodontitis in matrix metalloproteinase-8 null mice. J. Periodontal Res. 2011, 46, 58–66. [Google Scholar] [CrossRef]
- Van Lint, P.; Wielockx, B.; Puimege, L.; Noel, A.; Lopez-Otin, C.; Libert, C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J. Immunol. 2005, 175, 7642–7649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, H.; Yan, Y.; Jin, Y.H.; Meng, X.Y.; Mo, Y.Y.; Zeng, X.T. Matrix metalloproteinase gene polymorphisms and periodontitis susceptibility: A meta-analysis involving 6,162 individuals. Sci. Rep. 2016, 6, 24812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Cai, Q.; Ma, L.; Wang, M.; Ma, J.; Zhang, W.; Pan, Y.; Wang, L. Association between MMP-1 g.-1607dupG polymorphism and periodontitis susceptibility: A meta-analysis. PLoS ONE 2013, 8, e59513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef] [PubMed]
- Grzibovskis, M.; Urtane, I.; Pilmane, M. Specific signaling molecule expression in periodontal ligaments in different age groups: Pilot study. Stomatologija 2011, 13, 117–122. [Google Scholar]
- Tanimoto, K.; Ohkuma, S.; Tanne, Y.; Kunimatsu, R.; Hirose, N.; Mitsuyoshi, T.; Yoshimi, Y.; Su, S.; Tanne, K. Effects of bFGF on the Modulation of Apoptosis in Gingival Fibroblasts with Different Host Ages. Int. J. Dent. 2013, 2013, 619580. [Google Scholar] [CrossRef]
- An, S.; Huang, X.; Gao, Y.; Ling, J.; Huang, Y.; Xiao, Y. FGF-2 induces the proliferation of human periodontal ligament cells and modulates their osteoblastic phenotype by affecting Runx2 expression in the presence and absence of osteogenic inducers. Int. J. Mol. Med. 2015, 36, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, C.; Kanai, Y.; Masumoto, R.; Kitagaki, J.; Matsumoto, M.; Yamada, S.; Kajikawa, T.; Murakami, S. Fibroblast growth factor-2 inhibits CD40-mediated periodontal inflammation. J. Cell Physiol. 2019, 234, 7149–7160. [Google Scholar] [CrossRef]
- Kitamura, M.; Nakashima, K.; Kowashi, Y.; Fujii, T.; Shimauchi, H.; Sasano, T.; Furuuchi, T.; Fukuda, M.; Noguchi, T.; Shibutani, T.; et al. Periodontal tissue regeneration using fibroblast growth factor-2: Randomized controlled phase II clinical trial. PLoS ONE 2008, 3, e2611. [Google Scholar] [CrossRef]
- Kitamura, M.; Akamatsu, M.; Machigashira, M.; Hara, Y.; Sakagami, R.; Hirofuji, T.; Hamachi, T.; Maeda, K.; Yokota, M.; Kido, J.; et al. FGF-2 stimulates periodontal regeneration: Results of a multi-center randomized clinical trial. J. Dent. Res. 2011, 90, 35–40. [Google Scholar] [CrossRef]
- Kitamura, M.; Akamatsu, M.; Kawanami, M.; Furuichi, Y.; Fujii, T.; Mori, M.; Kunimatsu, K.; Shimauchi, H.; Ogata, Y.; Yamamoto, M.; et al. Randomized Placebo-Controlled and Controlled Non-Inferiority Phase III Trials Comparing Trafermin, a Recombinant Human Fibroblast Growth Factor 2, and Enamel Matrix Derivative in Periodontal Regeneration in Intrabony Defects. J. Bone Miner. Res. 2016, 31, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Oh, T.J.; Mills, M.P.; Clem, D.S.; McClain, P.K.; Schallhorn, R.A.; McGuire, M.K.; Scheyer, E.T.; Giannobile, W.V.; Reddy, M.S.; et al. A Randomized Clinical Trial Evaluating rh-FGF-2/beta-TCP in Periodontal Defects. J. Dent. Res. 2016, 95, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Elkon, K.B.; Ma, X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 2004, 21, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, O.; Jungas, T.; Verbeke, P.; Ojcius, D.M. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect. Immun. 2004, 72, 3743–3751. [Google Scholar] [CrossRef] [Green Version]
- Shoemark, D.K.; Allen, S.J. The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 725–738. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Dong, G.; Xiao, W.; Xiao, E.; Miao, F.; Syverson, A.; Missaghian, N.; Vafa, R.; Cabrera-Ortega, A.A.; Rossa, C., Jr.; et al. Effect of Aging on Periodontal Inflammation, Microbial Colonization, and Disease Susceptibility. J. Dent. Res. 2016, 95, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Jia, Z.; Tang, D.; Zhao, X.; Shi, J.; Jia, Q.; He, K.; Feng, Q. Time-Course Transcriptome Analysis for Drug Repositioning in Fusobacterium nucleatum-Infected Human Gingival Fibroblasts. Front. Cell Dev. Biol. 2019, 7, 204. [Google Scholar] [CrossRef]
- Domon, H.; Tabeta, K.; Nakajima, T.; Yamazaki, K. Age-related alterations in gene expression of gingival fibroblasts stimulated with Porphyromonas gingivalis. J. Periodontal Res. 2014, 49, 536–543. [Google Scholar] [CrossRef]
- Ahn, S.H.; Chun, S.; Park, C.; Lee, J.H.; Lee, S.W.; Lee, T.H. Transcriptome profiling analysis of senescent gingival fibroblasts in response to Fusobacterium nucleatum infection. PLoS ONE 2017, 12, e0188755. [Google Scholar] [CrossRef] [Green Version]
- Patini, R.; Staderini, E.; Lajolo, C.; Lopetuso, L.; Mohammed, H.; Rimondini, L.; Rocchetti, V.; Franceschi, F.; Cordaro, M.; Gallenzi, P. Relationship between oral microbiota and periodontal disease: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5775–5788. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Nolan, N.E.; Puckett, S.A. Longitudinal analysis of parotid and submandibular salivary flow rates in healthy, different-aged adults. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M285–M289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, R.S.; Challacombe, S.J.; Marsh, P.D. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J. Dent. Res. 1994, 73, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Park, I.S.; Kim, S.K.; Lim, J.Y.; Kim, Y.M. Analysis of age-related changes in the functional morphologies of salivary glands in mice. Arch. Oral Biol. 2013, 58, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.R.; Damante, J.H.; Lara, V.S.; Lauris, J.R. Age-related changes in human sublingual glands: A post mortem study. Arch. Oral Biol. 2005, 50, 565–574. [Google Scholar] [CrossRef]
- Lamster, I.B.; Asadourian, L.; Del Carmen, T.; Friedman, P.K. The aging mouth: Differentiating normal aging from disease. Periodontology 2000 2016, 72, 96–107. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Livada, R.; Shiloah, J. Calcium channel blocker-induced gingival enlargement. J. Hum. Hypertens. 2014, 28, 10–14. [Google Scholar] [CrossRef]
- Miller, C.S.; Damm, D.D. Incidence of verapamil-induced gingival hyperplasia in a dental population. J. Periodontol. 1992, 63, 453–456. [Google Scholar] [CrossRef]
- Barak, S.; Engelberg, I.S.; Hiss, J. Gingival hyperplasia caused by nifedipine. Histopathologic findings. J. Periodontol. 1987, 58, 639–642. [Google Scholar] [CrossRef]
- Lombardi, T.; Fiore-Donno, G.; Belser, U.; Di Felice, R. Felodipine-induced gingival hyperplasia: A clinical and histologic study. J. Oral Pathol. Med. 1991, 20, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.I.; Bartold, P.M. A clinical review of drug-induced gingival overgrowths. Aust. Dent. J. 1999, 44, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowson, C.S.; Matteson, E.L.; Myasoedova, E.; Michet, C.J.; Ernste, F.C.; Warrington, K.J.; Davis, J.M., 3rd; Hunder, G.G.; Therneau, T.M.; Gabriel, S.E. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011, 63, 633–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panayi, G.S. B cells: A fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology 2005, 44 (Suppl. 2), ii3–ii7. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.C.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coat, J.; Demoersman, J.; Beuzit, S.; Cornec, D.; Devauchelle-Pensec, V.; Saraux, A.; Pers, J.O. Anti-B lymphocyte immunotherapy is associated with improvement of periodontal status in subjects with rheumatoid arthritis. J. Clin. Periodontol. 2015, 42, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Zakikhani, M.; Dowling, R.; Fantus, I.G.; Sonenberg, N.; Pollak, M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006, 66, 10269–10273. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–1188. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, A.R.; Nagpal, K.; Karvekar, S.; Patnaik, K.; Naik, S.B.; Guruprasad, C.N. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2015, 86, 729–737. [Google Scholar] [CrossRef]
- Kang, W.; Wang, T.; Hu, Z.; Liu, F.; Sun, Y.; Ge, S. Metformin Inhibits Porphyromonas gingivalis Lipopolysaccharide-Influenced Inflammatory Response in Human Gingival Fibroblasts via Regulating Activating Transcription Factor-3 Expression. J. Periodontol. 2017, 88, e169–e178. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, J.; Jiang, Y.; Chen, X.; Li, J.; Chen, B.; Gao, J. The anti-periodontitis action of metformin via targeting NLRP3 inflammasome. Arch. Oral Biol. 2020, 114, 104692. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Hu, B.; Feng, G.; Xiang, M.; Deng, Y.; Tan, M.; Li, J.; Song, J. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 2020, 21, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Malta, F.S.; Garcia, R.P.; Azarias, J.S.; Ribeiro, G.; Miranda, T.S.; Shibli, J.A.; Bastos, M.F. Impact of hyperglycemia and treatment with metformin on ligature-induced bone loss, bone repair and expression of bone metabolism transcription factors. PLoS ONE 2020, 15, e0237660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liang, Q.; Kang, W.; Ge, S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biol. Int. 2019. [Google Scholar] [CrossRef]
- Jia, L.; Xiong, Y.; Zhang, W.; Ma, X.; Xu, X. Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway. Exp. Cell Res. 2020, 386, 111717. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.G.; Lee, S.M.; Bae, S.; Park, T.; Kim, H.; Jang, Y.; Moon, K.; Kim, H.; Lee, K.; Park, J.; et al. Effect of Aging on Homeostasis in the Soft Tissue of the Periodontium: A Narrative Review. J. Pers. Med. 2021, 11, 58. https://doi.org/10.3390/jpm11010058
Kim YG, Lee SM, Bae S, Park T, Kim H, Jang Y, Moon K, Kim H, Lee K, Park J, et al. Effect of Aging on Homeostasis in the Soft Tissue of the Periodontium: A Narrative Review. Journal of Personalized Medicine. 2021; 11(1):58. https://doi.org/10.3390/jpm11010058
Chicago/Turabian StyleKim, Yu Gyung, Sang Min Lee, Sungeun Bae, Taejun Park, Hyeonjin Kim, Yujeong Jang, Keonwoo Moon, Hyungmin Kim, Kwangmin Lee, Joonyoung Park, and et al. 2021. "Effect of Aging on Homeostasis in the Soft Tissue of the Periodontium: A Narrative Review" Journal of Personalized Medicine 11, no. 1: 58. https://doi.org/10.3390/jpm11010058
APA StyleKim, Y. G., Lee, S. M., Bae, S., Park, T., Kim, H., Jang, Y., Moon, K., Kim, H., Lee, K., Park, J., Byun, J.-S., & Kim, D.-Y. (2021). Effect of Aging on Homeostasis in the Soft Tissue of the Periodontium: A Narrative Review. Journal of Personalized Medicine, 11(1), 58. https://doi.org/10.3390/jpm11010058