The Pancreatic β-Cell: The Perfect Redox System
Abstract
1. Introduction
1.1. Emerging Concept of Redox Signaling
1.2. Traditional Classification of Insulin Secretion Mechanisms
2. Revisited Mechanism of Glucose-Stimulated Insulin Secretion
2.1. Elevations of ATP Plus H2O2 as the Fundamental Condition for GSIS
2.1.1. Discovery of NOX4-Mediated Redox Signaling in Pancreatic β-Cells
2.1.2. Glucose Equilibration and Specific Metabolism in Pancreatic β-Cells
2.1.3. Two Phases of GSIS
2.1.4. Inconsistencies in Considering Exclusive GSIS Dependence on Elevated ATP in Pancreatic β-Cells
2.2. Ion Channels Participating in GSIS
2.2.1. Plasma Membrane Potential and Ion Channels of Pancreatic β-Cells
2.2.2. Detailed Sequence of Events in Plasma Membrane upon GSIS
2.2.3. Ablations of KIR6.2 and SUR1 Support a Central but not Exclusive Role of KATP
2.2.4. Structure of KATP and Behavior with Insulin Non-Stimulating vs. Stimulating Glucose
2.2.5. Possible Modulation of KATP by Kinases and Phosphatases in Pancreatic β-Cells
2.3. Possible Redox Regulation of Ion Channels
2.3.1. Observed Redox Regulation of Ion Channels Participating in Insulin Secretion
2.3.2. Possible Redox-Target Residues of KIR6.2, SUR1 and TRPM2
2.4. Fine Tuning of the Glucose-Sensitivity Range
2.4.1. Inhibitory Factor IF1 as a Key Element Setting the Glucose-Sensitivity Range in Pancreatic β-Cells
2.4.2. Cristae Narrowing vs. Steepness of Glucose-Sensitivity Range in Pancreatic β-Cells
2.4.3. Responses of the Mitochondrial Matrix Calcium during GSIS
2.5. Amplifying Mechanisms
2.5.1. Receptor Mediated Amplification
2.5.2. PKA Pathway in Pancreatic β-cells
2.5.3. EPAC2 Pathway in Pancreatic β-Cells
2.5.4. Mitochondrial PKA Pathways in Pancreatic β-cells
2.5.5. Gαq/11-IP3 and Gαq/11-DAG-PKC Pathways
2.6. Biology of Insulin Granule Vesicles
2.6.1. Biogenesis of Insulin Granule Vesicles
2.6.2. Major Proteins of IGVs
2.6.3. Mechanism of IGV Exocytosis
2.6.4. Glutamate Promotion of Insulin Granule Vesicles Exocytosis
3. Incretins: GLP-1 and GIP
3.1. Incretin Potentiation of GSIS In Vivo
3.2. Glucagon-Like Peptide 1, GLP-1
3.2.1. GLP-1 Amplification of GSIS
3.2.2. GLP-1 Receptor Signaling
3.2.3. Two Phases of Insulin Secretion vs. GLP-1 Potentiation
3.2.4. Does GLP-1 Stimulate Insulin Secretion at Low Glucose?
3.3. Glucagon Inhibitory Peptide, GIP
4. Role of Redox Shuttles upon GSIS
4.1. Redox Shuttles Exporting Redox Equivalents to the Cytosol of Pancreatic β-Cells
4.1.1. Pyruvate/Malate Redox Shuttle
4.1.2. Pyruvate/Citrate Redox Shuttle
4.1.3. Pyruvate/Isocitrate Redox Shuttle
4.2. Specific Metabolism at Insulin-Non-Stimulating vs. Insulin-Stimulating Glucose in Pancreatic β-Cells
4.2.1. NADPH and NADH Homeostasis in Pancreatic β-Cells
4.2.2. Nicotine Nucleotide Translocase in Pancreatic β-Cells
4.2.3. Mitochondrial Matrix NADPH Homeostasis in Pancreatic β-Cells
4.2.4. Relationships to the Malate/Aspartate Shuttle
4.2.5. Acetoacetate and β-Hydroxybutyrate in Pancreatic β-Cells
4.2.6. Phosphoenolpyruvate Shuttle and the Role of Pyruvate Kinases
5. Mechanism of Insulin Secretion Stimulated by Branched-Chain Ketoacids
5.1. Superoxide Formation and Subsequent Redox Signaling upon β-Like Oxidation of Branched-Chain Ketoacids
5.1.1. Electron Transfer Flavoprotein: Ubiquinone Oxidoreductase (ETFQOR) as the Key Factor
5.1.2. Redox Signaling upon β-Like Oxidation of Branched-Chain Ketoacids
5.2. Metabolism of Branched-Chain Ketoacids
5.2.1. Branched-Chain Amino Acids as Precursors of Branched-Chain Ketoacids
5.2.2. β-Like Oxidation of Branched-Chain Ketoacids
5.3. Insulin Secretion Stimulated by Branched-Chain Ketoacids
5.3.1. Overview of Branched-Chain-Ketoacid-Stimulated Insulin Secretion
5.3.2. Physiological Context of Branched-Chain-Ketoacid-Stimulated Insulin Secretion
6. Mechanism of Fatty Acid-Stimulated Insulin Secretion
6.1. Relevancy of Fatty Acid-Stimulated Insulin Secretion (FASIS)
6.1.1. Experimental Approach vs. Physiology
6.1.2. GPR119 Pathway in Pancreatic β-Cells
6.1.3. Physiological Stimulation of GPR40 and GPR119 Receptors in Pancreatic β-Cells
6.1.4. Physiological Stimulation of Other Metabotropic Receptors in Pancreatic β-Cells
6.2. GPR40 and Metabolic Pathway upon Fatty Acid-Stimulated Insulin Secretion
6.2.1. GPR40 Pathway in Pancreatic β-Cells
6.2.2. β-Oxidation of Fatty Acids in Pancreatic β-Cells
6.2.3. β-Oxidation of Fatty Acids Produces Superoxide
6.3. Redox-Sensitive Mitochondrial Phospholipase iPLA2γ Amplifies FASIS
6.3.1. FASIS at Low Glucose vs. Dependence of Metabolic and Receptor Part of FASIS
6.3.2. Redox-Sensitive Mitochondrial Phospholipase iPLA2γ Amplifies FASIS
6.4. FASIS at High Glucose
6.4.1. Distinction from FASIS at Low Glucose
6.4.2. β-Oxidation vs. Triglyceride/Fatty Acid Cycle in Pancreatic β-Cells
6.5. GLP-1 as an Important Stimulus for Postprandial Insulin Secretion after High Fat Meal
6.6. Other Sectretagogues
7. Mechanisms of Transfer of Redox Signaling and Possible Insults
7.1. Mechanims of Transfer of Redox Signaling
7.2. Redox Status of Pancreatic β-Cells may Affect and Impact Redox Signaling Essential for Insulin Secretion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Klimova, T.A.; Eisenbart, J.; Moraes, C.T.; Murphy, M.P.; Budinger, G.R.; Chandel, N.S. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007, 177, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.J.; Koivunen, P.; Cao, S.; Backus, K.M.; Olenchock, B.A.; Patel, H.; Zhang, Q.; Signoretti, S.; Gerfen, G.J.; Richardson, A.L.; et al. Paracrine Induction of HIF by Glutamate in Breast Cancer: EglN1 Senses Cysteine. Cell 2016, 166, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Plecita-Hlavata, L.; Jaburek, M.; Holendova, B.; Tauber, J.; Pavluch, V.; Berkova, Z.; Cahova, M.; Schroeder, K.; Brandes, R.P.; Siemen, D.; et al. Glucose-Stimulated Insulin Secretion Fundamentally Requires H2O2 Signaling by NADPH Oxidase 4. Diabetes 2020. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Mori, Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. 2020, 146, 36–44. [Google Scholar] [CrossRef]
- Kakei, M.; Yoshida, M.; Dezaki, K.; Ito, K.; Yamada, H.; Funazaki, S.; Kawakami, M.; Sugawara, H.; Yada, T. Glucose and GTP-binding protein-coupled receptor cooperatively regulate transient receptor potential-channels to stimulate insulin secretion [Review]. Endocr. J. 2016, 63, 867–876. [Google Scholar] [CrossRef]
- Prentki, M.; Joly, E.; El-Assaad, W.; Roduit, R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: Role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes 2002, 51 (Suppl. 3), S405–S413. [Google Scholar] [CrossRef]
- Lenzen, S. Oxidative stress: The vulnerable beta-cell. Biochem. Soc. Trans. 2008, 36, 343–347. [Google Scholar] [CrossRef]
- Lenzen, S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic β-cells. Biochim. Et Biophys. Acta. Gen. Subj. 2017, 1861, 1929–1942. [Google Scholar] [CrossRef]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Welsh, N.; Margulis, B.; Borg, L.A.; Wiklund, H.J.; Saldeen, J.; Flodström, M.; Mello, M.A.; Andersson, A.; Pipeleers, D.G.; Hellerström, C. Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: Implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol. Med. (Camb. Mass.) 1995, 1, 806–820. [Google Scholar] [CrossRef]
- Ivarsson, R.; Quintens, R.; Dejonghe, S.; Tsukamoto, K.; Veld, P.; Renström, E.; Schuit, F.C. Redox control of exocytosis: Regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 2005, 54, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Reinbothe, T.M.; Ivarsson, R.; Li, D.-Q.; Niazi, O.; Jing, X.; Zhang, E.; Stenson, L.; Bryborn, U.; Renström, E. Glutaredoxin-1 Mediates NADPH-Dependent Stimulation of Calcium-Dependent Insulin Secretion. Mol. Endocrinol. 2009, 23, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Jezek, P.; Holendova, B.; Plecita-Hlavata, L. Redox Signaling from Mitochondria: Signal Propagation and Its Targets. Biomolecules 2020, 10, 93. [Google Scholar] [CrossRef]
- Woo, H.A.; Yim, S.H.; Shin, D.H.; Kang, D.; Yu, D.Y.; Rhee, S.G. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 2010, 140, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Ježek, P.; Jabůrek, M.; Plecitá-Hlavatá, L. Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes. Antioxid. Redox Signal. 2019. [Google Scholar] [CrossRef]
- Swisa, A.; Glaser, B.; Dor, Y. Metabolic Stress and Compromised Identity of Pancreatic Beta Cells. Front. Genet. 2017, 8, 21. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rorsman, P. Diabetes Mellitus and the β Cell: The Last Ten Years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef]
- Maechler, P. Mitochondrial function and insulin secretion. Mol. Cell. Endocrinol. 2013. [Google Scholar] [CrossRef]
- Prentki, M.; Matschinsky, F.M.; Madiraju, S.R.M. Metabolic Signaling in Fuel-Induced Insulin Secretion. Cell Metab. 2013, 18, 162–185. [Google Scholar] [CrossRef]
- Rutter, G.A.; Pullen, T.J.; Hodson, D.J.; Martinez-Sanchez, A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015, 466, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Straub, S.G.; Sharp, G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes/Metab. Res. Rev. 2002, 18, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.C. Regulation of insulin secretion: A matter of phase control and amplitude modulation. Diabetologia 2009, 52, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Sugawara, K.; Yokoi, N.; Takahashi, H. β-Cell signalling and insulin secretagogues: A path for improved diabetes therapy. DiabetesObes. Metab. 2017, 19 (Suppl. 1), 22–29. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, T.; Takahashi, T.; Takahashi, H.; Seino, S. Cooperation between cAMP signalling and sulfonylurea in insulin secretion. DiabetesObes. Metab. 2014, 16 (Suppl. 1), 118–125. [Google Scholar] [CrossRef] [PubMed]
- Seino, S. Cell signalling in insulin secretion: The molecular targets of ATP, cAMP and sulfonylurea. Diabetologia 2012, 55, 2096–2108. [Google Scholar] [CrossRef]
- Ježek, P.; Jabůrek, M.; Holendová, B.; Plecitá-Hlavatá, L. Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity. Molecules 2018, 23, 1483. [Google Scholar] [CrossRef]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.-L.; Ktorza, A.; Casteilla, L.; Penicaud, L. Mitochondrial Reactive Oxygen Species Are Obligatory Signals for Glucose-Induced Insulin Secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef]
- Saadeh, M.; Ferrante, T.C.; Kane, A.; Shirihai, O.; Corkey, B.E.; Deeney, J.T. Reactive Oxygen Species Stimulate Insulin Secretion in Rat Pancreatic Islets: Studies Using Mono-Oleoyl-Glycerol. PLoS ONE 2012, 7, e30200. [Google Scholar] [CrossRef]
- Rebelato, E.; Abdulkader, F.; Curi, R.; Carpinelli, A.R. Control of the Intracellular Redox State by Glucose Participates in the Insulin Secretion Mechanism. PLoS ONE 2011, 6, e24507. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive Oxygen Species as a Signal in Glucose-Stimulated Insulin Secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Rebelato, E.; Abdulkader, F.; Graciano, M.F.R.; Oliveira-Emilio, H.R.; Hirata, A.E.; Rocha, M.S.; Bordin, S.; Curi, R.; Carpinelli, A.R. Association of NAD(P)H Oxidase with Glucose-Induced Insulin Secretion by Pancreatic β-Cells. Endocrinology 2009, 150, 2197–2201. [Google Scholar] [CrossRef] [PubMed]
- Imoto, H.; Sasaki, N.; Iwase, M.; Nakamura, U.; Oku, M.; Sonoki, K.; Uchizono, Y.; Iida, M. Impaired Insulin Secretion by Diphenyleneiodium Associated with Perturbation of Cytosolic Ca 2+ Dynamics in Pancreatic β-Cells. Endocrinology 2008, 149, 5391–5400. [Google Scholar] [CrossRef]
- Syed, I.; Kyathanahalli, C.N.; Kowluru, A. Phagocyte-like NADPH oxidase generates ROS in INS 832/13 cells and rat islets: Role of protein prenylation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R756–R762. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, B.; Brun, T.; Deffert-Delbouille, C.; Mahiout, Z.; Daali, Y.; Ma, X.-J.; Krause, K.-H.; Maechler, P. NADPH Oxidase NOX2 Defines a New Antagonistic Role for Reactive Oxygen Species and cAMP/PKA in the Regulation of Insulin Secretion. Diabetes 2012, 61, 2842–2850. [Google Scholar] [CrossRef]
- Bouzakri, K.; Veyrat-Durebex, C.; Holterman, C.; Arous, C.; Barbieux, C.; Bosco, D.; Altirriba, J.; Alibashe, M.; Tournier, B.B.; Gunton, J.E.; et al. Beta-Cell-Specific Expression of Nicotinamide Adenine Dinucleotide Phosphate Oxidase 5 Aggravates High-Fat Diet-Induced Impairment of Islet Insulin Secretion in Mice. Antioxid. Redox Signal. 2020, 32, 618–635. [Google Scholar] [CrossRef]
- Plecitá-Hlavatá, L.; Engstová, H.; Holendová, B.; Tauber, J.; Špaček, T.; Petrásková, L.; Křen, V.; Špačková, J.; Gotvaldová, K.; Ježek, J.; et al. Mitochondrial Superoxide Production Decreases on Glucose-Stimulated Insulin Secretion in Pancreatic β Cells Due to Decreasing Mitochondrial Matrix NADH/NAD(+) Ratio. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Spégel, P.; Sharoyko, V.V.; Goehring, I.; Danielsson, A.P.; Malmgren, S.; Nagorny, C.L.; Andersson, L.E.; Koeck, T.; Sharp, G.W.; Straub, S.G.; et al. Time-resolved metabolomics analysis of β-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem. J. 2013, 450, 595–605. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Fórró, L.; Schlegel, W.; Krause, K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef]
- Di Fulvio, M.; Aguilar-Bryan, L. Chloride transporters and channels in β-cell physiology: Revisiting a 40-year-old model. Biochem. Soc. Trans. 2019, 47, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; McBride, E.L.; Zhang, G.; Xu, H.; Cai, T.; Notkins, A.L.; Aronova, M.A.; Leapman, R.D. Determination of secretory granule maturation times in pancreatic islet β-cells by serial block face scanning electron microscopy. J. Struct. Biol. 2020. [Google Scholar] [CrossRef]
- Ma, W.; Chang, J.; Tong, J.; Ho, U.; Yau, B.; Kebede, M.A.; Thorn, P. Arp2/3 nucleates F-actin coating of fusing insulin granules in pancreatic β cells to control insulin secretion. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Meda, P.; Bosco, D.; Chanson, M.; Giordano, E.; Vallar, L.; Wollheim, C.; Orci, L. Rapid and reversible secretion changes during uncoupling of rat insulin-producing cells. J. Clin. Investig. 1990, 86, 759–768. [Google Scholar] [CrossRef]
- Meda, P. The role of gap junction membrane channels in secretion and hormonal action. J. Bioenerg. Biomembr. 1996, 28, 369–377. [Google Scholar] [CrossRef]
- Ravier, M.A.; Güldenagel, M.; Charollais, A.; Gjinovci, A.; Caille, D.; Söhl, G.; Wollheim, C.B.; Willecke, K.; Henquin, J.C.; Meda, P. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005, 54, 1798–1807. [Google Scholar] [CrossRef]
- Jacob, S.; Köhler, M.; Tröster, P.; Visa, M.; García-Prieto, C.F.; Alanentalo, T.; Moede, T.; Leibiger, B.; Leibiger, I.B.; Berggren, P.O. In vivo Ca(2+) dynamics in single pancreatic β cells. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 945–959. [Google Scholar] [CrossRef]
- Johnston, N.R.; Mitchell, R.K.; Haythorne, E.; Pessoa, M.P.; Semplici, F.; Ferrer, J.; Piemonti, L.; Marchetti, P.; Bugliani, M.; Bosco, D.; et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 2016, 24, 389–401. [Google Scholar] [CrossRef]
- Rutter, G.A.; Hodson, D.J.; Chabosseau, P.; Haythorne, E.; Pullen, T.J.; Leclerc, I. Local and regional control of calcium dynamics in the pancreatic islet. DiabetesObes. Metab. 2017, 19 (Suppl. 1), 30–41. [Google Scholar] [CrossRef]
- Leturque, A.; Brot-Laroche, E.; Le Gall, M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E985–E992. [Google Scholar] [CrossRef]
- Kaminski, M.T.; Lenzen, S.; Baltrusch, S. Real-time analysis of intracellular glucose and calcium in pancreatic beta cells by fluorescence microscopy. Biochim. Et Biophys. Acta 2012, 1823, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.J.; Park, S.H.; Son, D.G.; Bae, J.H.; Kim, H.K.; Han, J.; Song, D.K. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic beta-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology 2012, 153, 574–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matschinsky, F.M.; Wilson, D.F. The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Front. Physiol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Schuit, F.; De Vos, A.; Farfari, S.; Moens, K.; Pipeleers, D.; Brun, T.; Prentki, M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J. Biol. Chem. 1997, 272, 18572–18579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liew, C.W.; Handy, D.E.; Zhang, Y.; Leopold, J.A.; Hu, J.; Guo, L.; Kulkarni, R.N.; Loscalzo, J.; Stanton, R.C. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 1497–1505. [Google Scholar] [CrossRef]
- Huang, M.; Joseph, J.W. Metabolomic analysis of pancreatic β-cell insulin release in response to glucose. Islets 2012, 4, 210–222. [Google Scholar] [CrossRef]
- Goehring, I.; Sauter, N.S.; Catchpole, G.; Assmann, A.; Shu, L.; Zien, K.S.; Moehlig, M.; Pfeiffer, A.F.H.; Oberholzer, J.; Willmitzer, L.; et al. Identification of an intracellular metabolic signature impairing beta cell function in the rat beta cell line INS-1E and human islets. Diabetologia 2011, 54, 2584–2594. [Google Scholar] [CrossRef]
- Ammon, H.P.; Steinke, J. 6-Amnionicotinamide (6-AN) as a diabetogenic agent. In vitro and in vivo studies in the rat. Diabetes 1972, 21, 143–148. [Google Scholar] [CrossRef]
- Verspohl, E.J.; Händel, M.; Ammon, H.P. Pentosephosphate shunt activity of rat pancreatic islets: Its dependence on glucose concentration. Endocrinology 1979, 105, 1269–1274. [Google Scholar] [CrossRef]
- Monte Alegre, S.; Saad, S.T.; Delatre, E.; Saad, M.J. Insulin secretion in patients deficient in glucose-6-phosphate dehydrogenase. Horm. Metab. Res. Horm. Und Stoffwechs. Horm. Et Metab. 1991, 23, 171–173. [Google Scholar] [CrossRef]
- Akhmedov, D.; De Marchi, U.; Wollheim, C.B.; Wiederkehr, A. Pyruvate dehydrogenase E1α phosphorylation is induced by glucose but does not control metabolism-secretion coupling in INS-1E clonal β-cells. Biochim. Et Biophys. Acta 2012, 1823, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.A.; El Azzouny, M.A.; Kennedy, R.T.; Burant, C.F. Metabolome response to glucose in the β-cell line INS-1 832/13. J. Biol. Chem. 2013, 288, 10923–10935. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.C.; Pongratz, R.L.; Zhao, X.; Yarborough, O.; Sereda, S.; Shirihai, O.; Cline, G.W.; Mason, G.; Kibbey, R.G. Integrated, Step-Wise, Mass-Isotopomeric Flux Analysis of the TCA Cycle. Cell Metab. 2015, 22, 936–947. [Google Scholar] [CrossRef]
- Ouyang, Q.; Nakayama, T.; Baytas, O.; Davidson, S.M.; Yang, C.; Schmidt, M.; Lizarraga, S.B.; Mishra, S.; Ei-Quessny, M.; Niaz, S.; et al. Mutations in mitochondrial enzyme GPT2 cause metabolic dysfunction and neurological disease with developmental and progressive features. Proc. Natl. Acad. Sci. USA 2016, 113, E5598–E5607. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Z.; Park, S.; Reagan, W.J.; Goldstein, R.; Zhong, S.; Lawton, M.; Rajamohan, F.; Qian, K.; Liu, L.; Gong, D.W. Alanine aminotransferase isoenzymes: Molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatol. (Baltim. Md.) 2009, 49, 598–607. [Google Scholar] [CrossRef]
- Maechler, P. Glutamate pathways of the beta-cell and the control of insulin secretion. Diabetes Res. Clin. Pract. 2017, 131, 149–153. [Google Scholar] [CrossRef]
- Takahashi, H.; Yokoi, N.; Seino, S. Glutamate as intracellular and extracellular signals in pancreatic islet functions. Proc. Jpn. Acad. Ser. BPhys. Biol. Sci. 2019, 95, 246–260. [Google Scholar] [CrossRef]
- Hoang, D.T.; Hara, M.; Jo, J. Design Principles of Pancreatic Islets: Glucose-Dependent Coordination of Hormone Pulses. PLoS ONE 2016, 11, e0152446. [Google Scholar] [CrossRef]
- Kalwat, M.A.; Cobb, M.H. Mechanisms of the amplifying pathway of insulin secretion in the β cell. Pharmacol. Ther. 2017, 179, 17–30. [Google Scholar] [CrossRef]
- Villard, O.; Brun, J.F.; Bories, L.; Molinari, N.; Benhamou, P.Y.; Berney, T.; Wojtusciszyn, A. The Second Phase of Insulin Secretion in Nondiabetic Islet-Grafted Recipients Is Altered and Can Predict Graft Outcome. J. Clin. Endocrinol. Metab. 2018, 103, 1310–1319. [Google Scholar] [CrossRef]
- Henquin, J.C.; Dufrane, D.; Kerr-Conte, J.; Nenquin, M. Dynamics of glucose-induced insulin secretion in normal human islets. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E640–E650. [Google Scholar] [CrossRef] [PubMed]
- Gembal, M.; Detimary, P.; Gilon, P.; Gao, Z.Y.; Henquin, J.C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. J. Clin. Investig. 1993, 91, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Takei, M.; Ishii, H.; Sato, Y. Glucose-stimulated insulin secretion: A newer perspective. J. Diabetes Investig. 2013, 4, 511–516. [Google Scholar] [CrossRef]
- Pedersen, M.G.; Tagliavini, A.; Henquin, J.C. Calcium signaling and secretory granule pool dynamics underlie biphasic insulin secretion and its amplification by glucose: Experiments and modeling. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E475–E486. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef]
- Daniel, S.; Noda, M.; Straub, S.G.; Sharp, G.W. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes 1999, 48, 1686–1690. [Google Scholar] [CrossRef]
- Rorsman, P.; Renström, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Ohara-Imaizumi, M.; Nakamichi, Y.; Kikuta, T.; Nishiwaki, C. Imaging docking and fusion of insulin granules induced by antidiabetes agents: Sulfonylurea and glinide drugs preferentially mediate the fusion of newcomer, but not previously docked, insulin granules. Diabetes 2006, 55, 2819–2825. [Google Scholar] [CrossRef]
- Ohara-Imaizumi, M.; Fujiwara, T.; Nakamichi, Y.; Okamura, T.; Akimoto, Y.; Kawai, J.; Matsushima, S.; Kawakami, H.; Watanabe, T.; Akagawa, K.; et al. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J. Cell Biol. 2007, 177, 695–705. [Google Scholar] [CrossRef]
- Kalwat, M.A.; Thurmond, D.C. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp. Mol. Med. 2013, 45, e37. [Google Scholar] [CrossRef]
- Mourad, N.I.; Nenquin, M.; Henquin, J.C. Metabolic amplifying pathway increases both phases of insulin secretion independently of beta-cell actin microfilaments. Am. J. Physiol. Cell Physiol. 2010, 299, C389–C398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Thurmond, D.C. Mechanisms of biphasic insulin-granule exocytosis-roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 2009, 122, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Mourad, N.I.; Nenquin, M.; Henquin, J.C. cAMP-mediated and metabolic amplification of insulin secretion are distinct pathways sharing independence of β-cell microfilaments. Endocrinology 2012, 153, 4644–4654. [Google Scholar] [CrossRef] [PubMed]
- Mourad, N.I.; Nenquin, M.; Henquin, J.C. Amplification of insulin secretion by acetylcholine or phorbol ester is independent of β-cell microfilaments and distinct from metabolic amplification. Mol. Cell. Endocrinol. 2013, 367, 11–20. [Google Scholar] [CrossRef]
- Shibasaki, T.; Takahashi, H.; Miki, T.; Sunaga, Y.; Matsumura, K.; Yamanaka, M.; Zhang, C.; Tamamoto, A.; Satoh, T.; Miyazaki, J.; et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl. Acad. Sci. USA 2007, 104, 19333–19338. [Google Scholar] [CrossRef]
- Leguina-Ruzzi, A.; Vodičková, A.; Holendová, B.; Pavluch, V.; Tauber, J.; Engstová, H.; Dlasková, A.; Ježek, P. Glucose-Induced Expression of DAPIT in Pancreatic β-Cells. Biomolecules 2020, 10, 1026. [Google Scholar] [CrossRef]
- Bränström, R.; Leibiger, I.B.; Leibiger, B.; Corkey, B.E.; Berggren, P.O.; Larsson, O. Long chain coenzyme A esters activate the pore-forming subunit (Kir6. 2) of the ATP-regulated potassium channel. J. Biol. Chem. 1998, 273, 31395–31400. [Google Scholar] [CrossRef]
- Bränström, R.; Corkey, B.E.; Berggren, P.O.; Larsson, O. Evidence for a unique long chain acyl-CoA ester binding site on the ATP-regulated potassium channel in mouse pancreatic beta cells. J. Biol. Chem. 1997, 272, 17390–17394. [Google Scholar] [CrossRef]
- Gribble, F.M.; Proks, P.; Corkey, B.E.; Ashcroft, F.M. Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J. Biol. Chem. 1998, 273, 26383–26387. [Google Scholar] [CrossRef]
- Prentki, M.; Vischer, S.; Glennon, M.C.; Regazzi, R.; Deeney, J.T.; Corkey, B.E. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J. Biol. Chem. 1992, 267, 5802–5810. [Google Scholar] [CrossRef]
- Yang, S.N.; Shi, Y.; Yang, G.; Li, Y.; Yu, J.; Berggren, P.O. Ionic mechanisms in pancreatic β cell signaling. Cell. Mol. Life Sci. Cmls 2014, 71, 4149–4177. [Google Scholar] [CrossRef] [PubMed]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Electrophysiology of Islet Cells. In Advances in Experimental Medicine and Biology; Islam, M., Ed.; Springer: Dordrecht, The Netherlands, 2010; Volume 654, pp. 115–163. [Google Scholar] [CrossRef]
- Bennett, K.; James, C.; Hussain, K. Pancreatic β-cell KATP channels: Hypoglycaemia and hyperglycaemia. Rev. Endocr. Metab. Disord. 2010, 11, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Szollosi, A.; Nenquin, M.; Henquin, J. Pharmacological stimulation and inhibition of insulin secretion in mouse islets lacking ATP-sensitive K+ channels. Br. J. Pharmacol. 2010, 159, 669–677. [Google Scholar] [CrossRef]
- Soty, M.; Visa, M.; Soriano, S.; del Carmen Carmona, M.; Nadal, Á.; Novials, A. Involvement of ATP-sensitive Potassium (KATP) Channels in the Loss of Beta-cell Function Induced by Human Islet Amyloid Polypeptide. J. Biol. Chem. 2011, 286, 40857–40866. [Google Scholar] [CrossRef]
- Rorsman, P.; Braun, M.; Zhang, Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium 2012, 51, 300–308. [Google Scholar] [CrossRef]
- MacDonald, P.E. Signal integration at the level of ion channel and exocytotic function in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1065–E1069. [Google Scholar] [CrossRef]
- Zhang, Q.; Chibalina, M.V.; Bengtsson, M.; Groschner, L.N.; Ramracheya, R.; Rorsman, N.J.; Leiss, V.; Nassar, M.A.; Welling, A.; Gribble, F.M.; et al. Na+ current properties in islet α- and β-cells reflect cell-specific Scn3a and Scn9a expression. J. Physiol. 2014, 592, 4677–4696. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Semplici, F.; Li, D.; Rizzuto, R.; Ravier, M.A.; Gilon, P.; Rutter, G.A. Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic β cells. Pflug. Arch. Eur. J. Physiol. 2013, 465, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, S.L.; Cardone, R.L.; Foster, H.R.; Ho, T.; Potapenko, E.; Poudel, C.; VanDeusen, H.R.; Sdao, S.M.; Alves, T.C.; Zhao, X.; et al. Pyruvate Kinase Controls Signal Strength in the Insulin Secretory Pathway. Cell Metab. 2020, 32, 736–750.e735. [Google Scholar] [CrossRef]
- Rorsman, P.; Braun, M. Regulation of insulin secretion in human pancreatic islets. Annu. Rev. Physiol. 2013, 75, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Ashcroft, F.M.; Rorsman, P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K(+)-currents in isolated mouse pancreatic beta-cells. Febs Lett. 1990, 261, 187–190. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Girard, C.A.; Ashcroft, F.M. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic beta-cells. Diabetes 2006, 55, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Schulla, V.; Renström, E.; Feil, R.; Feil, S.; Franklin, I.; Gjinovci, A.; Jing, X.J.; Laux, D.; Lundquist, I.; Magnuson, M.A.; et al. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. Embo J. 2003, 22, 3844–3854. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Li, D.Q.; Olofsson, C.S.; Salehi, A.; Surve, V.V.; Caballero, J.; Ivarsson, R.; Lundquist, I.; Pereverzev, A.; Schneider, T.; et al. CaV2.3 calcium channels control second-phase insulin release. J. Clin. Investig. 2005, 115, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Suga, S.; Wu, J.; Kimura, M.; Wakui, M. Intracellular cAMP potentiates voltage-dependent activation of L-type Ca2+ channels in rat islet beta-cells. Pflug. Arch. Eur. J. Physiol. 1998, 435, 578–580. [Google Scholar] [CrossRef]
- Rorsman, P.; Eliasson, L.; Kanno, T.; Zhang, Q.; Gopel, S. Electrophysiology of pancreatic β-cells in intact mouse islets of Langerhans. Prog. Biophys. Mol. Biol. 2011, 107, 224–235. [Google Scholar] [CrossRef]
- Best, L. Glucose-induced electrical activity in rat pancreatic beta-cells: Dependence on intracellular chloride concentration. J. Physiol. 2005, 568, 137–144. [Google Scholar] [CrossRef]
- Stuhlmann, T.; Planells-Cases, R.; Jentsch, T.J. LRRC8/VRAC anion channels enhance β-cell glucose sensing and insulin secretion. Nat. Commun. 2018, 9, 1974. [Google Scholar] [CrossRef]
- Colsoul, B.; Schraenen, A.; Lemaire, K.; Quintens, R.; Van Lommel, L.; Segal, A.; Owsianik, G.; Talavera, K.; Voets, T.; Margolskee, R.F.; et al. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5-/- mice. Proc. Natl. Acad. Sci. USA 2010, 107, 5208–5213. [Google Scholar] [CrossRef]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011, 589, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Masgrau, R.; Churchill, G.C.; Morgan, A.J.; Ashcroft, S.J.; Galione, A. NAADP: A new second messenger for glucose-induced Ca2+ responses in clonal pancreatic beta cells. Curr. Biol. 2003, 13, 247–251. [Google Scholar] [CrossRef]
- Ostapchenko, V.G.; Chen, M.; Guzman, M.S.; Xie, Y.F.; Lavine, N.; Fan, J.; Beraldo, F.H.; Martyn, A.C.; Belrose, J.C.; Mori, Y.; et al. The Transient Receptor Potential Melastatin 2 (TRPM2) Channel Contributes to β-Amyloid Oligomer-Related Neurotoxicity and Memory Impairment. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 15157–15169. [Google Scholar] [CrossRef] [PubMed]
- Miyanohara, J.; Kakae, M.; Nagayasu, K.; Nakagawa, T.; Mori, Y.; Arai, K.; Shirakawa, H.; Kaneko, S. TRPM2 Channel Aggravates CNS Inflammation and Cognitive Impairment via Activation of Microglia in Chronic Cerebral Hypoperfusion. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 3520–3533. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.J.; Hasan, N.M.; Longacre, M.J. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim. Et Biophys. Acta 2008, 1780, 966–972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilon, P.; Ravier, M.A.; Jonas, J.C.; Henquin, J.C. Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes 2002, 51 (Suppl. 1), S144–S151. [Google Scholar] [CrossRef]
- Beauvois, M.C.; Merezak, C.; Jonas, J.C.; Ravier, M.A.; Henquin, J.C.; Gilon, P. Glucose-induced mixed [Ca2+]c oscillations in mouse beta-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum. Am. J. Physiol Cell Physiol 2006, 290, C1503–C1511. [Google Scholar] [CrossRef]
- Sabourin, J.; Allagnat, F. Store-operated Ca2+ entry: A key component of the insulin secretion machinery. J. Mol. Endocrinol. 2016, 57, F35–F39. [Google Scholar] [CrossRef]
- Sabourin, J.; Le Gal, L.; Saurwein, L.; Haefliger, J.A.; Raddatz, E.; Allagnat, F. Store-operated Ca2+ Entry Mediated by Orai1 and TRPC1 Participates to Insulin Secretion in Rat β-Cells. J. Biol. Chem. 2015, 290, 30530–30539. [Google Scholar] [CrossRef]
- Rorsman, P.; Trube, G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J. Physiol. 1986, 374, 531–550. [Google Scholar] [CrossRef]
- Düfer, M.; Gier, B.; Wolpers, D.; Krippeit-Drews, P.; Ruth, P.; Drews, G. Enhanced glucose tolerance by SK4 channel inhibition in pancreatic beta-cells. Diabetes 2009, 58, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Vierra, N.C.; Dadi, P.K.; Jeong, I.; Dickerson, M.; Powell, D.R.; Jacobson, D.A. Type 2 Diabetes-Associated K+ Channel TALK-1 Modulates β-Cell Electrical Excitability, Second-Phase Insulin Secretion, and Glucose Homeostasis. Diabetes 2015, 64, 3818–3828. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.A.; Kuznetsov, A.; Lopez, J.P.; Kash, S.; Ammälä, C.E.; Philipson, L.H. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 2007, 6, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Rebelato, E.; Santos, L.R.; Carpinelli, A.R.; Rorsman, P.; Abdulkader, F. Short-term high glucose culture potentiates pancreatic beta cell function. Sci. Rep. 2018, 8, 13061. [Google Scholar] [CrossRef]
- Miki, T.; Nagashima, K.; Tashiro, F.; Kotake, K.; Yoshitomi, H.; Tamamoto, A.; Gonoi, T.; Iwanaga, T.; Miyazaki, J.; Seino, S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 10402–10406. [Google Scholar] [CrossRef]
- Ravier, M.A.; Nenquin, M.; Miki, T.; Seino, S.; Henquin, J.C. Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology 2009, 150, 33–45. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Long, R.K.; Ferrara, C.T.; Gitelman, S.E.; German, M.S.; Yang, S.B. A new familial form of a late-onset, persistent hyperinsulinemic hypoglycemia of infancy caused by a novel mutation in KCNJ11. Channels (AustinTex.) 2017, 11, 636–647. [Google Scholar] [CrossRef]
- Nenquin, M.; Szollosi, A.; Aguilar-Bryan, L.; Bryan, J.; Henquin, J.C. Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic beta-cells. J. Biol. Chem. 2004, 279, 32316–32324. [Google Scholar] [CrossRef]
- Seghers, V.; Nakazaki, M.; DeMayo, F.; Aguilar-Bryan, L.; Bryan, J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J. Biol. Chem. 2000, 275, 9270–9277. [Google Scholar] [CrossRef]
- Nakazaki, M.; Crane, A.; Hu, M.; Seghers, V.; Ullrich, S.; Aguilar-Bryan, L.; Bryan, J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes 2002, 51, 3440–3449. [Google Scholar] [CrossRef]
- Kikuta, T.; Ohara-Imaizumi, M.; Nakazaki, M.; Nishiwaki, C.; Nakamichi, Y.; Tei, C.; Aguilar-Bryan, L.; Bryan, J.; Nagamatsu, S. Docking and fusion of insulin secretory granules in SUR1 knock out mouse beta-cells observed by total internal reflection fluorescence microscopy. Febs Lett. 2005, 579, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, J.X.; Ding, D.; Cheng, J.; Gao, N.; Chen, L. Structure of a Pancreatic ATP-Sensitive Potassium Channel. Cell 2017, 168, 101–110.e110. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.M.; Yoshioka, C.; Rex, E.A.; Fay, J.F.; Xie, Q.; Whorton, M.R.; Chen, J.Z.; Shyng, S.L. Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, M.V.; Campbell, J.D.; de Wet, H.; Shimomura, K.; Zadek, B.; Collins, R.F.; Sansom, M.S.; Ford, R.C.; Ashcroft, F.M. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. Embo J. 2005, 24, 4166–4175. [Google Scholar] [CrossRef]
- Nichols, C.G. KATP channels as molecular sensors of cellular metabolism. Nature 2006, 440, 470–476. [Google Scholar] [CrossRef]
- Yang, H.Q.; Martinez-Ortiz, W.; Hwang, J.; Fan, X.; Cardozo, T.J.; Coetzee, W.A. Palmitoylation of the K(ATP) channel Kir6.2 subunit promotes channel opening by regulating PIP(2) sensitivity. Proc. Natl. Acad. Sci. USA 2020, 117, 10593–10602. [Google Scholar] [CrossRef]
- Shyng, S.; Ferrigni, T.; Nichols, C.G. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol 1997, 110, 643–654. [Google Scholar] [CrossRef]
- Vedovato, N.; Rorsman, O.; Hennis, K.; Ashcroft, F.M.; Proks, P. Role of the C-terminus of SUR in the differential regulation of β-cell and cardiac K(ATP) channels by MgADP and metabolism. J. Physiol. 2018, 596, 6205–6217. [Google Scholar] [CrossRef]
- Shyng, S.L.; Nichols, C.G. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 1998, 282, 1138–1141. [Google Scholar] [CrossRef]
- Baukrowitz, T.; Schulte, U.; Oliver, D.; Herlitze, S.; Krauter, T.; Tucker, S.J.; Ruppersberg, J.P.; Fakler, B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 1998, 282, 1141–1144. [Google Scholar] [CrossRef]
- Lin, Y.F.; Jan, Y.N.; Jan, L.Y. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. Embo J. 2000, 19, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Béguin, P.; Nagashima, K.; Nishimura, M.; Gonoi, T.; Seino, S. PKA-mediated phosphorylation of the human K(ATP) channel: Separate roles of Kir6.2 and SUR1 subunit phosphorylation. Embo J. 1999, 18, 4722–4732. [Google Scholar] [CrossRef]
- Kline, C.F.; Wright, P.J.; Koval, O.M.; Zmuda, E.J.; Johnson, B.L.; Anderson, M.E.; Hai, T.; Hund, T.J.; Mohler, P.J. βIV-Spectrin and CaMKII facilitate Kir6.2 regulation in pancreatic beta cells. Proc. Natl. Acad. Sci. USA 2013, 110, 17576–17581. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M.; Harrison, D.E.; Ashcroft, S.J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 1984, 312, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Yasui, S.; Mawatari, K.; Morizumi, R.; Furukawa, H.; Shimohata, T.; Harada, N.; Takahashi, A.; Nakaya, Y. Hydrogen peroxide inhibits insulin-induced ATP-sensitive potassium channel activation independent of insulin signaling pathway in cultured vascular smooth muscle cells. J. Med. Investig. JMI 2012, 59, 36–44. [Google Scholar] [CrossRef]
- Finol-Urdaneta, R.K.; Remedi, M.S.; Raasch, W.; Becker, S.; Clark, R.B.; Strüver, N.; Pavlov, E.; Nichols, C.G.; French, R.J.; Terlau, H. Block of Kv1.7 potassium currents increases glucose-stimulated insulin secretion. Embo Mol. Med. 2012, 4, 424–434. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; Salapatek, A.M.; Wheeler, M.B. Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic beta-cells. J. Physiol. 2003, 546, 647–653. [Google Scholar] [CrossRef]
- Mittal, M.; Gu, X.Q.; Pak, O.; Pamenter, M.E.; Haag, D.; Fuchs, D.B.; Schermuly, R.T.; Ghofrani, H.A.; Brandes, R.P.; Seeger, W.; et al. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic. Biol. Med. 2012, 52, 1033–1042. [Google Scholar] [CrossRef]
- Grupe, M.; Myers, G.; Penner, R.; Fleig, A. Activation of store-operated I(CRAC) by hydrogen peroxide. Cell Calcium 2010, 48, 1–9. [Google Scholar] [CrossRef]
- Kashio, M.; Tominaga, M. Redox Signal-mediated Enhancement of the Temperature Sensitivity of Transient Receptor Potential Melastatin 2 (TRPM2) Elevates Glucose-induced Insulin Secretion from Pancreatic Islets. J. Biol. Chem. 2015, 290, 12435–12442. [Google Scholar] [CrossRef]
- Llanos, P.; Contreras-Ferrat, A.; Barrientos, G.; Valencia, M.; Mears, D.; Hidalgo, C. Glucose-Dependent Insulin Secretion in Pancreatic β-Cell Islets from Male Rats Requires Ca2+ Release via ROS-Stimulated Ryanodine Receptors. PLoS ONE 2015, 10, e0129238. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Wakamori, M.; Ishii, M.; Maeno, E.; Nishida, M.; Yoshida, T.; Yamada, H.; Shimizu, S.; Mori, E.; Kudoh, J.; et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 2002, 9, 163–173. [Google Scholar] [CrossRef]
- Yosida, M.; Dezaki, K.; Uchida, K.; Kodera, S.; Lam, N.V.; Ito, K.; Rita, R.S.; Yamada, H.; Shimomura, K.; Ishikawa, S.E.; et al. Involvement of cAMP/EPAC/TRPM2 activation in glucose- and incretin-induced insulin secretion. Diabetes 2014, 63, 3394–3403. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968–983.e924. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Huang, Y.; Roth, B.; Lü, W.; Du, J. Ligand recognition and gating mechanism through three ligand-binding sites of human TRPM2 channel. eLife 2019, 8. [Google Scholar] [CrossRef]
- Kahancová, A.; Sklenář, F.; Ježek, P.; Dlasková, A. Regulation of glucose-stimulated insulin secretion by ATPase Inhibitory Factor 1 (IF1). Febs Lett. 2018, 592, 999–1009. [Google Scholar] [CrossRef]
- Kahancová, A.; Sklenář, F.; Ježek, P.; Dlasková, A. Overexpression of native IF1 downregulates glucose-stimulated insulin secretion by pancreatic INS-1E cells. Sci. Rep. 2020, 10, 1551. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, L.; Zong, S.; Guo, R.; Liu, T.; Yi, J.; Wang, P.; Zhuo, W.; Yang, M. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science 2019, 364, 1068–1075. [Google Scholar] [CrossRef]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. How the regulatory protein, IF(1), inhibits F(1)-ATPase from bovine mitochondria. Proc. Natl. Acad. Sci. USA 2007, 104, 15671–15676. [Google Scholar] [CrossRef]
- Esparza-Moltó, P.B.; Cuezva, J.M. Reprogramming Oxidative Phosphorylation in Cancer: A Role for RNA-Binding Proteins. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Shen, L.; Zhi, L.; Hu, W.; Wu, M.X. IEX-1 targets mitochondrial F1Fo-ATPase inhibitor for degradation. Cell Death Differ. 2009, 16, 603–612. [Google Scholar] [CrossRef] [PubMed]
- García-Aguilar, A.; Cuezva, J.M. A Review of the Inhibition of the Mitochondrial ATP Synthase by IF1 in vivo: Reprogramming Energy Metabolism and Inducing Mitohormesis. Front. Physiol. 2018, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- Dlaskova, A.; Spacek, T.; Engstova, H.; Spackova, J.; Schrofel, A.; Holendova, B.; Smolkova, K.; Plecita-Hlavata, L.; Jezek, P. Mitochondrial cristae narrowing upon higher 2-oxoglutarate load. Biochim. Et Biophys. Acta. Bioenerg. 2019, 1860, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, E.; Haythorne, E.; Dickerson, M.T.; Lopez-Noriega, L.; Pullen, T.J.; da Silva Xavier, G.; Davis, S.P.X.; Martinez-Sanchez, A.; Semplici, F.; Rizzuto, R.; et al. The pore-forming subunit MCU of the mitochondrial Ca(2+) uniporter is required for normal glucose-stimulated insulin secretion in vitro and in vivo in mice. Diabetologia 2020, 63, 1368–1381. [Google Scholar] [CrossRef]
- McCormack, J.G.; Halestrap, A.P.; Denton, R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990, 70, 391–425. [Google Scholar] [CrossRef]
- Drews, G.; Bauer, C.; Edalat, A.; Düfer, M.; Krippeit-Drews, P. Evidence against a Ca(2+)-induced potentiation of dehydrogenase activity in pancreatic beta-cells. Pflug. Arch. Eur. J. Physiol. 2015, 467, 2389–2397. [Google Scholar] [CrossRef]
- Rutter, G.A.; Pralong, W.F.; Wollheim, C.B. Regulation of mitochondrial glycerol-phosphate dehydrogenase by Ca2+ within electropermeabilized insulin-secreting cells (INS-1). Biochim. Et Biophys. Acta 1992, 1175, 107–113. [Google Scholar] [CrossRef]
- Alam, M.R.; Groschner, L.N.; Parichatikanond, W.; Kuo, L.; Bondarenko, A.I.; Rost, R.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J. Biol. Chem. 2012, 287, 34445–34454. [Google Scholar] [CrossRef]
- McKenna, J.P.; Ha, J.; Merrins, M.J.; Satin, L.S.; Sherman, A.; Bertram, R. Ca2+ Effects on ATP Production and Consumption Have Regulatory Roles on Oscillatory Islet Activity. Biophys J. 2016, 110, 733–742. [Google Scholar] [CrossRef]
- Tsuboi, T.; da Silva Xavier, G.; Holz, G.G.; Jouaville, L.S.; Thomas, A.P.; Rutter, G.A. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem. J. 2003, 369, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Hodson, D.J.; Tarasov, A.I.; Gimeno Brias, S.; Mitchell, R.K.; Johnston, N.R.; Haghollahi, S.; Cane, M.C.; Bugliani, M.; Marchetti, P.; Bosco, D.; et al. Incretin-modulated beta cell energetics in intact islets of Langerhans. Mol. Endocrinol. (Baltim. Md.) 2014, 28, 860–871. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, U.; Galindo, A.N.; Thevenet, J.; Hermant, A.; Bermont, F.; Lassueur, S.; Domingo, J.S.; Kussmann, M.; Dayon, L.; Wiederkehr, A. Mitochondrial lysine deacetylation promotes energy metabolism and calcium signaling in insulin-secreting cells. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 4660–4674. [Google Scholar] [CrossRef]
- Quan, X.; Nguyen, T.T.; Choi, S.K.; Xu, S.; Das, R.; Cha, S.K.; Kim, N.; Han, J.; Wiederkehr, A.; Wollheim, C.B.; et al. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. J. Biol. Chem. 2015, 290, 4086–4096. [Google Scholar] [CrossRef]
- Kennedy, E.D.; Rizzuto, R.; Theler, J.M.; Pralong, W.F.; Bastianutto, C.; Pozzan, T.; Wollheim, C.B. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J. Clin. Investig. 1996, 98, 2524–2538. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Semplici, F.; Ravier, M.A.; Bellomo, E.A.; Pullen, T.J.; Gilon, P.; Sekler, I.; Rizzuto, R.; Rutter, G.A. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS ONE 2012, 7, e39722. [Google Scholar] [CrossRef]
- Wiederkehr, A.; Szanda, G.; Akhmedov, D.; Mataki, C.; Heizmann, C.W.; Schoonjans, K.; Pozzan, T.; Spät, A.; Wollheim, C.B. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 2011, 13, 601–611. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Furman, B.; Ong, W.K.; Pyne, N.J. Cyclic AMP signaling in pancreatic islets. Adv. Exp. Med. Biol. 2010, 654, 281–304. [Google Scholar] [CrossRef]
- Lefkimmiatis, K.; Zaccolo, M. cAMP signaling in subcellular compartments. Pharmacol. Ther. 2014, 143, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Salloum, G.; Jaafar, L.; El-Sibai, M. Rho A and Rac1: Antagonists moving forward. Tissue Cell 2020, 65, 101364. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Ravier, M.A.; Bertrand, G. Emerging roles for β-arrestin-1 in the control of the pancreatic β-cell function and mass: New therapeutic strategies and consequences for drug screening. Cell. Signal. 2011, 23, 522–528. [Google Scholar] [CrossRef]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef]
- Taylor, S.S.; Ilouz, R.; Zhang, P.; Kornev, A.P. Assembly of allosteric macromolecular switches: Lessons from PKA. Nat. Rev. Mol. Cell Biol. 2012, 13, 646–658. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Qi, Y.; Xu, H. Mitochondrial cAMP signaling. Cell. Mol. Life Sci. Cmls 2016, 73, 4577–4590. [Google Scholar] [CrossRef]
- Ould Amer, Y.; Hebert-Chatelain, E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Et Biophys. Acta. Bioenerg. 2018, 1859, 868–877. [Google Scholar] [CrossRef]
- Härndahl, L.; Jing, X.J.; Ivarsson, R.; Degerman, E.; Ahrén, B.; Manganiello, V.C.; Renström, E.; Holst, L.S. Important role of phosphodiesterase 3B for the stimulatory action of cAMP on pancreatic beta-cell exocytosis and release of insulin. J. Biol. Chem. 2002, 277, 37446–37455. [Google Scholar] [CrossRef]
- Bünemann, M.; Gerhardstein, B.L.; Gao, T.; Hosey, M.M. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the beta(2) subunit. J. Biol. Chem. 1999, 274, 33851–33854. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; Wang, X.; Xia, F.; El-kholy, W.; Targonsky, E.D.; Tsushima, R.G.; Wheeler, M.B. Antagonism of rat beta-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. J. Biol. Chem. 2003, 278, 52446–52453. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Seshadri, M.; Ashraf, U.; Mdluli, T.; Mondal, P.; Keil, M.; Azevedo, M.; Kirschner, L.S.; Stratakis, C.A.; Hussain, M.A. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab. 2011, 13, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Somanath, S.; Partridge, C.J.; Marshall, C.; Rowe, T.; Turner, M.D. Snapin mediates insulin secretory granule docking, but not trans-SNARE complex formation. Biochem. Biophys. Res. Commun. 2016, 473, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Holz, G.G. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 2004, 53, 5–13. [Google Scholar] [CrossRef]
- Kang, G.; Leech, C.A.; Chepurny, O.G.; Coetzee, W.A.; Holz, G.G. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic beta-cells and rat INS-1 cells. J. Physiol. 2008, 586, 1307–1319. [Google Scholar] [CrossRef]
- de Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- Gloerich, M.; Bos, J.L. Epac: Defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 355–375. [Google Scholar] [CrossRef]
- Holz, G.G.; Leech, C.A.; Heller, R.S.; Castonguay, M.; Habener, J.F. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37). J. Biol. Chem. 1999, 274, 14147–14156. [Google Scholar] [CrossRef]
- Gilon, P.; Chae, H.Y.; Rutter, G.A.; Ravier, M.A. Calcium signaling in pancreatic β-cells in health and in Type 2 diabetes. Cell Calcium 2014, 56, 340–361. [Google Scholar] [CrossRef]
- Kang, G.; Chepurny, O.G.; Holz, G.G. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J. Physiol. 2001, 536, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, N.; Shibasaki, T.; Kashima, Y.; Miki, T.; Takahashi, K.; Ueno, H.; Sunaga, Y.; Yano, H.; Matsuura, Y.; Iwanaga, T.; et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat. Cell Biol. 2000, 2, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Kashima, Y.; Miki, T.; Shibasaki, T.; Ozaki, N.; Miyazaki, M.; Yano, H.; Seino, S. Critical role of cAMP-GEFII--Rim2 complex in incretin-potentiated insulin secretion. J. Biol. Chem. 2001, 276, 46046–46053. [Google Scholar] [CrossRef]
- Yasuda, T.; Shibasaki, T.; Minami, K.; Takahashi, H.; Mizoguchi, A.; Uriu, Y.; Numata, T.; Mori, Y.; Miyazaki, J.; Miki, T.; et al. Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab. 2010, 12, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; León, I.R.; Bak, S.; Mogensen, M.; Wrzesinski, K.; Højlund, K.; Jensen, O.N. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol. Cell. Proteom. Mcp 2011, 10, M110.000299. [Google Scholar] [CrossRef] [PubMed]
- Grimsrud, P.A.; Carson, J.J.; Hebert, A.S.; Hubler, S.L.; Niemi, N.M.; Bailey, D.J.; Jochem, A.; Stapleton, D.S.; Keller, M.P.; Westphall, M.S.; et al. A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Cell Metab. 2012, 16, 672–683. [Google Scholar] [CrossRef]
- De Rasmo, D.; Micelli, L.; Santeramo, A.; Signorile, A.; Lattanzio, P.; Papa, S. cAMP regulates the functional activity, coupling efficiency and structural organization of mammalian FOF1 ATP synthase. Biochim. Et Biophys. Acta 2016, 1857, 350–358. [Google Scholar] [CrossRef]
- Acin-Perez, R.; Russwurm, M.; Günnewig, K.; Gertz, M.; Zoidl, G.; Ramos, L.; Buck, J.; Levin, L.R.; Rassow, J.; Manfredi, G.; et al. A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J. Biol. Chem. 2011, 286, 30423–30432. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, Y.; Zhou, K.; Zhang, G.; Linask, K.; Xu, H. The cAMP phosphodiesterase Prune localizes to the mitochondrial matrix and promotes mtDNA replication by stabilizing TFAM. Embo Rep. 2015, 16, 520–527. [Google Scholar] [CrossRef]
- García-Bermúdez, J.; Sánchez-Aragó, M.; Soldevilla, B.; Del Arco, A.; Nuevo-Tapioles, C.; Cuezva, J.M. PKA Phosphorylates the ATPase Inhibitory Factor 1 and Inactivates Its Capacity to Bind and Inhibit the Mitochondrial H(+)-ATP Synthase. Cell Rep. 2015, 12, 2143–2155. [Google Scholar] [CrossRef]
- DiPilato, L.M.; Cheng, X.; Zhang, J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA 2004, 101, 16513–16518. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Scalzotto, E.; Mongillo, M.; Pozzan, T. Mitochondrial Ca²⁺ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 2013, 17, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Lefkimmiatis, K.; Leronni, D.; Hofer, A.M. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J. Cell Biol. 2013, 202, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Agnes, R.S.; Jernigan, F.; Shell, J.R.; Sharma, V.; Lawrence, D.S. Suborganelle sensing of mitochondrial cAMP-dependent protein kinase activity. J. Am. Chem. Soc. 2010, 132, 6075–6080. [Google Scholar] [CrossRef]
- Srinivasan, S.; Spear, J.; Chandran, K.; Joseph, J.; Kalyanaraman, B.; Avadhani, N.G. Oxidative stress induced mitochondrial protein kinase A mediates cytochrome c oxidase dysfunction. PLoS ONE 2013, 8, e77129. [Google Scholar] [CrossRef]
- Rosca, M.; Minkler, P.; Hoppel, C.L. Cardiac mitochondria in heart failure: Normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim. Et Biophys. Acta 2011, 1807, 1373–1382. [Google Scholar] [CrossRef]
- Parkkila, A.K.; Scarim, A.L.; Parkkila, S.; Waheed, A.; Corbett, J.A.; Sly, W.S. Expression of carbonic anhydrase V in pancreatic beta cells suggests role for mitochondrial carbonic anhydrase in insulin secretion. J. Biol. Chem. 1998, 273, 24620–24623. [Google Scholar] [CrossRef]
- Shigeto, M.; Ramracheya, R.; Tarasov, A.I.; Cha, C.Y.; Chibalina, M.V.; Hastoy, B.; Philippaert, K.; Reinbothe, T.; Rorsman, N.; Salehi, A.; et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J. Clin. Investig. 2015, 125, 4714–4728. [Google Scholar] [CrossRef]
- Barker, C.J.; Berggren, P.O. New horizons in cellular regulation by inositol polyphosphates: Insights from the pancreatic β-cell. Pharm. Rev. 2013, 65, 641–669. [Google Scholar] [CrossRef]
- Warwar, N.; Efendic, S.; Ostenson, C.G.; Haber, E.P.; Cerasi, E.; Nesher, R. Dynamics of glucose-induced localization of PKC isoenzymes in pancreatic beta-cells: Diabetes-related changes in the GK rat. Diabetes 2006, 55, 590–599. [Google Scholar] [CrossRef]
- Seed Ahmed, M.; Pelletier, J.; Leumann, H.; Gu, H.F.; Östenson, C.G. Expression of Protein Kinase C Isoforms in Pancreatic Islets and Liver of Male Goto-Kakizaki Rats, a Model of Type 2 Diabetes. PLoS ONE 2015, 10, e0135781. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, A.; Yu, Q.; Tengholm, A. Autocrine Signaling Underlies Fast Repetitive Plasma Membrane Translocation of Conventional and Novel Protein Kinase C Isoforms in β Cells. J. Biol. Chem. 2016, 291, 14986–14995. [Google Scholar] [CrossRef]
- Hashimoto, T.; Mogami, H.; Tsuriya, D.; Morita, H.; Sasaki, S.; Kumada, T.; Suzuki, Y.; Urano, T.; Oki, Y.; Suda, T. G-protein-coupled receptor 40 agonist GW9508 potentiates glucose-stimulated insulin secretion through activation of protein kinase Cα and ε in INS-1 cells. PLoS ONE 2019, 14, e0222179. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, L.L.; Kunkel, M.T.; Newton, A.C. Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J. Biol. Chem. 2006, 281, 30947–30956. [Google Scholar] [CrossRef] [PubMed]
- Santo-Domingo, J.; Chareyron, I.; Dayon, L.; Núñez Galindo, A.; Cominetti, O.; Pilar Giner Giménez, M.; De Marchi, U.; Canto, C.; Kussmann, M.; Wiederkehr, A. Coordinated activation of mitochondrial respiration and exocytosis mediated by PKC signaling in pancreatic β cells. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1028–1045. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Alippe, Y.; Carreras, M.C.; Poderoso, J.J. Mitochondrial kinases in cell signaling: Facts and perspectives. Adv. Drug Deliv. Rev. 2009, 61, 1234–1249. [Google Scholar] [CrossRef]
- Straub, S.G.; Shanmugam, G.; Sharp, G.W. Stimulation of insulin release by glucose is associated with an increase in the number of docked granules in the beta-cells of rat pancreatic islets. Diabetes 2004, 53, 3179–3183. [Google Scholar] [CrossRef]
- Vakilian, M.; Tahamtani, Y.; Ghaedi, K. A review on insulin trafficking and exocytosis. Gene 2019, 706, 52–61. [Google Scholar] [CrossRef]
- Hutton, J.C.; Penn, E.J.; Peshavaria, M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem. J. 1983, 210, 297–305. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Lai, F.A.; Rutter, G.A. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic beta-cells (MIN6). J. Biol. Chem. 2003, 278, 11057–11064. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Okamoto, H. Translational control of proinsulin synthesis by glucose. Nature 1980, 283, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Dodson, G.; Steiner, D. The role of assembly in insulin’s biosynthesis. Curr. Opin. Struct. Biol. 1998, 8, 189–194. [Google Scholar] [CrossRef]
- Orci, L.; Halban, P.; Perrelet, A.; Amherdt, M.; Ravazzola, M.; Anderson, R.G. pH-independent and -dependent cleavage of proinsulin in the same secretory vesicle. J. Cell Biol. 1994, 126, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.V. Zinc and insulin in pancreatic beta-cells. Endocrine 2014, 45, 178–189. [Google Scholar] [CrossRef]
- Trogden, K.P.; Zhu, X.; Lee, J.S.; Wright, C.V.E.; Gu, G.; Kaverina, I. Regulation of Glucose-Dependent Golgi-Derived Microtubules by cAMP/EPAC2 Promotes Secretory Vesicle Biogenesis in Pancreatic β Cells. Curr. Biol. 2019, 29, 2339–2350.e2335. [Google Scholar] [CrossRef]
- Li, M.; Du, W.; Zhou, M.; Zheng, L.; Song, E.; Hou, J. Proteomic analysis of insulin secretory granules in INS-1 cells by protein correlation profiling. Biophys. Rep. 2018, 4, 329–338. [Google Scholar] [CrossRef]
- Davidson, H.W.; Wenzlau, J.M.; O’Brien, R.M. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol. Metab. Tem 2014, 25, 415–424. [Google Scholar] [CrossRef]
- Geng, X.; Li, L.; Watkins, S.; Robbins, P.D.; Drain, P. The insulin secretory granule is the major site of K(ATP) channels of the endocrine pancreas. Diabetes 2003, 52, 767–776. [Google Scholar] [CrossRef][Green Version]
- Geng, X.; Lou, H.; Wang, J.; Li, L.; Swanson, A.L.; Sun, M.; Beers-Stolz, D.; Watkins, S.; Perez, R.G.; Drain, P. α-Synuclein binds the K(ATP) channel at insulin-secretory granules and inhibits insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E276–E286. [Google Scholar] [CrossRef]
- Colsoul, B.; Nilius, B.; Vennekens, R. Transient receptor potential (TRP) cation channels in diabetes. Curr. Top. Med. Chem. 2013, 13, 258–269. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Pinton, P.; Varadi, A.; Tacchetti, C.; Ainscow, E.K.; Pozzan, T.; Rizzuto, R.; Rutter, G.A. Dense core secretory vesicles revealed as a dynamic Ca(2+) store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J. Cell Biol. 2001, 155, 41–51. [Google Scholar] [CrossRef]
- Blondel, O.; Moody, M.M.; Depaoli, A.M.; Sharp, A.H.; Ross, C.A.; Swift, H.; Bell, G.I. Localization of inositol trisphosphate receptor subtype 3 to insulin and somatostatin secretory granules and regulation of expression in islets and insulinoma cells. Proc. Natl. Acad. Sci. USA 1994, 91, 7777–7781. [Google Scholar] [CrossRef]
- Dai, F.F.; Bhattacharjee, A.; Liu, Y.; Batchuluun, B.; Zhang, M.; Wang, X.S.; Huang, X.; Luu, L.; Zhu, D.; Gaisano, H.; et al. A Novel GLP1 Receptor Interacting Protein ATP6ap2 Regulates Insulin Secretion in Pancreatic Beta Cells. J. Biol. Chem. 2015, 290, 25045–25061. [Google Scholar] [CrossRef]
- Boland, B.B.; Rhodes, C.J.; Grimsby, J.S. The dynamic plasticity of insulin production in β-cells. Mol. Metab. 2017, 6, 958–973. [Google Scholar] [CrossRef]
- Song, S.H.; McIntyre, S.S.; Shah, H.; Veldhuis, J.D.; Hayes, P.C.; Butler, P.C. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 2000, 85, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Fujita, T.; Gomi, H.; Izumi, T. Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic (Cph. Den.) 2008, 9, 1191–1203. [Google Scholar] [CrossRef]
- Lai, Y.; Choi, U.B.; Leitz, J.; Rhee, H.J.; Lee, C.; Altas, B.; Zhao, M.; Pfuetzner, R.A.; Wang, A.L.; Brose, N.; et al. Molecular Mechanisms of Synaptic Vesicle Priming by Munc13 and Munc18. Neuron 2017, 95, 591–607.e510. [Google Scholar] [CrossRef]
- Rizo, J.; Xu, J. The Synaptic Vesicle Release Machinery. Annu. Rev. Biophys. 2015, 44, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Choi, U.B.; Gong, J.; Yang, X.; Li, Y.; Wang, A.L.; Yang, X.; Brunger, A.T.; Ma, C. Conformational change of syntaxin linker region induced by Munc13s initiates SNARE complex formation in synaptic exocytosis. Embo J. 2017, 36, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Walker, E.M.; Dadi, P.K.; Hu, R.; Xu, Y.; Zhang, W.; Sanavia, T.; Mun, J.; Liu, J.; Nair, G.G.; et al. Synaptotagmin 4 Regulates Pancreatic β Cell Maturation by Modulating the Ca(2+) Sensitivity of Insulin Secretion Vesicles. Dev. Cell 2018, 45, 347–361.e345. [Google Scholar] [CrossRef]
- Maechler, P.; Wollheim, C.B. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 1999, 402, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Høy, M.; Maechler, P.; Efanov, A.M.; Wollheim, C.B.; Berggren, P.O.; Gromada, J. Increase in cellular glutamate levels stimulates exocytosis in pancreatic beta-cells. Febs Lett. 2002, 531, 199–203. [Google Scholar] [CrossRef]
- Casimir, M.; Lasorsa, F.M.; Rubi, B.; Caille, D.; Palmieri, F.; Meda, P.; Maechler, P. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J. Biol. Chem. 2009, 284, 25004–25014. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Fahien, L.A. Glutamate is not a messenger in insulin secretion. J. Biol. Chem. 2000, 275, 34025–34027. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, G.; Ishiyama, N.; Nenquin, M.; Ravier, M.A.; Henquin, J.C. The elevation of glutamate content and the amplification of insulin secretion in glucose-stimulated pancreatic islets are not causally related. J. Biol. Chem. 2002, 277, 32883–32891. [Google Scholar] [CrossRef]
- Gheni, G.; Ogura, M.; Iwasaki, M.; Yokoi, N.; Minami, K.; Nakayama, Y.; Harada, K.; Hastoy, B.; Wu, X.; Takahashi, H.; et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014, 9, 661–673. [Google Scholar] [CrossRef]
- Aspinwall, C.A.; Brooks, S.A.; Kennedy, R.T.; Lakey, J.R. Effects of intravesicular H+ and extracellular H+ and Zn2+ on insulin secretion in pancreatic beta cells. J. Biol. Chem. 1997, 272, 31308–31314. [Google Scholar] [CrossRef] [PubMed]
- Gammelsaeter, R.; Coppola, T.; Marcaggi, P.; Storm-Mathisen, J.; Chaudhry, F.A.; Attwell, D.; Regazzi, R.; Gundersen, V. A role for glutamate transporters in the regulation of insulin secretion. PLoS ONE 2011, 6, e22960. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.; Yokoi, N.; Takahashi, H.; Gheni, G.; Okechi, O.S.; Hayami, T.; Murao, N.; Hidaka, S.; Minami, K.; Mizoguchi, A.; et al. Inhibition of SNAT5 Induces Incretin-Responsive State From Incretin-Unresponsive State in Pancreatic β-Cells: Study of β-Cell Spheroid Clusters as a Model. Diabetes 2018, 67, 1795–1806. [Google Scholar] [CrossRef]
- Elrick, H.; Stimmler, L.; Hlad, C.J., Jr.; Arai, Y. Plasma insulin response to oral and intravenous glucose administration. J. Clin. Endocrinol. Metab. 1964, 24, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Ebert, R.; Unger, H.; Creutzfeldt, W. Preservation of incretin activity after removal of gastric inhibitory polypeptide (GIP) from rat gut extracts by immunoadsorption. Diabetologia 1983, 24, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Scrocchi, L.A.; Brown, T.J.; MaClusky, N.; Brubaker, P.L.; Auerbach, A.B.; Joyner, A.L.; Drucker, D.J. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 1996, 2, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Scrocchi, L.A.; Marshall, B.A.; Cook, S.M.; Brubaker, P.L.; Drucker, D.J. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes 1998, 47, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.J.; Park, S.; Kim, D.K.; Cho, E.B.; Hwang, J.I.; Vaudry, H.; Seong, J.Y. Structural and molecular conservation of glucagon-like Peptide-1 and its receptor confers selective ligand-receptor interaction. Front. Endocrinol. 2012, 3, 141. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Hartmann, B.; Deacon, C.F.; Holst, J.J. Measurement of the incretin hormones: Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J. Diabetes Its Complicat. 2015, 29, 445–450. [Google Scholar] [CrossRef]
- Teraoku, H.; Lenzen, S. Dynamics of Insulin Secretion from EndoC-βH1 β-Cell Pseudoislets in Response to Glucose and Other Nutrient and Nonnutrient Secretagogues. J. Diabetes Res. 2017, 2017, 2309630. [Google Scholar] [CrossRef]
- Graaf, C.d.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.-M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef]
- Moran, B.M.; Abdel-Wahab, Y.H.; Flatt, P.R.; McKillop, A.M. Activation of GPR119 by fatty acid agonists augments insulin release from clonal β-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol. Chem. 2014, 395, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Philippe, J.; Mojsov, S.; Chick, W.L.; Habener, J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 3434–3438. [Google Scholar] [CrossRef]
- Weir, G.C.; Mojsov, S.; Hendrick, G.K.; Habener, J.F. Glucagonlike peptide I (7-37) actions on endocrine pancreas. Diabetes 1989, 38, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Hjøllund, K.R.; Deacon, C.F.; Holst, J.J. Dipeptidyl peptidase-4 inhibition increases portal concentrations of intact glucagon-like peptide-1 (GLP-1) to a greater extent than peripheral concentrations in anaesthetised pigs. Diabetologia 2011, 54, 2206–2208. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Göke, R.; Richter, G.; Fehmann, H.C.; Arnold, R.; Göke, B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995, 56, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Reynolds, C.A.; Smith, K.J.; Mobarec, J.C.; Koole, C.; Savage, E.E.; Pabreja, K.; Simms, J.; Sridhar, R.; Furness, S.G.B.; et al. The Extracellular Surface of the GLP-1 Receptor Is a Molecular Trigger for Biased Agonism. Cell 2016, 165, 1632–1643. [Google Scholar] [CrossRef]
- Sonoda, N.; Imamura, T.; Yoshizaki, T.; Babendure, J.L.; Lu, J.C.; Olefsky, J.M. Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6614–6619. [Google Scholar] [CrossRef]
- Montrose-Rafizadeh, C.; Avdonin, P.; Garant, M.J.; Rodgers, B.D.; Kole, S.; Yang, H.; Levine, M.A.; Schwindinger, W.; Bernier, M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 1999, 140, 1132–1140. [Google Scholar] [CrossRef]
- Light, P.E.; Manning Fox, J.E.; Riedel, M.J.; Wheeler, M.B. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol. Endocrinol. (Baltim. Md.) 2002, 16, 2135–2144. [Google Scholar] [CrossRef]
- Kang, G.; Joseph, J.W.; Chepurny, O.G.; Monaco, M.; Wheeler, M.B.; Bos, J.L.; Schwede, F.; Genieser, H.G.; Holz, G.G. Epac-selective cAMP analog 8-pCPT-2’-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J. Biol. Chem. 2003, 278, 8279–8285. [Google Scholar] [CrossRef]
- Thompson, A.; Kanamarlapudi, V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Gαq pathway. Biochem. Pharm. 2015, 93, 72–84. [Google Scholar] [CrossRef]
- MacDonald, P.E.; Salapatek, A.M.; Wheeler, M.B. Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K(+) currents in beta-cells: A possible glucose-dependent insulinotropic mechanism. Diabetes 2002, 51 (Suppl. 3), S443–S447. [Google Scholar] [CrossRef]
- Vierra, N.C.; Dickerson, M.T.; Philipson, L.H.; Jacobson, D.A. Simultaneous Real-Time Measurement of the β-Cell Membrane Potential and Ca(2+) Influx to Assess the Role of Potassium Channels on β-Cell Function. Methods Mol. Biol. (CliftonN.J.) 2018, 1684, 73–84. [Google Scholar] [CrossRef]
- Fernandez, J.; Valdeolmillos, M. Glucose-dependent stimulatory effect of glucagon-like peptide 1(7-36) amide on the electrical activity of pancreatic beta-cells recorded in vivo. Diabetes 1999, 48, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Valdeolmillos, M. Synchronous glucose-dependent [Ca(2+)](i) oscillations in mouse pancreatic islets of Langerhans recorded in vivo. Febs Lett. 2000, 477, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, H.; Gounko, N.V.; Zhou, Z.; Xu, A.; Hong, W.; Han, W. Detection of insulin granule exocytosis by an electrophysiology method with high temporal resolution reveals enlarged insulin granule pool in BIG3-knockout mice. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E611–E618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dupre, J.; Ross, S.A.; Watson, D.; Brown, J.C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J. Clin. Endocrinol. Metab. 1973, 37, 826–828. [Google Scholar] [CrossRef]
- Hinke, S.A.; Pauly, R.P.; Ehses, J.; Kerridge, P.; Demuth, H.U.; McIntosh, C.H.; Pederson, R.A. Role of glucose in chronic desensitization of isolated rat islets and mouse insulinoma (betaTC-3) cells to glucose-dependent insulinotropic polypeptide. J. Endocrinol. 2000, 165, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ehses, J.A.; Pelech, S.L.; Pederson, R.A.; McIntosh, C.H. Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J. Biol. Chem. 2002, 277, 37088–37097. [Google Scholar] [CrossRef]
- McIntosh, C.H.; Widenmaier, S.; Kim, S.J. Glucose-dependent insulinotropic polypeptide signaling in pancreatic β-cells and adipocytes. J. Diabetes Investig. 2012, 3, 96–106. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Wutthisathapornchai, A.; Wallace, J.C.; MacDonald, M.J. Regulation of insulin secretion: Role of mitochondrial signalling. Diabetologia 2010, 53, 1019–1032. [Google Scholar] [CrossRef]
- Joseph, J.W.; Jensen, M.V.; Ilkayeva, O.; Palmieri, F.; Alárcon, C.; Rhodes, C.J.; Newgard, C.B. The Mitochondrial Citrate/Isocitrate Carrier Plays a Regulatory Role in Glucose-stimulated Insulin Secretion. J. Biol. Chem. 2006, 281, 35624–35632. [Google Scholar] [CrossRef]
- Odegaard, M.L.; Joseph, J.W.; Jensen, M.V.; Lu, D.; Ilkayeva, O.; Ronnebaum, S.M.; Becker, T.C.; Newgard, C.B. The Mitochondrial 2-Oxoglutarate Carrier Is Part of a Metabolic Pathway That Mediates Glucose- and Glutamine-stimulated Insulin Secretion. J. Biol. Chem. 2010, 285, 16530–16537. [Google Scholar] [CrossRef] [PubMed]
- Ronnebaum, S.M.; Ilkayeva, O.; Burgess, S.C.; Joseph, J.W.; Lu, D.; Stevens, R.D.; Becker, T.C.; Sherry, A.D.; Newgard, C.B.; Jensen, M.V. A Pyruvate Cycling Pathway Involving Cytosolic NADP-dependent Isocitrate Dehydrogenase Regulates Glucose-stimulated Insulin Secretion. J. Biol. Chem. 2006, 281, 30593–30602. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Mulder, H.; Zhao, P.; Burgess, S.C.; Jensen, M.V.; Kamzolova, S.; Newgard, C.B.; Sherry, A.D. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc. Natl. Acad. Sci. USA 2002, 99, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Madiraju, S.R.; Aumais, A.; Joly, E.; Prentki, M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J. Biol. Chem. 2007, 282, 35657–35665. [Google Scholar] [CrossRef]
- Pongratz, R.L.; Kibbey, R.G.; Shulman, G.I.; Cline, G.W. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J. Biol. Chem. 2007, 282, 200–207. [Google Scholar] [CrossRef]
- Farfari, S.; Schulz, V.; Corkey, B.; Prentki, M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: Possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 2000, 49, 718–726. [Google Scholar] [CrossRef]
- El Azzouny, M.; Longacre, M.J.; Ansari, I.H.; Kennedy, R.T.; Burant, C.F.; MacDonald, M.J. Knockdown of ATP citrate lyase in pancreatic beta cells does not inhibit insulin secretion or glucose flux and implicates the acetoacetate pathway in insulin secretion. Mol. Metab. 2016, 5, 980–987. [Google Scholar] [CrossRef]
- Chen, W.W.; Freinkman, E.; Wang, T.; Birsoy, K.; Sabatini, D.M. Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell 2016, 166, 1324–1337.e1311. [Google Scholar] [CrossRef]
- Rydström, J. Mitochondrial NADPH, transhydrogenase and disease. Biochim. Et Biophys. Acta 2006, 1757, 721–726. [Google Scholar] [CrossRef]
- Santos, L.R.B.; Muller, C.; de Souza, A.H.; Takahashi, H.K.; Spégel, P.; Sweet, I.R.; Chae, H.; Mulder, H.; Jonas, J.-C. NNT reverse mode of operation mediates glucose control of mitochondrial NADPH and glutathione redox state in mouse pancreatic β-cells. Mol. Metab. 2017, 6, 535–547. [Google Scholar] [CrossRef]
- Freeman, H.C.; Hugill, A.; Dear, N.T.; Ashcroft, F.M.; Cox, R.D. Deletion of nicotinamide nucleotide transhydrogenase: A new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 2006, 55, 2153–2156. [Google Scholar] [CrossRef]
- Toye, A.A.; Lippiat, J.D.; Proks, P.; Shimomura, K.; Bentley, L.; Hugill, A.; Mijat, V.; Goldsworthy, M.; Moir, L.; Haynes, A.; et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia 2005, 48, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.; Shimomura, K.; Cox, R.D.; Ashcroft, F.M. Nicotinamide nucleotide transhydrogenase: A link between insulin secretion, glucose metabolism and oxidative stress. Biochem. Soc. Trans. 2006, 34, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Blair, A.R.; Morahan, G.; Andrikopoulos, S. The deletion variant of nicotinamide nucleotide transhydrogenase (Nnt) does not affect insulin secretion or glucose tolerance. Endocrinology 2010, 151, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.M.; Longacre, M.J.; Stoker, S.W.; Kendrick, M.A.; MacDonald, M.J. Mitochondrial malic enzyme 3 is important for insulin secretion in pancreatic β-cells. Mol. Endocrinol. (Baltim. Md.) 2015, 29, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Spégel, P.; Mulder, H. Metabolomics Analysis of Nutrient Metabolism in β-Cells. J. Mol. Biol. 2020, 432, 1429–1445. [Google Scholar] [CrossRef]
- El-Azzouny, M.; Evans, C.R.; Treutelaar, M.K.; Kennedy, R.T.; Burant, C.F. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. J. Biol. Chem. 2014, 289, 13575–13588. [Google Scholar] [CrossRef]
- Ježek, J.; Dlasková, A.; Zelenka, J.; Jabůrek, M.; Ježek, P. H2O2-Activated Mitochondrial Phospholipase iPLA2γ Prevents Lipotoxic Oxidative Stress in Synergy with UCP2, Amplifies Signaling via G-Protein–Coupled Receptor GPR40, and Regulates Insulin Secretion in Pancreatic β-Cells. Antioxid. Redox Signal. 2015, 23, 958–972. [Google Scholar] [CrossRef]
- Bränström, R.; Aspinwall, C.A.; Välimäki, S.; Ostensson, C.G.; Tibell, A.; Eckhard, M.; Brandhorst, H.; Corkey, B.E.; Berggren, P.O.; Larsson, O. Long-chain CoA esters activate human pancreatic beta-cell KATP channels: Potential role in Type 2 diabetes. Diabetologia 2004, 47, 277–283. [Google Scholar] [CrossRef][Green Version]
- Joseph, J.W.; Odegaard, M.L.; Ronnebaum, S.M.; Burgess, S.C.; Muehlbauer, J.; Sherry, A.D.; Newgard, C.B. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J. Biol. Chem. 2007, 282, 31592–31600. [Google Scholar] [CrossRef]
- Bender, K.; Maechler, P.; McClenaghan, N.H.; Flatt, P.R.; Newsholme, P. Overexpression of the malate-aspartate NADH shuttle member Aralar1 in the clonal beta-cell line BRIN-BD11 enhances amino-acid-stimulated insulin secretion and cell metabolism. Clin. Sci. 2009, 117, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rubi, B.; del Arco, A.; Bartley, C.; Satrustegui, J.; Maechler, P. The malate-aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells. J. Biol. Chem. 2004, 279, 55659–55666. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Mitok, K.A.; Freiberger, E.C.; Schueler, K.L.; Rabaglia, M.E.; Stapleton, D.S.; Kwiecien, N.W.; Malec, P.A.; Hebert, A.S.; Broman, A.T.; Kennedy, R.T.; et al. Islet proteomics reveals genetic variation in dopamine production resulting in altered insulin secretion. J. Biol. Chem. 2018, 293, 5860–5877. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Cardone, R.L.; Stark, R.; Lewandowski, S.L.; Zhao, X.; Hillion, J.; Ma, L.; Sehgal, R.; Alves, T.C.; Thomas, C.; et al. Multi-Tissue Acceleration of the Mitochondrial Phosphoenolpyruvate Cycle Improves Whole-Body Metabolic Health. Cell Metab. 2020, 32, 751–766.e711. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Ashcroft, S.J.; Harrison, D.E. Effects of 2-ketoisocaproate on insulin release and single potassium channel activity in dispersed rat pancreatic beta-cells. J. Physiol. 1987, 385, 517–529. [Google Scholar] [CrossRef]
- Panten, U.; Früh, E.; Reckers, K.; Rustenbeck, I. Acute metabolic amplification of insulin secretion in mouse islets: Role of cytosolic acetyl-CoA. Metab. Clin. Exp. 2016, 65, 1225–1229. [Google Scholar] [CrossRef]
- Panten, U.; Willenborg, M.; Schumacher, K.; Hamada, A.; Ghaly, H.; Rustenbeck, I. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metab. Clin. Exp. 2013, 62, 1375–1386. [Google Scholar] [CrossRef]
- McClenaghan, N.H.; Flatt, P.R. Glucose and non-glucidic nutrients exert permissive effects on 2-keto acid regulation of pancreatic beta-cell function. Biochim. Et Biophys. Acta 1999, 1426, 110–118. [Google Scholar] [CrossRef]
- Heissig, H.; Urban, K.A.; Hastedt, K.; Zünkler, B.J.; Panten, U. Mechanism of the insulin-releasing action of alpha-ketoisocaproate and related alpha-keto acid anions. Mol. Pharmacol. 2005, 68, 1097–1105. [Google Scholar] [CrossRef]
- Gurgul-Convey, E.; Kaminski, M.T.; Lenzen, S. Physiological characterization of the human EndoC-βH1 β-cell line. Biochem. Biophys. Res. Commun. 2015, 464, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V.I. Redox-Driven Signaling: 2-Oxo Acid Dehydrogenase Complexes as Sensors and Transmitters of Metabolic Imbalance. Antioxid. Redox Signal. 2019, 30, 1911–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Frerman, F.E.; Kim, J.J. Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool. Proc. Natl. Acad. Sci. USA 2006, 103, 16212–16217. [Google Scholar] [CrossRef] [PubMed]
- Watmough, N.J.; Frerman, F.E. The electron transfer flavoprotein: Ubiquinone oxidoreductases. Biochim. Et Biophys. Acta 2010, 1797, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Husen, P.; Nielsen, C.; Martino, C.F.; Solov’yov, I.A. Molecular Oxygen Binding in the Mitochondrial Electron Transfer Flavoprotein. J. Chem. Inf. Modeling 2019, 59, 4868–4879. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Hull, J.; Hindy, M.E.; Kehoe, P.G.; Chalmers, K.; Love, S.; Conway, M.E. Distribution of the branched chain aminotransferase proteins in the human brain and their role in glutamate regulation. J. Neurochem. 2012, 123, 997–1009. [Google Scholar] [CrossRef]
- Gao, Z.; Young, R.A.; Li, G.; Najafi, H.; Buettger, C.; Sukumvanich, S.S.; Wong, R.K.; Wolf, B.A.; Matschinsky, F.M. Distinguishing features of leucine and alpha-ketoisocaproate sensing in pancreatic beta-cells. Endocrinology 2003, 144, 1949–1957. [Google Scholar] [CrossRef]
- Cheng, Q.; Beltran, V.D.; Chan, S.M.; Brown, J.R.; Bevington, A.; Herbert, T.P. System-L amino acid transporters play a key role in pancreatic β-cell signalling and function. J. Mol. Endocrinol. 2016, 56, 175–187. [Google Scholar] [CrossRef]
- Giroix, M.H.; Saulnier, C.; Portha, B. Decreased pancreatic islet response to L-leucine in the spontaneously diabetic GK rat: Enzymatic, metabolic and secretory data. Diabetologia 1999, 42, 965–977. [Google Scholar] [CrossRef][Green Version]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Et Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef]
- Ævarsson, A.; Chuang, J.L.; Wynn, R.M.; Turley, S.; Chuang, D.T.; Hol, W.G. Crystal structure of human branched-chain alpha-ketoacid dehydrogenase and the molecular basis of multienzyme complex deficiency in maple syrup urine disease. Structure 2000, 8, 277–291. [Google Scholar] [CrossRef]
- Noguchi, S.; Kondo, Y.; Ito, R.; Katayama, T.; Kazama, S.; Kadota, Y.; Kitaura, Y.; Harris, R.A.; Shimomura, Y. Ca(2+)-dependent inhibition of branched-chain α-ketoacid dehydrogenase kinase by thiamine pyrophosphate. Biochem. Biophys. Res. Commun. 2018, 504, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Manders, R.J.; Little, J.P.; Forbes, S.C.; Candow, D.G. Insulinotropic and muscle protein synthetic effects of branched-chain amino acids: Potential therapy for type 2 diabetes and sarcopenia. Nutrients 2012, 4, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chi, Y.; Burkhardt, B.R.; Guan, Y.; Wolf, B.A. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr. Rev. 2010, 68, 270–279. [Google Scholar] [CrossRef]
- Stein, D.T.; Stevenson, B.E.; Chester, M.W.; Basit, M.; Daniels, M.B.; Turley, S.D.; McGarry, J.D. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Investig. 1997, 100, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Nyrén, R.; Chang, C.L.; Lindström, P.; Barmina, A.; Vorrsjö, E.; Ali, Y.; Juntti-Berggren, L.; Bensadoun, A.; Young, S.G.; Olivecrona, T.; et al. Localization of lipoprotein lipase and GPIHBP1 in mouse pancreas: Effects of diet and leptin deficiency. BMC Physiol. 2012, 12, 14. [Google Scholar] [CrossRef]
- Cen, J.; Sargsyan, E.; Bergsten, P. Fatty acids stimulate insulin secretion from human pancreatic islets at fasting glucose concentrations via mitochondria-dependent and -independent mechanisms. Nutr. Metab. 2016, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Valdeolmillos, M. Increased levels of free fatty acids in fasted mice stimulate in vivo beta-cell electrical activity. Diabetes 1998, 47, 1707–1712. [Google Scholar] [CrossRef]
- Frayn, K.N. Metabolic Regulation: A Human Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ee, L.C.; Zheng, S.; Yao, L.; Tso, P. Lymphatic absorption of fatty acids and cholesterol in the neonatal rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G325–G331. [Google Scholar] [CrossRef]
- Nauli, A.M.; Nassir, F.; Zheng, S.; Yang, Q.; Lo, C.M.; Vonlehmden, S.B.; Lee, D.; Jandacek, R.J.; Abumrad, N.A.; Tso, P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 2006, 131, 1197–1207. [Google Scholar] [CrossRef]
- Moss, C.E.; Glass, L.L.; Diakogiannaki, E.; Pais, R.; Lenaghan, C.; Smith, D.M.; Wedin, M.; Bohlooly, Y.M.; Gribble, F.M.; Reimann, F. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides 2016, 77, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Moriguchi, R.; Yamada, Y.; Fujita, M.; Yamato, T.; Oumi, M.; Holst, J.J.; Seino, Y. High saturated fatty acid intake induces insulin secretion by elevating gastric inhibitory polypeptide levels in healthy individuals. Nutr. Res. 2014, 34, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Winzell, M.S.; Ström, K.; Holm, C.; Ahrén, B. Glucose-stimulated insulin secretion correlates with beta-cell lipolysis. Nutr. Metab. Cardiovasc. Dis. Nmcd 2006, 16 (Suppl. 1), S11–S16. [Google Scholar] [CrossRef]
- Cruz, W.S.; Kwon, G.; Marshall, C.A.; McDaniel, M.L.; Semenkovich, C.F. Glucose and insulin stimulate heparin-releasable lipoprotein lipase activity in mouse islets and INS-1 cells. A potential link between insulin resistance and beta-cell dysfunction. J. Biol. Chem. 2001, 276, 12162–12168. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.A.; Tordjman, K.; Host, H.H.; Ensor, N.J.; Kwon, G.; Marshall, C.A.; Coleman, T.; McDaniel, M.L.; Semenkovich, C.F. Relative hypoglycemia and hyperinsulinemia in mice with heterozygous lipoprotein lipase (LPL) deficiency. Islet LPL regulates insulin secretion. J. Biol. Chem. 1999, 274, 27426–27432. [Google Scholar] [CrossRef] [PubMed]
- Peyot, M.L.; Guay, C.; Latour, M.G.; Lamontagne, J.; Lussier, R.; Pineda, M.; Ruderman, N.B.; Haemmerle, G.; Zechner, R.; Joly, E.; et al. Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion. J. Biol. Chem. 2009, 284, 16848–16859. [Google Scholar] [CrossRef]
- Fujiwara, K.; Maekawa, F.; Yada, T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: Mediation by PLC and L-type Ca2+ channel and link to insulin release. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E670–E677. [Google Scholar] [CrossRef]
- Khan, S.; Kowluru, A. CD36 mediates lipid accumulation in pancreatic beta cells under the duress of glucolipotoxic conditions: Novel roles of lysine deacetylases. Biochem. Biophys. Res. Commun. 2018, 495, 2221–2226. [Google Scholar] [CrossRef]
- Veprik, A.; Laufer, D.; Weiss, S.; Rubins, N.; Walker, M.D. GPR41 modulates insulin secretion and gene expression in pancreatic β-cells and modifies metabolic homeostasis in fed and fasting states. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3860–3869. [Google Scholar] [CrossRef]
- Pujol, J.B.; Christinat, N.; Ratinaud, Y.; Savoia, C.; Mitchell, S.E.; Dioum, E.H.M. Coordination of GPR40 and Ketogenesis Signaling by Medium Chain Fatty Acids Regulates Beta Cell Function. Nutrients 2018, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.M.; Abdel-Wahab, Y.H.; Flatt, P.R.; McKillop, A.M. Evaluation of the insulin-releasing and glucose-lowering effects of GPR120 activation in pancreatic β-cells. DiabetesObes. Metab. 2014, 16, 1128–1139. [Google Scholar] [CrossRef]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; Di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Luckmann, M.; et al. GPR40 (FFAR1)-Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.D.; Bertrand, G.; Vivot, K.; Carpentier, É.; Tremblay, C.; Ghislain, J.; Bouvier, M.; Poitout, V. β-Arrestin Recruitment and Biased Agonism at Free Fatty Acid Receptor 1. J. Biol. Chem. 2015, 290, 21131–21140. [Google Scholar] [CrossRef] [PubMed]
- Graciano, M.F.; Valle, M.M.; Curi, R.; Carpinelli, A.R. Evidence for the involvement of GPR40 and NADPH oxidase in palmitic acid-induced superoxide production and insulin secretion. Islets 2013, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sabrautzki, S.; Kaiser, G.; Przemeck, G.K.H.; Gerst, F.; Lorza-Gil, E.; Panse, M.; Sartorius, T.; Hoene, M.; Marschall, S.; Haring, H.U.; et al. Point mutation of Ffar1 abrogates fatty acid-dependent insulin secretion, but protects against HFD-induced glucose intolerance. Mol. Metab. 2017, 6, 1304–1312. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshida, M.; Ito, K.; Dezaki, K.; Yada, T.; Ishikawa, S.E.; Kakei, M. Potentiation of Glucose-stimulated Insulin Secretion by the GPR40-PLC-TRPC Pathway in Pancreatic β-Cells. Sci. Rep. 2016, 6, 25912. [Google Scholar] [CrossRef]
- Qian, J.; Gu, Y.; Wu, C.; Yu, F.; Chen, Y.; Zhu, J.; Yao, X.; Bei, C.; Zhu, Q. Agonist-induced activation of human FFA1 receptor signals to extracellular signal-regulated kinase 1 and 2 through Gq- and Gi-coupled signaling cascades. Cell. Mol. Biol. Lett. 2017, 22, 13. [Google Scholar] [CrossRef]
- Kristinsson, H.; Bergsten, P.; Sargsyan, E. Free fatty acid receptor 1 (FFAR1/GPR40) signaling affects insulin secretion by enhancing mitochondrial respiration during palmitate exposure. Biochim. Et Biophys. Acta 2015, 1853, 3248–3257. [Google Scholar] [CrossRef]
- Tomita, T.; Hosoda, K.; Fujikura, J.; Inagaki, N.; Nakao, K. The G-Protein-Coupled Long-Chain Fatty Acid Receptor GPR40 and Glucose Metabolism. Front. Endocrinol. 2014, 5, 152. [Google Scholar] [CrossRef]
- Vilas-Boas, E.A.; Karabacz, N.; Marsiglio-Librais, G.N.; Valle, M.M.R.; Nalbach, L.; Ampofo, E.; Morgan, B.; Carpinelli, A.R.; Roma, L.P. Chronic activation of GPR40 does not negatively impact upon BRIN-BD11 pancreatic β-cell physiology and function. Pharmacol. Rep. 2020, 72, 1725–1737. [Google Scholar] [CrossRef]
- Bergeron, V.; Ghislain, J.; Poitout, V. The P21-activated kinase PAK4 is implicated in fatty-acid potentiation of insulin secretion downstream of free fatty acid receptor 1. Islets 2016, 8, 157–164. [Google Scholar] [CrossRef][Green Version]
- Ferdaoussi, M.; Bergeron, V.; Zarrouki, B.; Kolic, J.; Cantley, J.; Fielitz, J.; Olson, E.N.; Prentki, M.; Biden, T.; MacDonald, P.E.; et al. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 2012, 55, 2682–2692. [Google Scholar] [CrossRef]
- Ribas, G.S.; Vargas, C.R. Evidence that Oxidative Disbalance and Mitochondrial Dysfunction are Involved in the Pathophysiology of Fatty Acid Oxidation Disorders. Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nunes Marsiglio-Librais, G.; Aparecida Vilas-Boas, E.; Carlein, C.; Hoffmann, M.D.A.; Roma, L.P.; Carpinelli, A.R. Evidence for NADPH oxidase activation by GPR40 in pancreatic β-cells. Redox Rep. Commun. Free Radic. Res. 2020, 25, 41–50. [Google Scholar] [CrossRef]
- Masiello, P.; Novelli, M.; Bombara, M.; Fierabracci, V.; Vittorini, S.; Prentki, M.; Bergamini, E. The antilipolytic agent 3,5-dimethylpyrazole inhibits insulin release in response to both nutrient secretagogues and cyclic adenosine monophosphate agonists in isolated rat islets. Metab. Clin. Exp. 2002, 51, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.; Yang, S.; Winzell, M.S.; Holm, C.; Ahrén, B. Inhibition of lipase activity and lipolysis in rat islets reduces insulin secretion. Diabetes 2004, 53, 122–128. [Google Scholar] [CrossRef]
- Fex, M.; Haemmerle, G.; Wierup, N.; Dekker-Nitert, M.; Rehn, M.; Ristow, M.; Zechner, R.; Sundler, F.; Holm, C.; Eliasson, L.; et al. A beta cell-specific knockout of hormone-sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia 2009, 52, 271–280. [Google Scholar] [CrossRef]
- Mugabo, Y.; Zhao, S.; Seifried, A.; Gezzar, S.; Al-Mass, A.; Zhang, D.; Lamontagne, J.; Attane, C.; Poursharifi, P.; Iglesias, J.; et al. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 2016, 113, E430–E439. [Google Scholar] [CrossRef]
- Zhao, S.; Mugabo, Y.; Iglesias, J.; Xie, L.; Delghingaro-Augusto, V.; Lussier, R.; Peyot, M.L.; Joly, E.; Taïb, B.; Davis, M.A.; et al. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 2014, 19, 993–1007. [Google Scholar] [CrossRef]
- Mugabo, Y.; Zhao, S.; Lamontagne, J.; Al-Mass, A.; Peyot, M.L.; Corkey, B.E.; Joly, E.; Madiraju, S.R.M.; Prentki, M. Metabolic fate of glucose and candidate signaling and excess-fuel detoxification pathways in pancreatic β-cells. J. Biol. Chem. 2017, 292, 7407–7422. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Joly, E.; Pepin, E.; Barbeau, A.; Hentsch, L.; Pineda, M.; Madiraju, S.R.; Brunengraber, H.; Prentki, M. A role for cytosolic isocitrate dehydrogenase as a negative regulator of glucose signaling for insulin secretion in pancreatic ß-cells. PLoS ONE 2013, 8, e77097. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Nielsen, S. Insulin dose response analysis of free fatty acid kinetics. Metab. Clin. Exp. 2007, 56, 68–76. [Google Scholar] [CrossRef] [PubMed]
- van der Vusse, G.J. Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Flachs, P.; Brauner, P.; Sponarova, J.; Matejkova, O.; Prazak, T.; Ruzickova, J.; Bardova, K.; Kuda, O.; Kopecky, J. Role of energy charge and AMP-activated protein kinase in adipocytes in the control of body fat stores. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28 (Suppl. 4), S38–S44. [Google Scholar] [CrossRef]
- Thams, P.; Capito, K. L-arginine stimulation of glucose-induced insulin secretion through membrane depolarization and independent of nitric oxide. Eur. J. Endocrinol. 1999, 140, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Wu, Y.; Lenchik, N.I.; Gerling, I.; Quarles, L.D. GPRC6A Mediates the Effects of l-Arginine on Insulin Secretion in Mouse Pancreatic Islets. Endocrinology 2012, 153, 4608–4615. [Google Scholar] [CrossRef]
- Gooding, J.R.; Jensen, M.V.; Dai, X.; Wenner, B.R.; Lu, D.; Arumugam, R.; Ferdaoussi, M.; MacDonald, P.E.; Newgard, C.B. Adenylosuccinate Is an Insulin Secretagogue Derived from Glucose-Induced Purine Metabolism. Cell Rep. 2015, 13, 157–167. [Google Scholar] [CrossRef]
- Ferdaoussi, M.; Dai, X.; Jensen, M.V.; Wang, R.; Peterson, B.S.; Huang, C.; Ilkayeva, O.; Smith, N.; Miller, N.; Hajmrle, C.; et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J. Clin. Investig. 2015, 125, 3847–3860. [Google Scholar] [CrossRef]
- Stocker, S.; Van Laer, K.; Mijuskovic, A.; Dick, T.P. The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxid. Redox Signal. 2018, 28, 558–573. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A.; Kang, D. The Role of Peroxiredoxins in the Transduction of H2O2 Signals. Antioxid. Redox Signal. 2018, 28, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, M.C.; Liou, W.; Stocker, S.; Talwar, D.; Oehler, M.; Ruppert, T.; Scharf, A.N.; Dick, T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015, 11, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, R.M.; Hughes, S.M.; Ledgerwood, E.C. Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic. Biol. Med. 2012, 53, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Stancill, J.S.; Broniowska, K.A.; Oleson, B.J.; Naatz, A.; Corbett, J.A. Pancreatic beta-cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system. J. Biol. Chem. 2019, 294, 4843–4853. [Google Scholar] [CrossRef]
- Stancill, J.S.; Happ, J.T.; Broniowska, K.A.; Hogg, N.; Corbett, J.A. Peroxiredoxin 1 plays a primary role in protecting pancreatic β-cells from hydrogen peroxide and peroxynitrite. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R1004–R1013. [Google Scholar] [CrossRef]
- Grankvist, K.; Marklund, S.L.; Täljedal, I.B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981, 199, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schröder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Li, N.; Stojanovski, S.; Maechler, P. Mitochondrial hormesis in pancreatic β cells: Does uncoupling protein 2 play a role? Oxidative Med. Cell. Longev. 2012, 2012, 740849. [Google Scholar] [CrossRef]
- Sharma, K. Mitochondrial hormesis and diabetic complications. Diabetes 2015, 64, 663–672. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Hypoglycemic Effect of Resveratrol: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 69. [Google Scholar] [CrossRef]
- Bagetta, D.; Maruca, A.; Lupia, A.; Mesiti, F.; Catalano, R.; Romeo, I.; Moraca, F.; Ambrosio, F.A.; Costa, G.; Artese, A.; et al. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur. J. Med. Chem. 2020, 186, 111903. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H.B. Effects and Mechanisms of Tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tuomilehto, J. Olive Oil Nutraceuticals in the Prevention and Management of Diabetes: From Molecules to Lifestyle. Int. J. Mol. Sci. 2018, 19, 2024. [Google Scholar] [CrossRef] [PubMed]
- Roma, L.P.; Jonas, J.C. Nutrient Metabolism, Subcellular Redox State, and Oxidative Stress in Pancreatic Islets and beta-Cells. J. Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Smolková, K.; Mikó, E.; Kovács, T.; Leguina-Ruzzi, A.; Sipos, A.; Bai, P. Nuclear Factor Erythroid 2-Related Factor 2 in Regulating Cancer Metabolism. Antioxid. Redox Signal. 2020, 33, 966–997. [Google Scholar] [CrossRef]
- Las, G.; Oliveira, M.F.; Shirihai, O.S. Emerging roles of β-cell mitochondria in type-2-diabetes. Mol. Asp. Med. 2020, 71, 100843. [Google Scholar] [CrossRef]
- Mirabelli, M.; Russo, D.; Brunetti, A. The Role of Diet on Insulin Sensitivity. Nutrients 2020, 12, 3042. [Google Scholar] [CrossRef]
- Cremonini, E.; Fraga, C.G.; Oteiza, P.I. (-)-Epicatechin in the control of glucose homeostasis: Involvement of redox-regulated mechanisms. Free Radic. Biol. Med. 2019, 130, 478–488. [Google Scholar] [CrossRef]
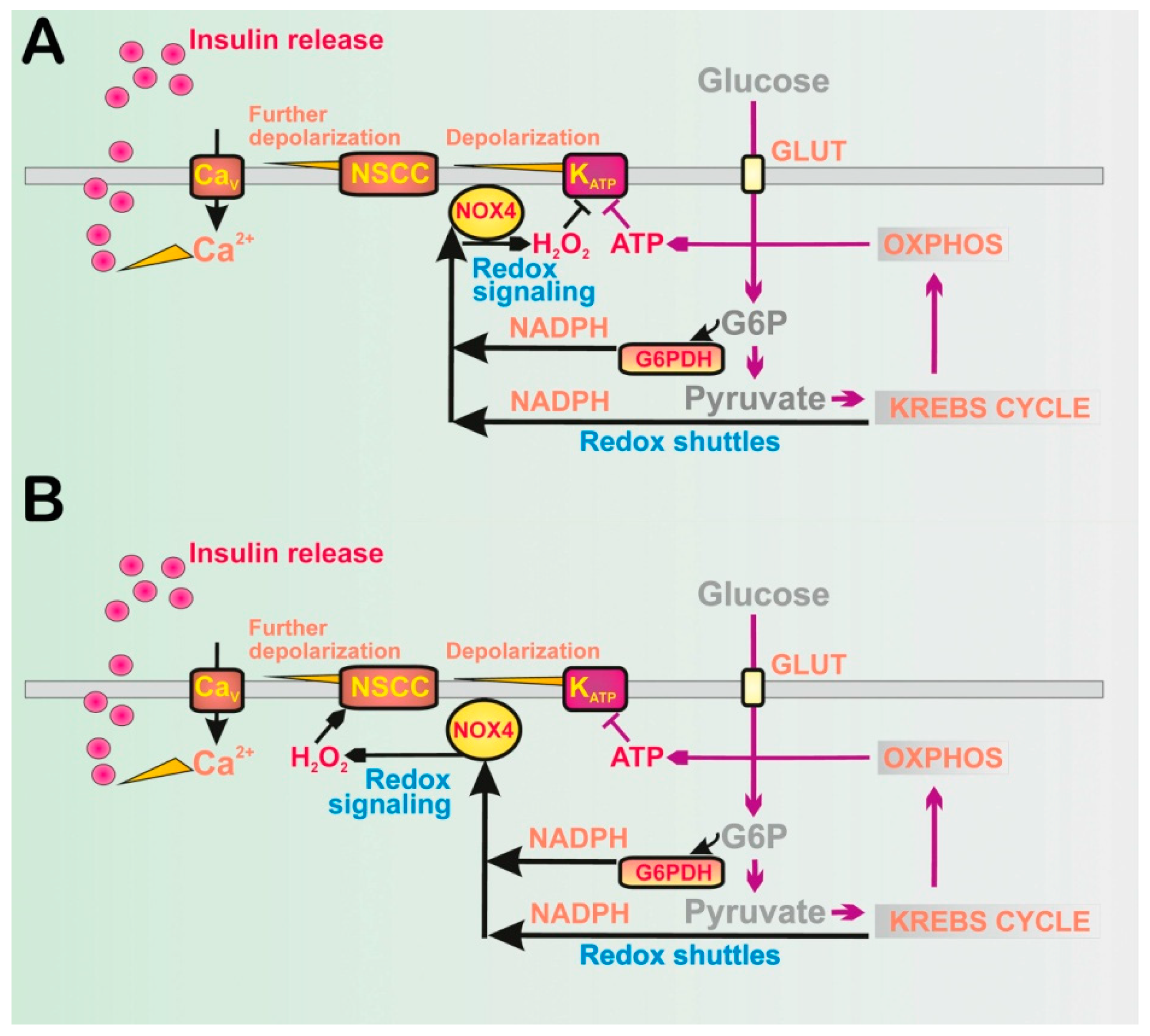



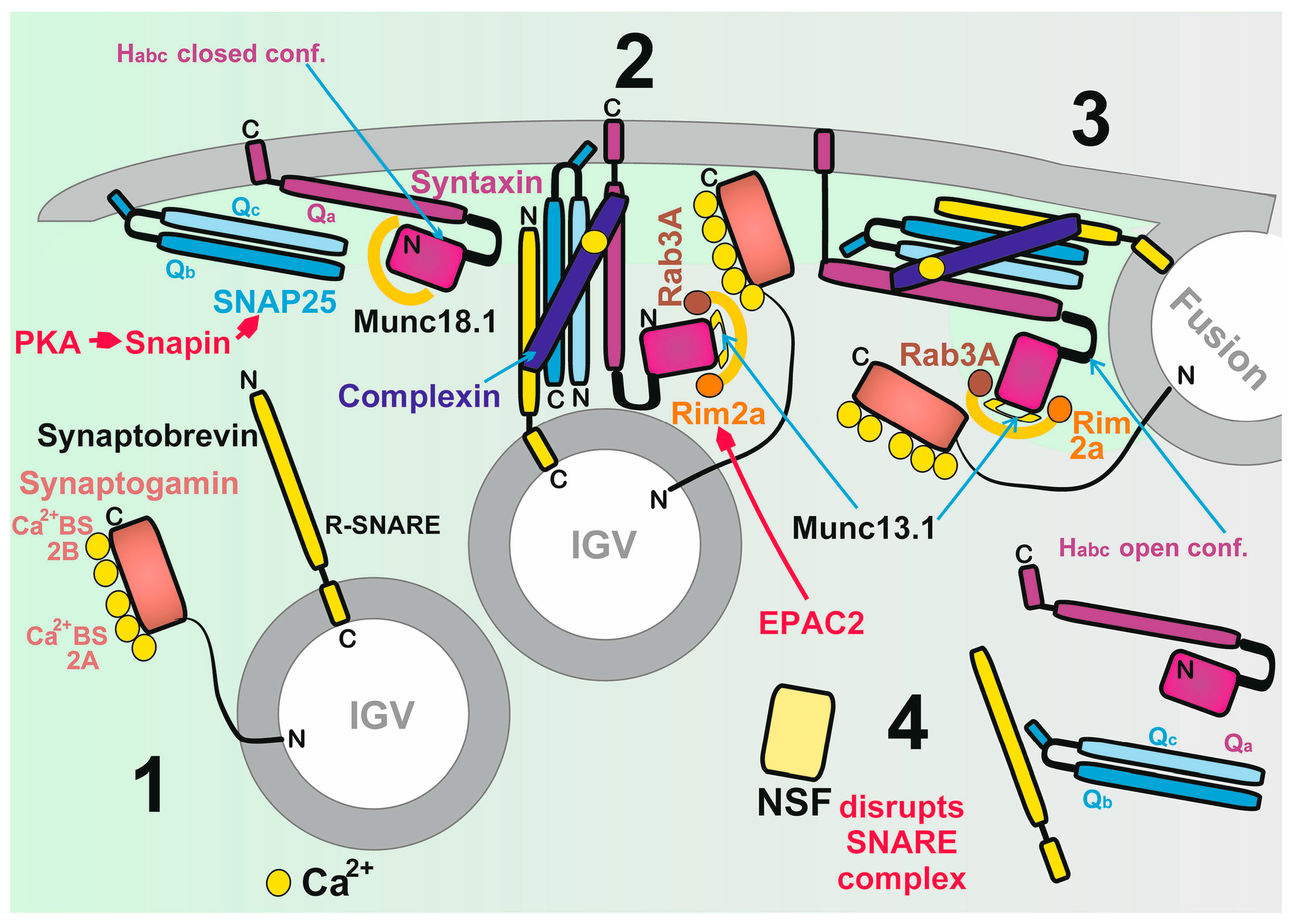


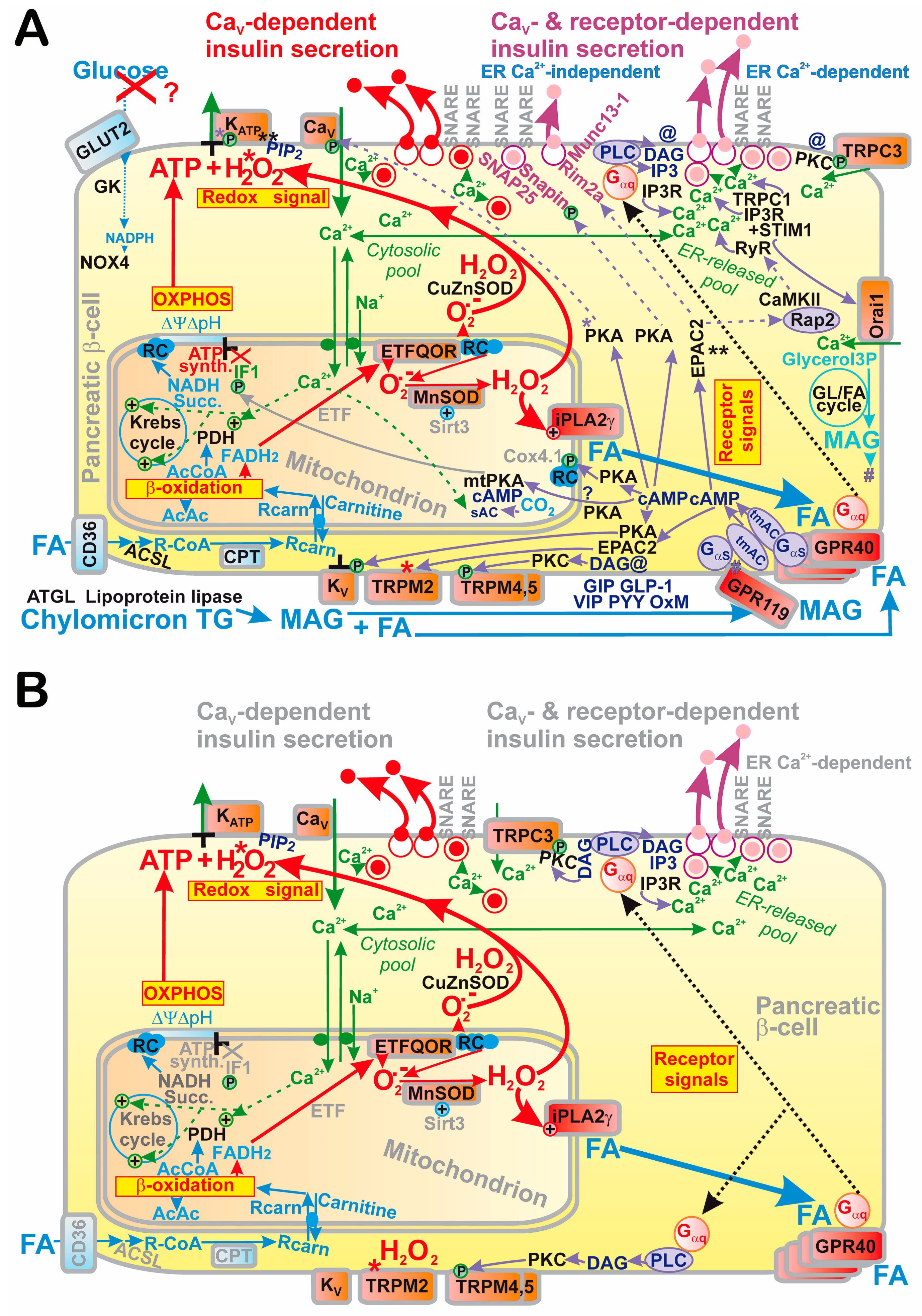
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ježek, P.; Holendová, B.; Jabůrek, M.; Tauber, J.; Dlasková, A.; Plecitá-Hlavatá, L. The Pancreatic β-Cell: The Perfect Redox System. Antioxidants 2021, 10, 197. https://doi.org/10.3390/antiox10020197
Ježek P, Holendová B, Jabůrek M, Tauber J, Dlasková A, Plecitá-Hlavatá L. The Pancreatic β-Cell: The Perfect Redox System. Antioxidants. 2021; 10(2):197. https://doi.org/10.3390/antiox10020197
Chicago/Turabian StyleJežek, Petr, Blanka Holendová, Martin Jabůrek, Jan Tauber, Andrea Dlasková, and Lydie Plecitá-Hlavatá. 2021. "The Pancreatic β-Cell: The Perfect Redox System" Antioxidants 10, no. 2: 197. https://doi.org/10.3390/antiox10020197
APA StyleJežek, P., Holendová, B., Jabůrek, M., Tauber, J., Dlasková, A., & Plecitá-Hlavatá, L. (2021). The Pancreatic β-Cell: The Perfect Redox System. Antioxidants, 10(2), 197. https://doi.org/10.3390/antiox10020197






