Electrochemical Biosensor Based on Chitosan- and Thioctic-Acid-Modified Nanoporous Gold Co-Immobilization Enzyme for Glycerol Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instruments
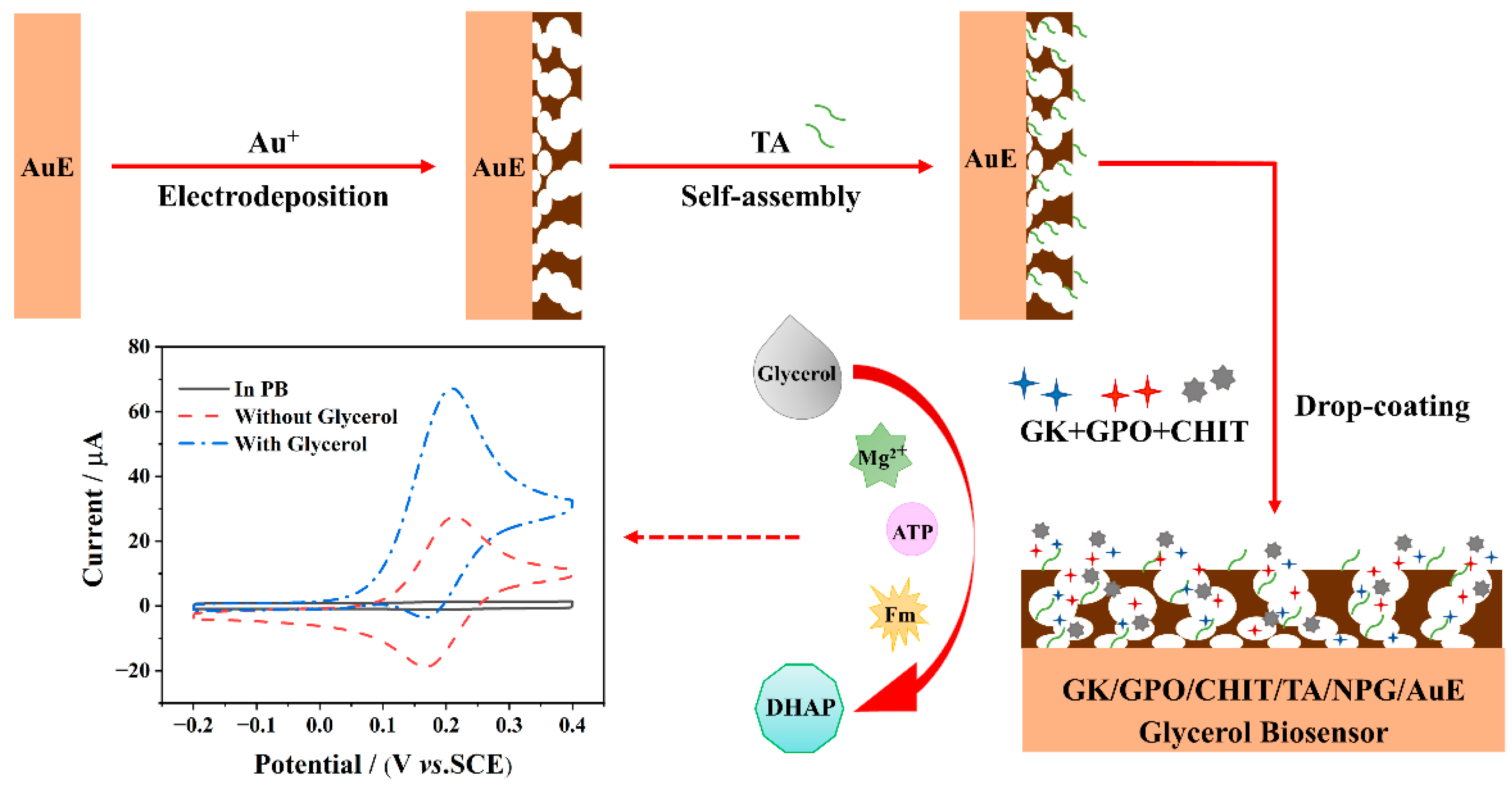
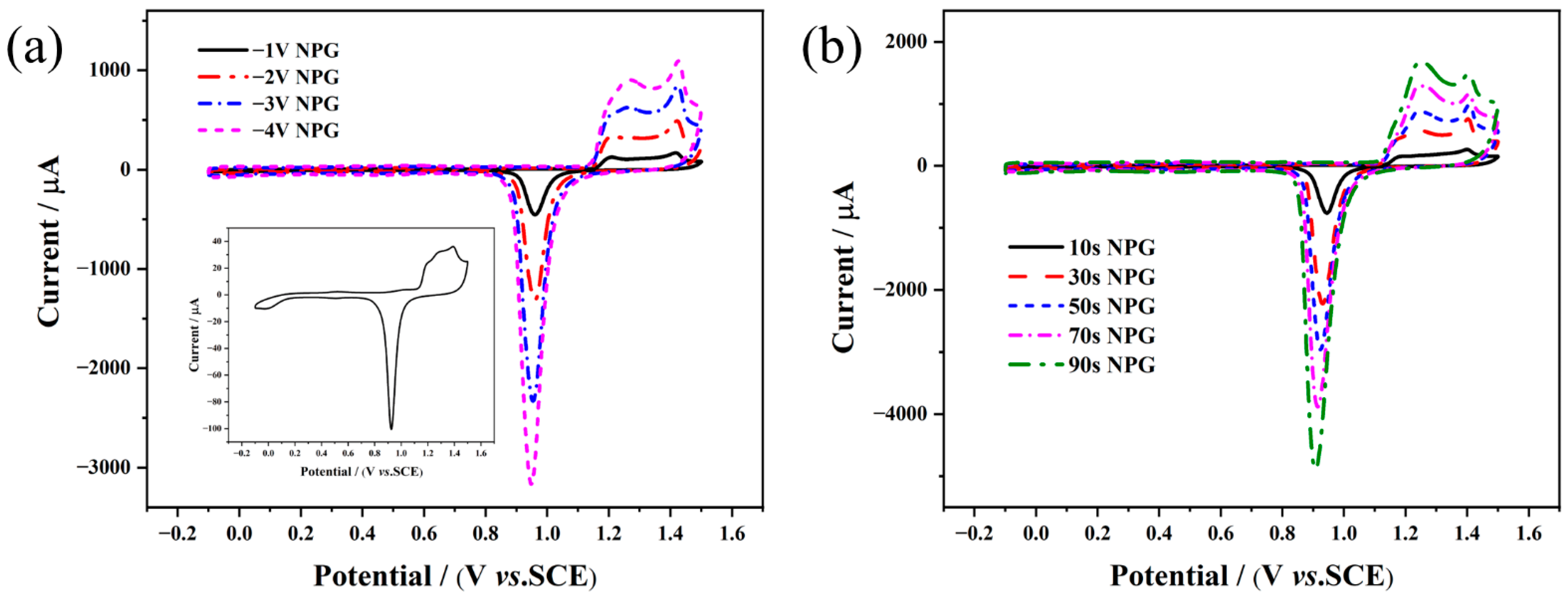
2.3. Preparation of NPG/AuE-Modified Electrode
2.4. Construction of the Enzymatic Electrochemical Biosensor
2.5. Application of Glycerol Biosensor in Real Samples
3. Results and Discussion
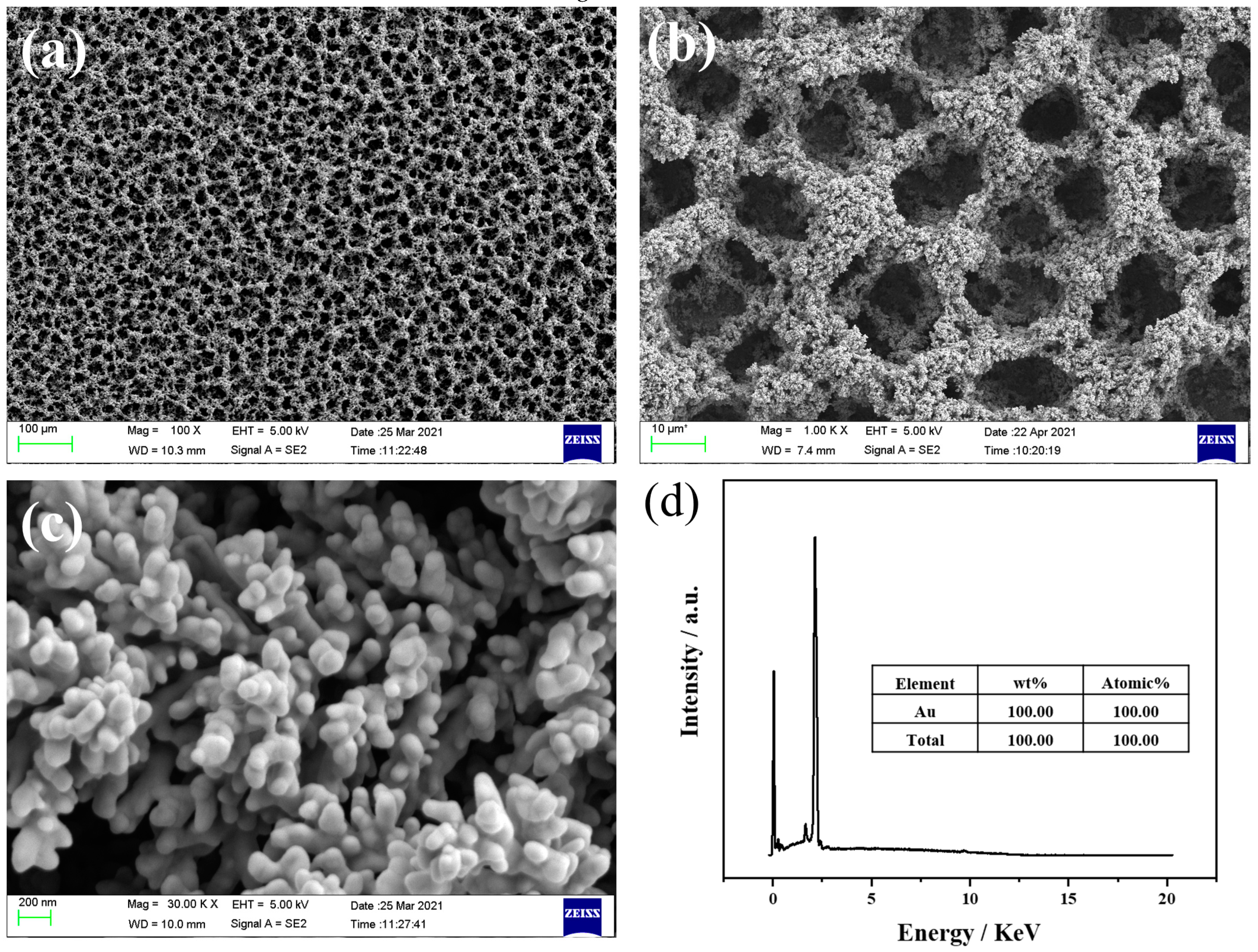
3.1. Structural and Morphological Characterization of the As-pPrepared NPG
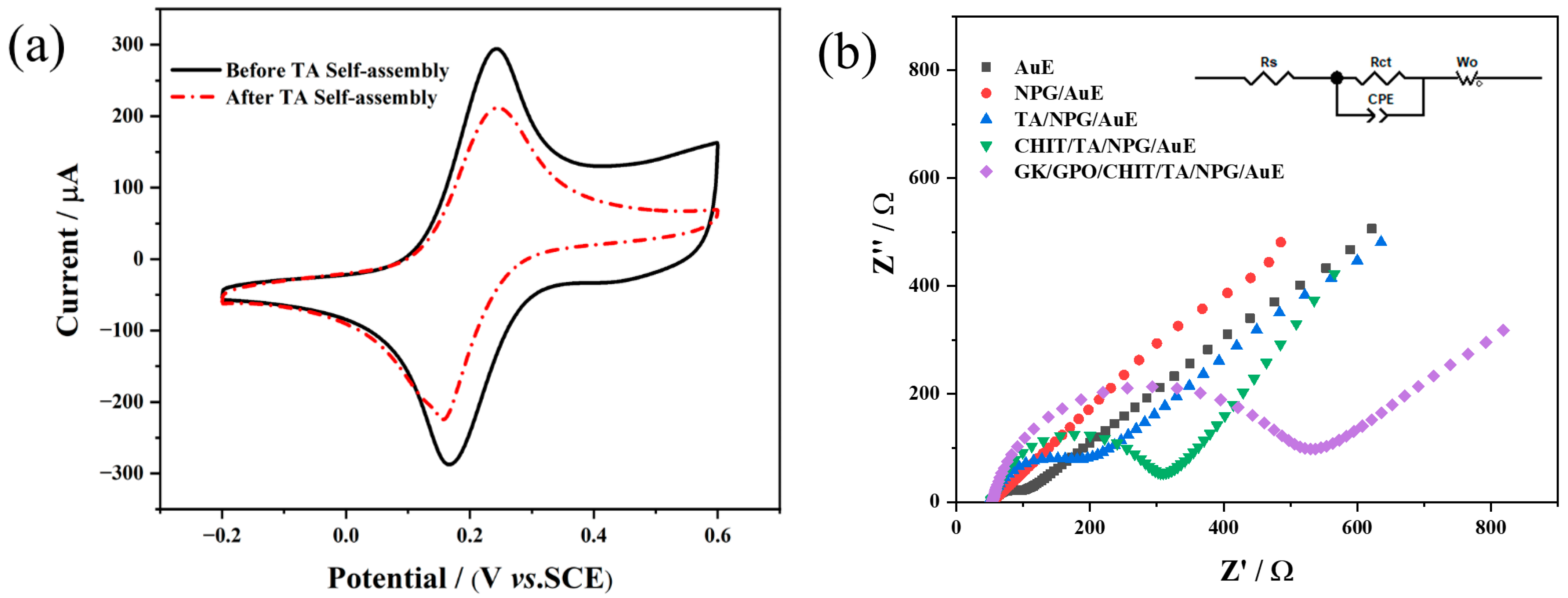
3.2. Electrochemical Properties of Different Modified Electrodes
3.3. Electrocatalytic Performance of the Fabricated Biosensor toward Glycerol
3.4. Analytical Performance of the Fabricated Biosensor
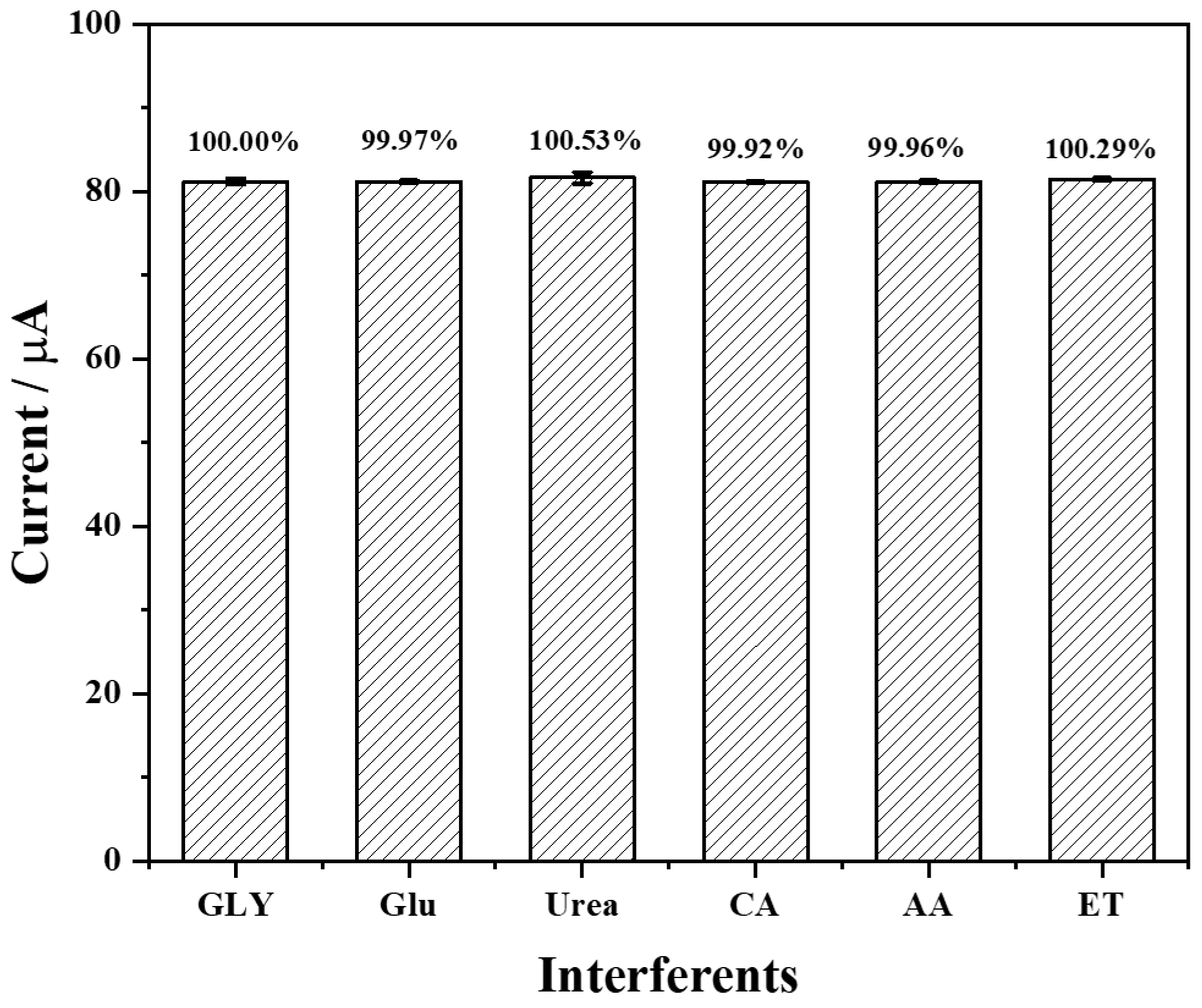
3.5. Selectivity, Repeatability, and Stability of the Fabricated Biosensor
3.6. Application of the Biosensor in Actual Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gamella, M.; Campuzano, S.; Reviejo, A.J.; Pingarrón, J.M. Integrated multienzyme electrochemical biosensors for the determination of glycerol in wines. Anal. Chim. Acta 2008, 609, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Gawel, R.; Sluyter, S.V.; Waters, E.J. The effects of ethanol and glycerol on the body and other sensory characteristics of Riesling wines. Aust. J. Grape Wine Res. 2007, 13, 38–45. [Google Scholar] [CrossRef]
- Jones, P.R.; Gawel, R.; Francis, I.L.; Waters, E.J. The influence of interactions between major white wine components on the aroma, flavour and texture of model white wine. Food Qual. Prefer. 2008, 19, 596–607. [Google Scholar] [CrossRef]
- Compagnone, D.; Esti, M.; Messia, M.C.; Peluso, E.; Palleschi, G. Development of a biosensor for monitoring of glycerol during alcoholic fermentation. Biosens. Bioelectron. 1998, 13, 875–880. [Google Scholar] [CrossRef]
- Šehović, D.; Petravić, V.; Marić, V. Glycerol and wine industry clycerol determination in grape must and wine. Kem. U Ind./J. Chem. Chem. Eng. 2004, 53, 505–516. [Google Scholar]
- Dias, A.N.; Cerqueira, M.B.R.; Moura, R.R.D.; Kurz, M.H.S.; Clementin, R.M.; D’oca, M.G.M.; Primel, E.G. Optimization of a method for the simultaneous determination of glycerides, free and total glycerol in biodiesel ethyl esters from castor oil using gas chromatography. Fuel 2012, 94, 178–183. [Google Scholar] [CrossRef] [Green Version]
- De Almeida Cozendey, D.; De Oliveira Muniz, R.; Cavalcante Dos Santos, R.; Gimenes De Souza, C.; França De Andrade, D.; Antonio D’avila, L. Quantitative analysis of free glycerol in biodiesel using solid-phase extraction and high-performance liquid chromatography. Microchem. J. 2021, 168, 106347. [Google Scholar] [CrossRef]
- Fernandes, E.N.; De Campos Moura, M.N.; Costa Lima, J.L.F.; Reis, B.F. Automatic flow procedure for the determination of glycerol in wine using enzymatic reaction and spectrophotometry. Microchem. J. 2004, 77, 107–112. [Google Scholar] [CrossRef]
- Monošík, R.; Magdolen, P.; Streďanský, M.; Šturdík, E. Monitoring of monosaccharides, oligosaccharides, ethanol and glycerol during wort fermentation by biosensors, HPLC and spectrophotometry. Food Chem. 2013, 138, 220–226. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. Development and Applications of a Bienzymatic Amperometric Glycerol Biosensor Based on a Poly (Neutral Red) Modified Carbon Film Electrode. Anal. Lett. 2006, 39, 1527–1542. [Google Scholar] [CrossRef]
- Pereira, D.F.; Santana, E.R.; Spinelli, A. Electrochemical paper-based analytical devices containing magnetite nanoparticles for the determination of vitamins B2 and B6. Microchem. J. 2022, 179, 107588. [Google Scholar] [CrossRef]
- Narwal, V.; Pundir, C.S. Fabrication of glycerol biosensor based on co-immobilization of enzyme nanoparticles onto pencil graphite electrode. Anal. Biochem. 2018, 555, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wu, W.; Jiang, Y.; Hu, B. Chitosan coated on the layers’ glucose oxidase immobilized on cysteamine/Au electrode for use as glucose biosensor. Biosens. Bioelectron. 2014, 60, 271–276. [Google Scholar] [CrossRef]
- Felipe, Q.J.A.; Javier, Q.B.; Diego, C.A.; Emilia, M. Metal free electrochemical glucose biosensor based on N-doped porous carbon material. Electrochim. Acta 2020, 367, 137434. [Google Scholar] [CrossRef]
- Wu, L.; Gao, J.; Lu, X.; Huang, C.; Dhanjai; Chen, J. Graphdiyne: A new promising member of 2D all-carbon nanomaterial as robust electrochemical enzyme biosensor platform. Carbon 2020, 156, 568–575. [Google Scholar] [CrossRef]
- Wonyong, J.; Hyughan, K.; Youngbong, C. Development of a Glucose Sensor Based on Glucose Dehydrogenase Using Polydopamine-Functionalized Nanotubes. Membranes 2021, 11, 384. [Google Scholar] [CrossRef]
- Sethuraman, V.; Sridhar, T.; Sasikumar, R. Development of an electrochemical biosensor for determination of dopamine by gold modified poly (thiophene-3-boronic acid)-polyphenol oxidase modified electrode. Mater. Lett. 2021, 302, 130387. [Google Scholar] [CrossRef]
- Sukeri, A.; Bertotti, M. Electrodeposited honeycomb-like dendritic porous gold surface: An efficient platform for enzyme-free hydrogen peroxide sensor at low overpotential. J. Electroanal. Chem. 2017, 805, 18–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, W.; Wu, D.; Yang, Y.; Feng, X.; Gao, C.; Meng, L.; Shen, X.; Zhang, Y.; Tang, X. An electrochemical method for determination of amaranth in drinks using functionalized graphene oxide/chitosan/ionic liquid nanocomposite supported nanoporous gold. Food Chem. 2022, 367, 130727. [Google Scholar] [CrossRef]
- Yang, X.N.; Huang, X.B.; Hang, R.Q.; Zhang, X.Y.; Qin, L.; Tang, B. Improved catalytic performance of porcine pancreas lipase immobilized onto nanoporous gold via covalent coupling. J. Mater. Sci. 2016, 51, 6428–6435. [Google Scholar] [CrossRef]
- Ng, A.K.; Welborn, S.S.; Detsi, E. Time-dependent power law function for the post-dealloying chemical coarsening of nanoporous gold derived using small-angle X-ray scattering. Scr. Mater. 2022, 206, 114215. [Google Scholar] [CrossRef]
- Welborn, S.S.; Simafranca, A.; Wang, Z.; Wei, H.; Detsi, E. Chelation-mediated synthesis of nanoporous gold at near-neutral pH and room temperature by free corrosion dealloying of gold-copper alloy driven by oxygen reduction. Scr. Mater. 2021, 200, 113901. [Google Scholar] [CrossRef]
- Mie, Y.; Takayama, H.; Hirano, Y. Facile control of surface crystallographic orientation of anodized nanoporous gold catalyst and its application for highly efficient hydrogen evolution reaction. J. Catal. 2020, 389, 476–482. [Google Scholar] [CrossRef]
- Rebbecchi, T.A.; Chen, Y. Template-based fabrication of nanoporous metals. J. Mater. Res. 2018, 33, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Plowman, B.J.; O’mullane, A.P.; Selvakannan, P.R.; Bhargava, S.K. Honeycomb nanogold networks with highly active sites. Chem. Commun. 2010, 46, 9182–9184. [Google Scholar] [CrossRef] [Green Version]
- Cherevko, S.; Chung, C.-H. Direct electrodeposition of nanoporous gold with controlled multimodal pore size distribution. Electrochem. Commun. 2011, 13, 16–19. [Google Scholar] [CrossRef]
- Kolodziej, A.; Fernandez-Trillo, F.; Rodriguez, P. Determining the parameters governing the electrochemical stability of thiols and disulfides self-assembled monolayer on gold electrodes in physiological medium. J. Electroanal. Chem. 2018, 819, 51–57. [Google Scholar] [CrossRef]
- Zhong, X.M.; Wang, F.; Piao, J.H.; Chen, Y.T. Fabrication and application of a novel electrochemical biosensor based on a mesoporous carbon sphere@UiO-66-NH2/Lac complex enzyme for tetracycline detection. Analyst 2021, 146, 2825–2833. [Google Scholar] [CrossRef]
- Narwal, V.; Pundir, C.S. Development of glycerol biosensor based on co-immobilization of enzyme nanoparticles onto graphene oxide nanoparticles decorated pencil graphite electrode. Int. J. Biol. Macromol. 2019, 127, 57–65. [Google Scholar] [CrossRef]
- He, F.; Qiao, Z.; Qin, X.; Chao, L.; Tan, Y.; Xie, Q.; Yao, S. Dynamic gas bubble template electrodeposition mechanisms and amperometric glucose sensing performance of three kinds of three-dimensional honeycomb-like porous nano-golds. Sens. Actuators B Chem. 2019, 296, 126679. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Furtado, V.L.; Gonçalves, J.M.; Bannitz-Fernandes, R.; Netto, L.E.S.; Araki, K.; Bertotti, M. Amperometric microsensor based on nanoporous gold for ascorbic acid detection in highly acidic biological extracts. Anal. Chim. Acta 2020, 1095, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Parra-Alfambra, A.M.; Casero, E.; Vázquez, L.; Quintana, C.; Del Pozo, M.; Petit-Domínguez, M.D. MoS2 nanosheets for improving analytical performance of lactate biosensors. Sens. Actuators B Chem. 2018, 274, 310–317. [Google Scholar] [CrossRef]
- Kumar, A.; Gonçalves, J.M.; Sukeri, A.; Araki, K.; Bertotti, M. Correlating surface growth of nanoporous gold with electrodeposition parameters to optimize amperometric sensing of nitrite. Sens. Actuators B Chem. 2018, 263, 237–247. [Google Scholar] [CrossRef]
- Monošík, R.; Ukropcová, D.; Streďanský, M.; Šturdík, E. Multienzymatic amperometric biosensor based on gold and nanocomposite planar electrodes for glycerol determination in wine. Anal. Biochem. 2012, 421, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ramonas, E.; Ratautas, D.; Dagys, M.; Meškys, R.; Kulys, J. Highly sensitive amperometric biosensor based on alcohol dehydrogenase for determination of glycerol in human urine. Talanta 2019, 200, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Fernando, S. An improved glycerol biosensor with an Au-FeS-NAD-glycerol-dehydrogenase anode. Biosens. Bioelectron. 2017, 92, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Motia, S.; Bouchikhi, B.; Llobet, E.; El Bari, N. Synthesis and characterization of a highly sensitive and selective electrochemical sensor based on molecularly imprinted polymer with gold nanoparticles modified screen-printed electrode for glycerol determination in wastewater. Talanta 2020, 216, 120953. [Google Scholar] [CrossRef]


| Sensors | Sensitivity (μA mM−1) | Linear Range (mM) | LOD (μM) | References |
|---|---|---|---|---|
| GK/CK/CRE/HRP/GPE/AuE | 0.80 | 0.005~0.64 | 1.96 | [35] |
| ADH/TTF/CNT/GE | 2.06 * | 0.05~1 | 18 | [36] |
| FeS/NAD+/GIDH | 0.025 | 1~25 | 160 | [37] |
| GKNPs/GPONPs/GrONPs/PGE | 8.59 * | 0.001~60 | 0.002 | [29] |
| AAM/NNMBA/AuNPs/Au-SPE | 0.58 | 0.22~2.47 | 0.01 | [38] |
| GK/GPO/CHIT/TA/NPG/AuE | 9.17 | 0.1~5 | 77.08 | This work |
| Samples | Original (mM) | Added (mM) | Detected (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Red wine | 0.74 | 0.5 | 1.27 | 102.76 | 4.14 |
| 1 | 1.75 | 101.03 | 1.65 | ||
| White wine | 0.41 | 0.5 | 0.96 | 105.07 | 2.06 |
| 1 | 1.42 | 100.49 | 3.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Jin, K.; Luo, X.; Piao, J.; Wang, F. Electrochemical Biosensor Based on Chitosan- and Thioctic-Acid-Modified Nanoporous Gold Co-Immobilization Enzyme for Glycerol Determination. Chemosensors 2022, 10, 258. https://doi.org/10.3390/chemosensors10070258
Yan C, Jin K, Luo X, Piao J, Wang F. Electrochemical Biosensor Based on Chitosan- and Thioctic-Acid-Modified Nanoporous Gold Co-Immobilization Enzyme for Glycerol Determination. Chemosensors. 2022; 10(7):258. https://doi.org/10.3390/chemosensors10070258
Chicago/Turabian StyleYan, Caiyun, Kaifeng Jin, Xiangyi Luo, Jinhua Piao, and Fang Wang. 2022. "Electrochemical Biosensor Based on Chitosan- and Thioctic-Acid-Modified Nanoporous Gold Co-Immobilization Enzyme for Glycerol Determination" Chemosensors 10, no. 7: 258. https://doi.org/10.3390/chemosensors10070258
APA StyleYan, C., Jin, K., Luo, X., Piao, J., & Wang, F. (2022). Electrochemical Biosensor Based on Chitosan- and Thioctic-Acid-Modified Nanoporous Gold Co-Immobilization Enzyme for Glycerol Determination. Chemosensors, 10(7), 258. https://doi.org/10.3390/chemosensors10070258






