MoSe2 as Electrode Material for Super-Capacitor, Hydrogen Evolution, and Electrochemical Sensing Applications: A Review
Abstract
:1. Introduction
2. MoSe2-Based Materials for Super-Capacitors
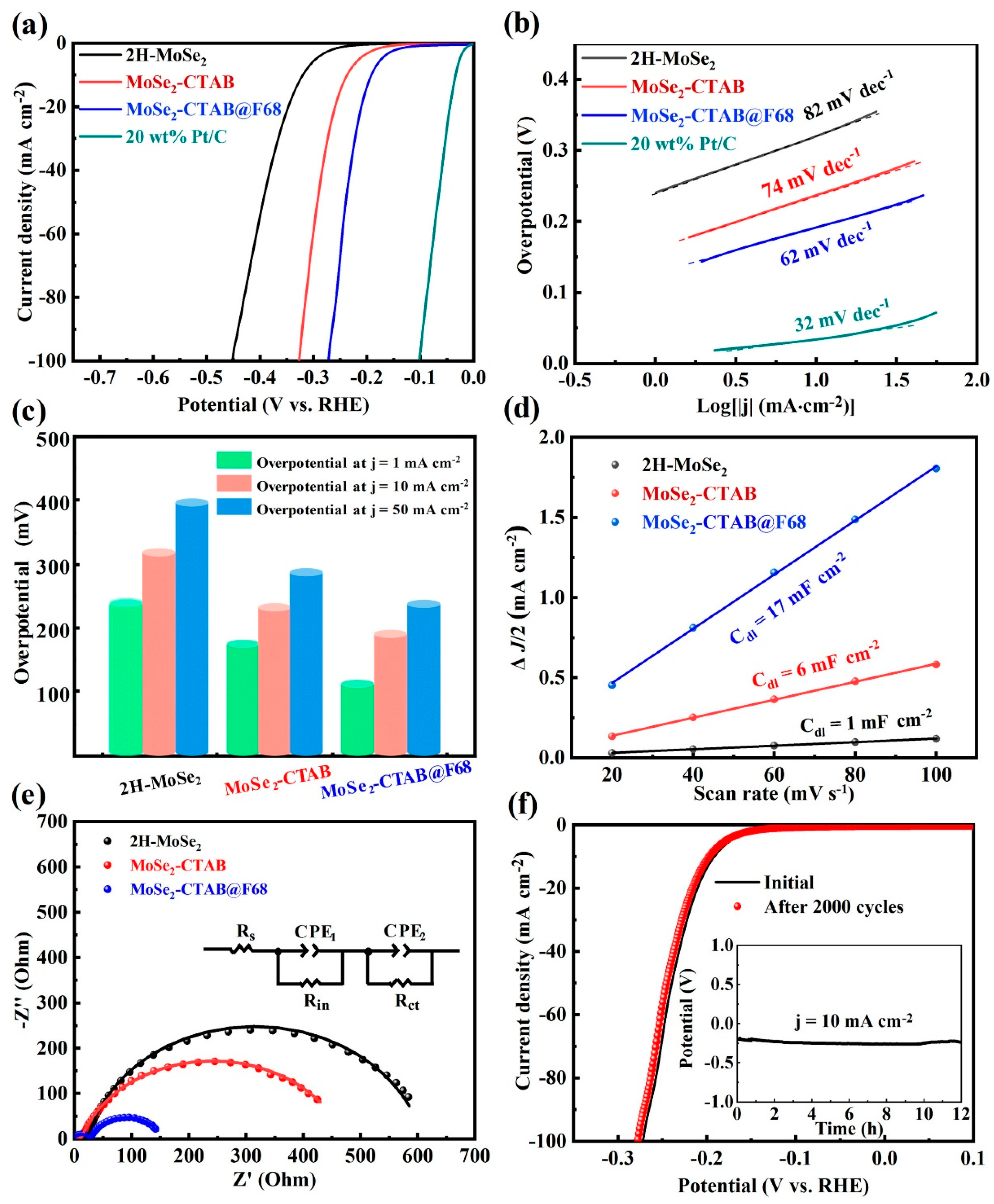
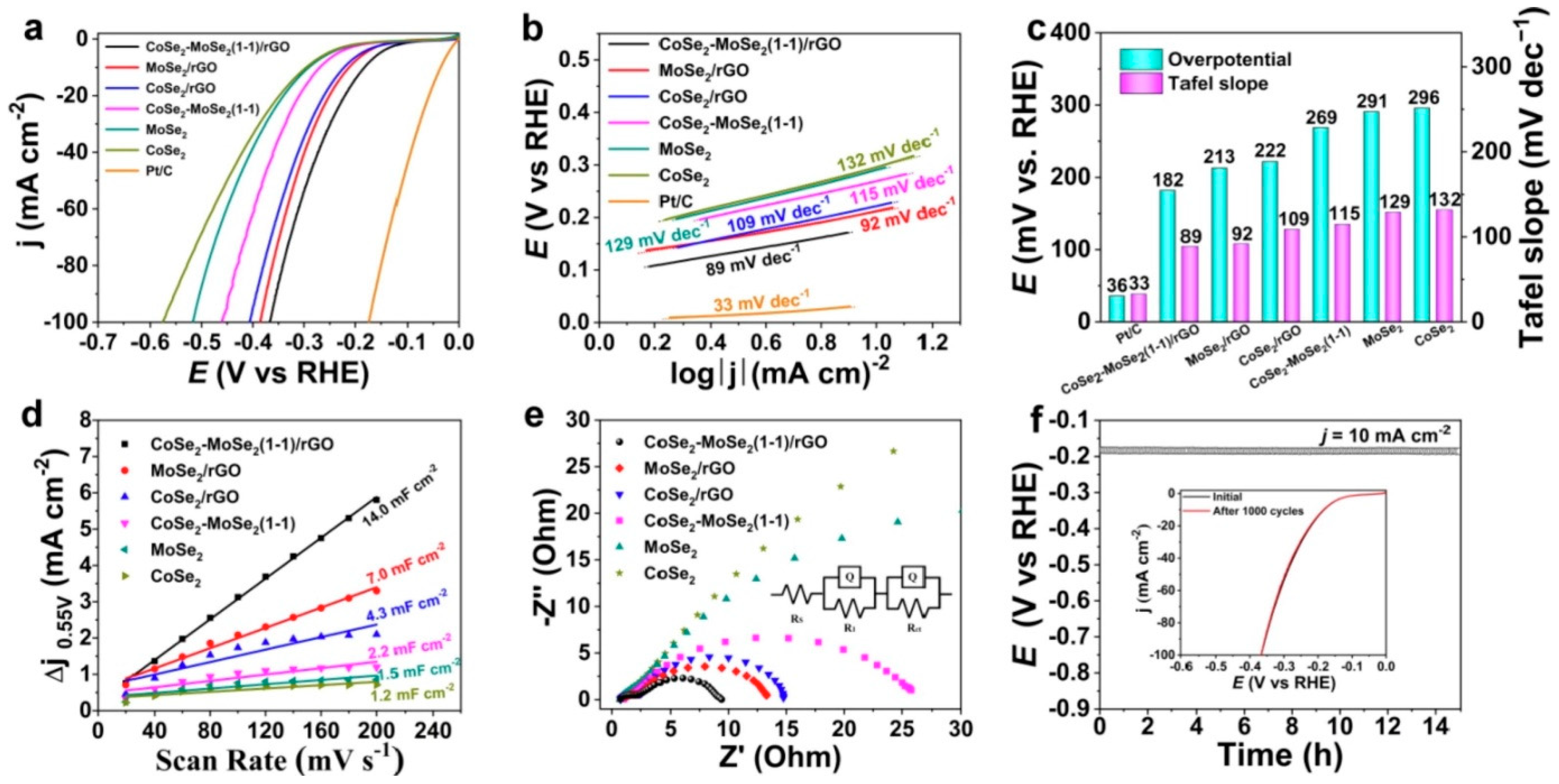
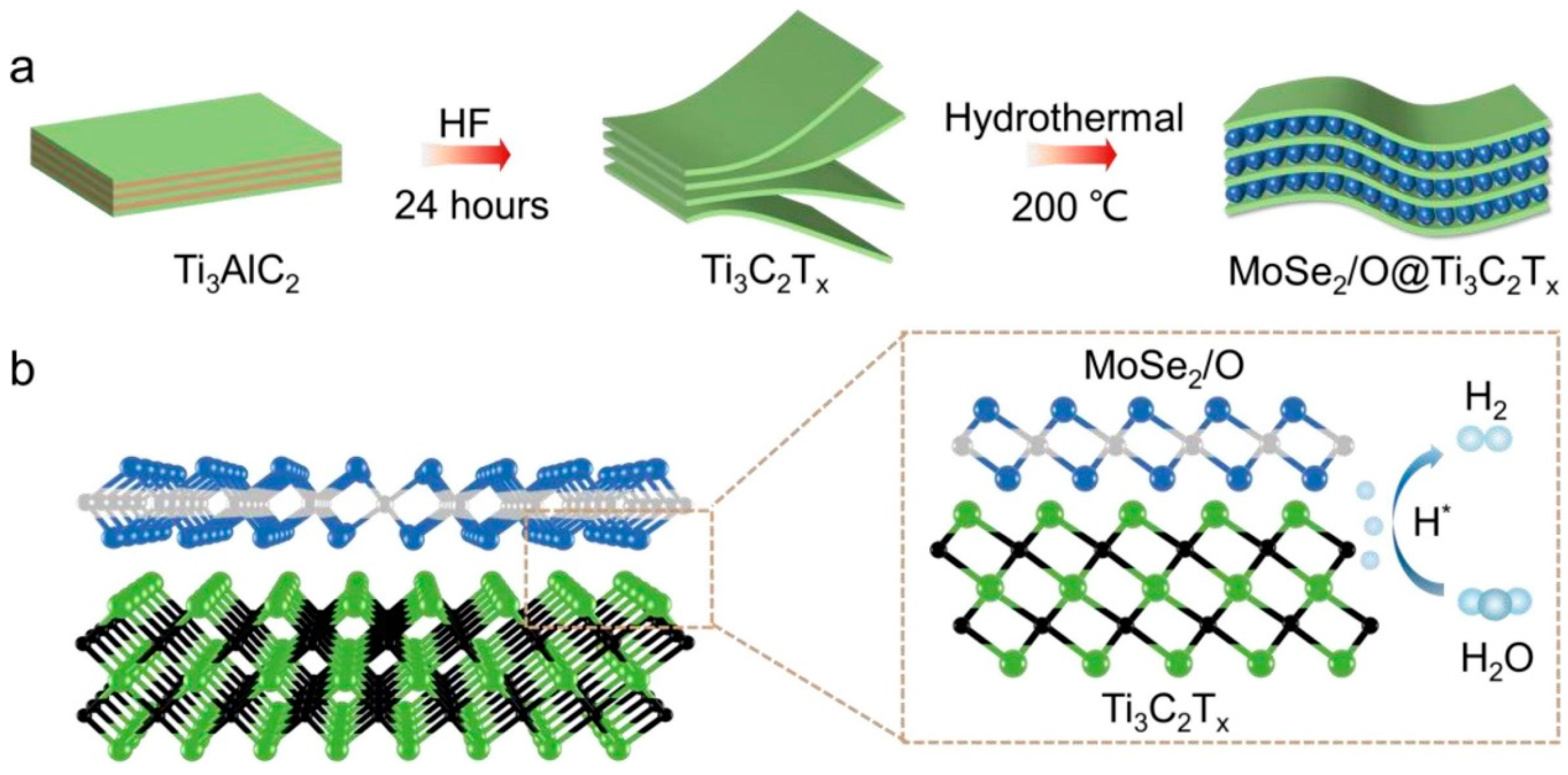
3. Hydrogen Evolution Reactions Using MoSe2-Based Materials
4. Electrochemical Sensing Applications
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
Acronyms
| CO2 | Carbon dioxide |
| Gt | Gigatons |
| SC | Super-capacitors |
| H2 | Hydrogen |
| HER | Hydrogen evolution reactions |
| TMDs | Transition metal dichalcogenides |
| MoSe2 | Molybdenum diselenide |
| HRTEM | High-resolution transmission electron microscopy |
| SEM | Scanning electron microscopy |
| HHM | Hierarchical hollow microspheres |
| NPs | Nanoparticles |
| ASC | Asymmetric super-capacitor |
| EIS | Electrochemical impedance spectroscopy |
| rGO | Reduced graphene oxide |
| LEDs | Light emitting diodes |
| AC | Activated carbon |
| M6AC | Six days aged sample activated carbon |
| CV | Cyclic Voltammetry |
| MOFs | Metal–organic frameworks |
| TMBs | Transition Metal Borides |
| CoB | Cobalt Boride |
| gCN | Graphitic Carbon Nitride |
| CNTs | Carbon Nanotubes |
| AFCNTs | Activated Functional Carbon Nanotubes |
| CVD | Chemical Vapor Deposition |
| H2SO4 | Sulfuric Acid |
| CTAB | Hexadecyl Trimethyl Ammonium Bromide |
| F68 | Polyethylene-Polypropylene Glycol |
| Cdl | Double Layer Capacitance |
| ECSA | Electrochemical Surface Area |
| SEA | Surface Electron Accumulation |
| 2H phase | Semiconducting |
| 1T phase | Metallic |
| CMT | Carbon Microtube |
| NF | Nickel Foam |
| MoSe2/O@Ti3C2Tx | Oxygen-modified 2H-phase MoSe2 on titanium carbide Mxene |
| CP | Carbon Paper |
| NiV | Nickle-Vanadium-Modulated |
| CANS | Carbon Aerogel Nanospheres |
| DPV | Differential Pulse Voltammetry |
| CV | Cyclic Voltammetry |
| DPA | Diphenylamine |
| BPA | Bisphenol A |
| LOD | Limit Of Detection |
| AFP | Alpha-Fetoprotein |
| DNR | Dendritic Nanorods |
| NSG | N, S doped graphene |
| MIPs | Molecularly Imprinted Polymers |
| RIF | Rifampicin |
| β-cd | β-cyclodextrin |
| miRNA-21 | MicroRNA-21 |
| En | Endosulfan |
| H2O2 | Hydrogen Peroxide |
| CPS | Chlorpyrifos |
| NCs | Nanoclusters |
| PCs | Porous Carbons |
| SPCE | Screen Printed Carbon Electrode |
| SWASV | Square Wave Anodic Stripping Voltammetry |
| TB | Toluidine Blue |
| POP | Porous Organic Polymer |
References
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Rothenberg, G. A realistic look at CO2 emissions, climate change and the role of sustainable chemistry. Sustain. Chem. Clim. Action 2023, 2, 100012. [Google Scholar] [CrossRef]
- Jeje, S.O.; Marazani, T.; Obiko, J.O.; Shongwe, M.B. Advancing the hydrogen production economy: A comprehensive review of technologies. sustainability, and future prospects. Int. J. Hydrogen Energy 2024, 78, 642–661. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Eltaweil, A.S.; Hosny, M.; Farghali, M.; Al-Fatesh, A.S.; Rooney, D.W.; El-Monaem, E.M.A. Advances in hydrogen storage materials: Harnessing innovative technology, from machine learning to computational chemistry, for energy storage solutions. Int. J. Hydrogen Energy 2024, 67, 1270–1294. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Sangmesh, B.; Karuppasamy, K.S.K. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Dissanayake, K.; Kularatna-Abeywardana, D. A review of supercapacitors: Materials, technology, challenges, and renewable energy applications. J. Energy Storage 2024, 96, 112563. [Google Scholar] [CrossRef]
- Upadhyay, S.; Pandey, O.P. Studies on 2D-molybdenum diselenide (MoSe2) based electrode materials for supercapacitor and batteries: A critical analysis. J. Energy Storage 2021, 40, 102809. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Girirajan, M.; Bojarajan, A.K.; Pulidindi, I.N.; Hui, K.N.; Sangaraju, S. An insight into the nanoarchitecture of electrode materials on the performance of supercapacitors. Coord. Chem. Rev. 2024, 518, 216080. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, H. Nanostructured Electrode Materials for Electrochemical Capacitor Applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for renewable energy applications: A review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Sammed, K.A.; Farid, A.; Mustafa, S.; Kumar, A.; Tabish, M.; Khan, A.A.; Ajmal, S.; Mo, Z.; Akbar, A.R.; Yasin, G.; et al. Developing next-generation supercapacitor electrodes by coordination chemistry-based advanced functional carbon nanostructures: Progress, Current challenges and prospects. Fuel Process. Technol. 2023, 250, 107896. [Google Scholar] [CrossRef]
- Mahmoudi-Qashqay, S.; Zamani-Meymian, M.R.; Maleki, A. A simple method of fabrication hybrid electrodes for supercapacitors. Sci. Rep. 2024, 14, 29105. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-Performance Supercapacitors: A Comprehensive Review on Paradigm Shift of Conventional Energy Storage Devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Qadeer, M.A.; Zhang, X.; Farid, M.A.; Tanveer, M.; Yan, Y.; Du, S.; Huang, Z.-F.; Tahir, M.; Zou, J.-J. A review on fundamentals for designing hydrogen evolution electrocatalyst. J. Power Sources 2024, 613, 234856. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H.; Kolhe, M.L. Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies 2021, 14, 8535. [Google Scholar] [CrossRef]
- Yusuf, B.A.; Yaseen, W.; Xie, M.; Zayyan, R.S.; Muhammad, A.I.; Nankya, R.; Xie, J.; Xu, Y. Recent advances in understanding and design of efficient hydrogen evolution electrocatalysts for water splitting: A comprehensive review. Adv. Colloid Interface Sci. 2023, 311, 102811. [Google Scholar] [CrossRef]
- Haridas, H.; Somapur, B.; Akkera, H.S.; Neella, N.; Kambhala, N. Review on Recent Advances of Nickel Sulfide Nano Electrocatalysts for Hydrogen Evolution. ChemistrySelect 2024, 9, e202402550. [Google Scholar] [CrossRef]
- Ogunkunle, S.A.; Mortier, F.; Bouzid, A.; Hinsch, J.J.; Zhang, L.; Wu, Z.; Bernard, S.; Zhu, Y.; Wang, Y. Navigating Alkaline Hydrogen Evolution Reaction Descriptors for Electrocatalyst Design. Catalysts 2024, 14, 608. [Google Scholar] [CrossRef]
- Joseph, S.; Mohan, J.; Lakshmy, S.; Thomas, S.; Chakraborty, B.; Thomas, S.; Kalarikkal, N. A review of the synthesis, properties, and applications of 2D transition metal dichalcogenides and their heterostructures. Mater. Chem. Phys. 2023, 297, 127332. [Google Scholar] [CrossRef]
- Lin, L.; Lei, W.; Zhang, S.; Liu, Y.; Wallace, G.G.; Chen, J. Two-dimensional transition metal dichalcogenides in supercapacitors and secondary batteries. Energy Storage Mater. 2019, 19, 408–423. [Google Scholar] [CrossRef]
- Sukanya, R.; da Silva Alves, D.C.; Breslin, C.B. Review—Recent Developments in the Applications of 2D Transition Metal Dichalcogenides as Electrocatalysts in the Generation of Hydrogen for Renewable Energy Conversion. J. Electrochem. Soc. 2022, 169, 064504. [Google Scholar] [CrossRef]
- Robertson, B.; Sapna, R.; Hegde, V.; Hareesh, K. A comprehensive review on MoSe2 nanostructures with an overview of machine learning techniques for supercapacitor applications. RSC Adv. 2024, 14, 37644–37675. [Google Scholar]
- Loo, A.H.; Bonanni, A.; Sofer, Z.; Pumera, M. Exfoliated transition metal dichalcogenides (MoS2, MoSe2, WS2, WSe2): An electrochemical impedance spectroscopic investigation. Electrochem. Commun. 2015, 50, 39–42. [Google Scholar] [CrossRef]
- Dau, M.T.; Gay, M.; Di Felice, D.; Vergnaud, C.; Marty, A.; Beigné, C.; Renaud, G.; Renault, O.; Mallet, P.; Le Quang, T.; et al. Beyond van der Waals Interaction: The Case of MoSe2 Epitaxially Grown on Few-Layer Graphene. ACS Nano 2018, 12, 2319–2331. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Qu, G. Molybdenum-Based Electrode Materials Applied in High-Performance Supercapacitors. Batteries 2023, 9, 479. [Google Scholar] [CrossRef]
- Bui, H.T.; Lam, N.D.; Linh, D.C.; Mai, N.T.; Chang, H.; Han, S.-H.; Oanh, V.T.K.; Pham, A.T.; Patil, S.A.; Tung, N.T.; et al. Escalating Catalytic Activity for Hydrogen Evolution Reaction on MoSe2@Graphene Functionalization. Nanomaterials 2023, 13, 2139. [Google Scholar] [CrossRef]
- Za’abar, F.I.; Yusoff, Y.; Mohamed, H.; Abdullah, S.F.; Mahmood Zuhdi, A.W.; Amin, N.; Chelvanathan, P.; Bahrudin, M.S.; Rahman, K.S.; Samsudin, N.A.; et al. A Numerical Investigation on the Combined Effects of MoSe2 Interface Layer and Graded Bandgap Absorber in CIGS Thin Film Solar Cells. Coatings 2021, 11, 930. [Google Scholar] [CrossRef]
- Narang, J.; Mishra, A.; Pilloton, R.; VV, A.; Wadhwa, S.; Pundir, C.S.; Khanuja, M. Development of MoSe2 Nano-Urchins as a Sensing Platform for a Selective Bio-Capturing of Escherichia coli Shiga Toxin DNA. Biosensors 2018, 8, 77. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Devarayapalli, K.C.; Nagajyothi, P.C.; Shim, J. Microwave synthesized dry leaf-like mesoporous MoSe2 nanostructure as an efficient catalyst for enhanced hydrogen evolution and supercapacitor applications. Microchem. J. 2020, 153, 104446. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Xu, Q.; Shi, Y.; Tian, Z.; Wang, R.; Zhang, G.; Chen, J.; Wang, Z.; Zheng, W. Controllable synthesis of NiSe/MoSe2/MoO2 3D hierarchical hollow microspheres with enhanced performance for asymmetric supercapacitors. Chem. Eng. J. 2020, 387, 124121. [Google Scholar] [CrossRef]
- Alam, A.; Saeed, G.; Lim, S. One-step synthesis of 2D–2D Co(OH)2–MoSe2 hybrid nanosheets as an efficient electrode material for high-performance asymmetric supercapacitor. J. Electroanal. Chem. 2020, 879, 114775. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Huang, B.; Yang, Z.; Cai, W.; Sui, J.; Cao, G.; Wang, H.-E. Dual interface coupled molybdenum diselenide for high-performance sodium ion batteries and capacitors. J. Power Sources 2020, 446, 227298. [Google Scholar] [CrossRef]
- Upadhyay, S.; Pandey, O.P. Synthesis of layered 2H–MoSe2 nanosheets for the high-performance supercapacitor electrode material. J. Alloys Compd. 2021, 857, 157522. [Google Scholar] [CrossRef]
- Gowrisankar, A.; Sherryn, A.L.; Selvaraju, T. In situ integrated 2D reduced graphene oxide nanosheets with MoSSe for hydrogen evolution reaction and supercapacitor application. Appl. Surf. Sci. Adv. 2021, 3, 100054. [Google Scholar] [CrossRef]
- Rahul; Arora, S.K. MoSe2 nanosheets as an efficient electrode material for supercapacitors. Mater. Today Proc. 2022, 54, 728–732. [Google Scholar] [CrossRef]
- Tanwar, S.; Arya, A.; Sharma, A.L. MoSe2-FeOOH nanocomposite as hybrid electrode material for high-performance symmetric supercapacitor. Mater. Res. Bull. 2023, 160, 112144. [Google Scholar] [CrossRef]
- Tanwar, S.; Sharma, A.L. Aging impact on morphological and electrochemical performance of MoSe2 composite for super-capacitor application. Ceram. Int. 2023, 49, 18281–18295. [Google Scholar] [CrossRef]
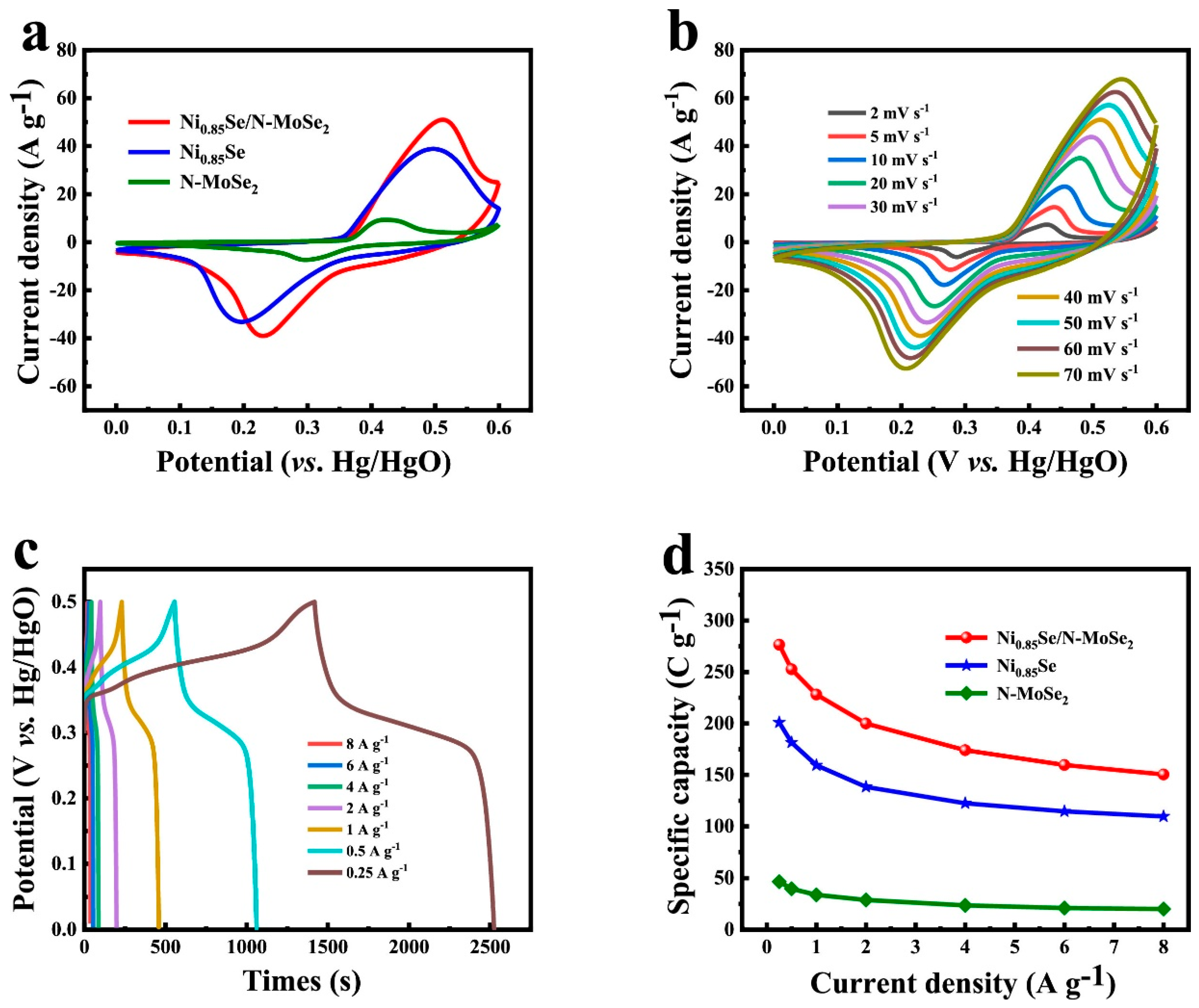
- Yu, J.; Su, H.; Shi, C.; Qiu, G.; Bai, L.; Li, Z. Ni0.85Se anchored on N-doped MoSe2 hybrids for long-life asymmetric supercapacitors. Electrochim. Acta 2023, 471, 143392. [Google Scholar] [CrossRef]
- Shui, J.; Bai, B.; Jiang, X.; Du, P. High-performance MoSe2/rGO composites based on interface and phase engineering for all-solid-state symmetric supercapacitors. Electrochim. Acta 2023, 469, 143257. [Google Scholar] [CrossRef]
- Arulkumar, C.; Gandhi, R.; Vadivel, S. Ultra-thin nanosheets of Ti3C2Tx MXene/MoSe2 nanocomposite electrode for asymmetric supercapacitor and electrocatalytic water splitting. Electrochim. Acta 2023, 462, 142742. [Google Scholar] [CrossRef]
- Velpandian, M.; Gupta, P.; Kuttasseri, A.; Mahata, A.; Basu, S. Unraveling the phase transition and electrochemical application of MoSe2 material for energy conversion and storage devices. Appl. Surf. Sci. 2024, 677, 160990. [Google Scholar] [CrossRef]
- Layek, R.; Mondal, K.; Karmakar, S.; Sarkar, R.; Chattopadhyay, D.; Kumbhakar, P. Synergistic effect in chemically synthesized noble metal nanoparticles and 2D MoSe2 nanocomposite for enhanced electrochemical performance. Mater. Today Commun. 2024, 38, 108342. [Google Scholar] [CrossRef]
- Singh, R.P.; Alegaonkar, P.S.; Devi, C.; Yogesh, G.K.; Yadav, K. Selenium-enriched MoSe2 as an effective electrode material for supercapacitors and a photocatalyst for dye degradation. Mater. Today Commun. 2024, 40, 109671. [Google Scholar] [CrossRef]
- Guo, H.; Ren, H.; Tian, J.; Xu, J.; Hao, Y.; Peng, L.; Liu, Y.; Yang, W. Polyoxometalate/MOF-derived MoSe2/(Ni, Co)Se2 for battery-type electrodes of high-performance supercapacitors. J. Alloys Compd. 2024, 1000, 175107. [Google Scholar] [CrossRef]
- Shahidani, H.S.; Seifi, M.; Askari, M.B. Design of NiSe2@MoSe2 nanocomposite anchored on multi-walled carbon nanotubes as advanced supercapacitor applications. Inorg. Chem. Commun. 2024, 170, 113218. [Google Scholar] [CrossRef]
- Singal, S.; Yadav, A.; Sharma, R.K. A high-performance asymmetric supercapacitor based on morphologically tuned CoB anode and heterostructured graphitic carbon nitride/MoSe2 cathode. J. Energy Storage 2024, 80, 110300. [Google Scholar] [CrossRef]
- Szkoda, M.; Ilnicka, A. Enhanced electrochemical capacitance of TiO2 nanotubes/MoSe2 composite obtained by hydrothermal route. Appl. Surf. Sci. 2025, 681, 161490. [Google Scholar] [CrossRef]
- Huang, M.; Yu, J.; Su, H.; Wu, Z.; Li, Z. Coupling (NixCo1-x)0.85Se with N-doped MoSe2 for hybrid supercapacitors with remarkable cyclic durability. Appl. Surf. Sci. 2025, 681, 161547. [Google Scholar] [CrossRef]
- Su, H.; Niu, C.; Zhang, R.; Huang, M.; Li, Z. Construction of a Ni2P/NiSe2/MoSe2 hybrid for advanced supercapacitors. J. Alloys Compd. 2025, 1010, 178074. [Google Scholar] [CrossRef]
- Dhanasekaran, G.; Parthiban, N.; Keerthana, T.; Gopal, R.; Sangaraju, S.; Chakrabortty, S.; Thangavel, E. Enhanced electrochemical performance of (MoSe2@NiSe2) (0D/1D) hybrid nanostructures for supercapacitors. Mater. Sci. Eng. B 2025, 313, 117975. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, H.; Jia, P.; Li, S.; Yang, Y.; Zhang, X. A dual-activation strategy for enhancing the energy storage performance of MoSe2/AFCNTs in symmetric supercapacitors. J. Alloys Compd. 2025, 1010, 177943. [Google Scholar] [CrossRef]
- Burragoni, S.G.; Koyyada, G.; Vattikuti, S.V.P.; Nam, N.D.; Kim, J.H. One-pot synthesis of flower-like MoSe2 nanoflakes for photocatalytic hydrogen evolution and methanol oxidation. Mater. Lett. 2021, 282, 128678. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.-Y.; Mei, B.; Zhang, X.; Liu, Y.; Wang, J.; Li, W. Synthesis of hollow CoSe2/MoSe2 nanospheres for efficient hydrazine-assisted hydrogen evolution. Chem. Eng. J. 2021, 404, 126529. [Google Scholar] [CrossRef]
- Tan, Y.; Yi, M.; Zhu, Z.; Zhang, X.; Qin, K.; Zhang, J.; Zhu, R. Carbon-coated MoSe2/Mo2CTx (MXene) heterostructure for efficient hydrogen evolution. Mater. Sci. Eng. B 2021, 271, 115239. [Google Scholar] [CrossRef]
- Chouki, T.; Donkova, B.; Aktarla, B.; Stefanov, P.; Emin, S. Growth of MoSe2 electrocatalyst from metallic molybdenum nanoparticles for efficient hydrogen evolution. Mater. Today Commun. 2021, 26, 101976. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Fan, X.-H.; Gao, J.; Qiu, S.-Y.; Zhang, L.-S.; Gu, L.-L.; Wang, C.; Wang, K.-X.; Mao, Y.-C. Controllable construction of Ag/MoSe2 hybrid architectures for efficient hydrogen evolution and advanced lithium anode. Chem. Eng. Sci. 2021, 233, 116404. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Li, H.; Li, H.; Wu, Z.; Liang, C.; Zhu, X.; Sun, Y. Dual surfactants applied in synthesis of MoSe2 for high-efficiency hydrogen evolution reaction. J. Alloys Compd. 2021, 863, 158092. [Google Scholar] [CrossRef]
- Xiao, D.; Bao, D.-L.; Liang, X.; Wang, Y.; Shen, J.; Cheng, C.; Chu, P.K. Experimental and theoretical investigation of the control and balance of active sites on oxygen plasma-functionalized MoSe2 nanosheets for efficient hydrogen evolution reaction. Appl. Catal. B Environ. 2021, 288, 119983. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chen, C.Y.; Ho, C.J.; Cheng, C.M.; Chen, H.R.; Fu, T.Y.; Huang, Y.T.; Ke, S.W.; Du, H.Y.; Lee, K.Y.; et al. Surface electron accumulation and enhanced hydrogen evolution reaction in MoSe2 basal planes. Nano Energy 2021, 84, 105922. [Google Scholar] [CrossRef]
- Rahul; Singh, H.; Lalla, N.P.; Deshpande, U.; Arora, S.K. Engineered MoSe2/WSe2 based heterostructures for efficient hydrogen evolution reaction. Mater. Today Proc. 2021, 45, 4787–4791. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Vinoth, V.; Pecchi, G.; Delgado, E.J.; Valdés, H.; Mangalaraja, R.V. Novel MoSe2–Ni(OH)2 nanocomposite as an electrocatalyst for high efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 32471–32479. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Li, H.; Yu, L.; Sun, L.; Xue, J.; Xu, R.; Chen, G. In-situ phase conversion of composited 1T@2H–MoSe2 nanosheets with enhanced HER performance. Mater. Chem. Phys. 2022, 278, 125657. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, Q.; Bai, X.; Cai, H.; Zhao, J.; Yan, Y.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.; et al. Construction of reduced graphene oxide coupled with CoSe2-MoSe2 heterostructure for enhanced electrocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 608, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.-Y.; Li, F.-L.; Chen, B.; Geng, H.; Zhang, W.; Xu, W.-Y.; Gu, H.; Braunstein, P.; Lang, J.-P. Engineering multiphasic MoSe2/NiSe heterostructure interfaces for superior hydrogen production electrocatalysis. Appl. Catal. B Environ. 2022, 312, 121434. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, L.; Wang, C.; Duan, W.; Zhang, L.; Zhang, F.; Zhang, J.; Zhen, Y.; Gao, L.; Fu, F.; et al. Large-scale synthetic Mo@(2H-1T)-MoSe2 monolithic electrode for efficient hydrogen evolution in all pH scale ranges and seawater. Appl. Catal. B Environ. 2022, 304, 120993. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, C.; Luo, L.; Ji, D.; Wang, W.; Chen, Z. Microwave hydrothermal synthesis of hierarchical Ce-doped MoSe2@CNTs as an efficient non-precious catalyst for hydrogen evolution in both acidic and alkaline media. Mater. Res. Bull. 2022, 146, 111625. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Le, P.-A.; Nguyen, V.-T.; Lu, Y.-C.; Sung, C.-W.; Cheng, H.-W.; Hsiao, C.-Y.; Dang, V.D.; Chiu, P.-W.; Wei, K.-H. Surface plasma–induced tunable nitrogen doping through precursors provides 1T-2H MoSe2/graphene sheet composites as electrocatalysts for the hydrogen evolution reaction. Electrochim. Acta 2022, 426, 140767. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, Y.; Yan, Q.; Zhu, K.; Ye, K.; Yan, J.; Wang, Q.; Cao, D.; Wang, G. Coupling of Ru nanoclusters decorated mixed-phase (1T and 2H) MoSe2 on biomass-derived carbon substrate for advanced hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 617, 594–603. [Google Scholar] [CrossRef]
- Li, H.; Zhu, L.; Li, C.; Wu, Z.; Li, H.; Chen, Q.; Huang, Y.; Zhu, X.; Sun, Y. S-doping induced phase engineering of MoSe2 for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 30371–30377. [Google Scholar] [CrossRef]
- Liu, S.; Lv, X.; Liu, G.; Li, C.; Thummavichaia, K.; Li, Z.; Zhang, L.; Bin, Z.; Wang, N.; Zhu, Y. In-situ fabrication of NixSey/MoSe2 hollow rod array for enhanced catalysts for efficient hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 617, 611–619. [Google Scholar] [CrossRef]
- Salehi, S.; Molaei, M.; Karimipour, M. Mo-rich rGO–2H–MoSe2 nanocomposites with novel crystal growth plane of (106) for high hydrogen evolution reaction activity. Mater. Sci. Semicond. Process. 2022, 142, 106475. [Google Scholar] [CrossRef]
- Luo, H.; Gao, H.; Zhang, X.; Yang, F.; Liu, C.; Xu, K.; Guo, D. Caterpillar-like 3D graphene nanoscrolls@CNTs hybrids decorated with Co-doped MoSe2 nanosheets for electrocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2023, 136, 43–53. [Google Scholar] [CrossRef]
- Kuang, Y.; Qiao, W.; Yang, F.; Feng, L. Electrochemical hydrogen evolution efficiently boosted by interfacial charge redistribution in Ru/MoSe2 embedded mesoporous hollow carbon spheres. J. Energy Chem. 2023, 85, 447–454. [Google Scholar] [CrossRef]
- Guo, F.-B.; Zhao, X.-Y.; Yu, Y.-M.; Cheng, J.; Liu, K.-K.; Zhang, L.-X. NiMOF-derived MoSe2@NiSe2 heterostructure with hollow core-shell for efficient hydrogen evolution reaction. J. Alloys Compd. 2023, 947, 169513. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Zheng, Q.; Ali, T.; Raza, S. Vacancy engineering in tungsten induced electronic structure optimization of MoSe2 for enhanced electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 35433–35442. [Google Scholar] [CrossRef]
- Dogra, N.; Agrawal, P.; Pathak, S.; Saini, R.; Sharma, S. Hydrothermally synthesized MoSe2/ZnO composite with enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 26210–26220. [Google Scholar] [CrossRef]
- Alahmadi, M.; Aoun, S.B. One-pot in-situ hydrothermal synthesis of VSe2/MoSe2 nanocomposite for enhanced hydrogen evolution reaction. Arab. J. Chem. 2023, 16, 104846. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Fan, A.; Li, L.; Geng, D.; Hu, W. Enhancing electrocatalytic hydrogen evolution performance through homogeneous deposition of 2H-Phase MoSe2 on Ti3C2Tx. FlatChem 2024, 47, 100705. [Google Scholar] [CrossRef]
- Alshgari, R.A.; Ahmad, N.; ALOthman, Z.A.; Alotibi, A.M.; Mohandoss, S.; Alothman, A.A.; Ouladsmane, M.; Khan, M.R. Titanium carbide (Ti3C2Tx) decorated molybdenum diselenide (MoSe2) nanoflower composite enhanced photo-electrocatalytic activity in hydrogen evolution. Ceram. Int. 2024, 50, 10928–10939. [Google Scholar] [CrossRef]
- Li, P.; Liang, J.; Zhu, Z.; Jiang, P.; Zhang, L.; Gao, Z.; Hou, X.; Zheng, X.; Yao, Y.; Sun, Q.; et al. Phase engineering and N doping modulated MoSe2 nanosheets for large-current–density hydrogen evolution in simulated seawater. Mater. Lett. 2024, 365, 136413. [Google Scholar] [CrossRef]
- Ma, T.; Wang, P.; Niu, H.-J.; Che, Z.; Li, G.; Zhou, W. Single Ru atoms dispersed on MoSe2/MXene nanosheets with multiple interfaces for enhanced acidic hydrogen evolution. Carbon 2024, 218, 118758. [Google Scholar] [CrossRef]
- Kozarenko, L.A.; Dyadyun, V.S.; Koshechko, V.G.; Pokhodenko, V.D. Ball milling-engineered nanostructured MoSe2/graphite electrocatalyst: Achieving superior hydrogen evolution with a high density of active sites. Int. J. Hydrogen Energy 2024, 63, 749–758. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, H.; Yang, C.; Deng, Q.; Liu, H.; Huang, J.; Zhang, Y. RuSe2 nanoparticles loaded MoSe2 hybrid rods via interface engineering as an efficient electrocatalyst for hydrogen evolution electrocatalysis. Appl. Surf. Sci. 2024, 659, 159916. [Google Scholar] [CrossRef]
- Qin, S.; Sun, J.; Meng, X. Modulating the electronic structure at the interface of CoSe and MoSe2 for enhanced electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 51, 415–422. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Santhoshkumar, P.; Alfantazi, A.; Jung, J.; Kim, H.-S. Facile fabrication of MoS2 and MoSe2 layered structures on Mo foil for the efficient photocatalytic dye degradation and electrocatalytic hydrogen evolution reaction. J. Water Process Eng. 2024, 60, 105127. [Google Scholar] [CrossRef]
- Kumar, G.; Francis, M.K.; Bhargav, P.B.; Ahmed, N. Silicon infused Molybedenum di-selinide (MoSe2) nanosheets for enhanced hydrogen evolution reaction (HER) and lithium-ion battery (LIB) applications. Int. J. Hydrogen Energy 2024, 51, 1448–1461. [Google Scholar]
- Zhang, H.; Yang, X.; Wang, H.; Wang, Y.; Li, R.; Fu, H.; Zhou, H.; Wu, D.; Yuang, P.; He, M.; et al. Vanadium modulated Ni-MoSe2 as highly efficient electrocatalyst for alkaline hydrogen evolution. J. Mol. Struct. 2025, 1326, 141132. [Google Scholar] [CrossRef]
- He, B. Molybdenum diselenide nanosheets wraping carbon aerogel nanospheres as an advanced material for supercapacitor and electrochemical sensing. Electrochim. Acta 2017, 257, 301–310. [Google Scholar] [CrossRef]
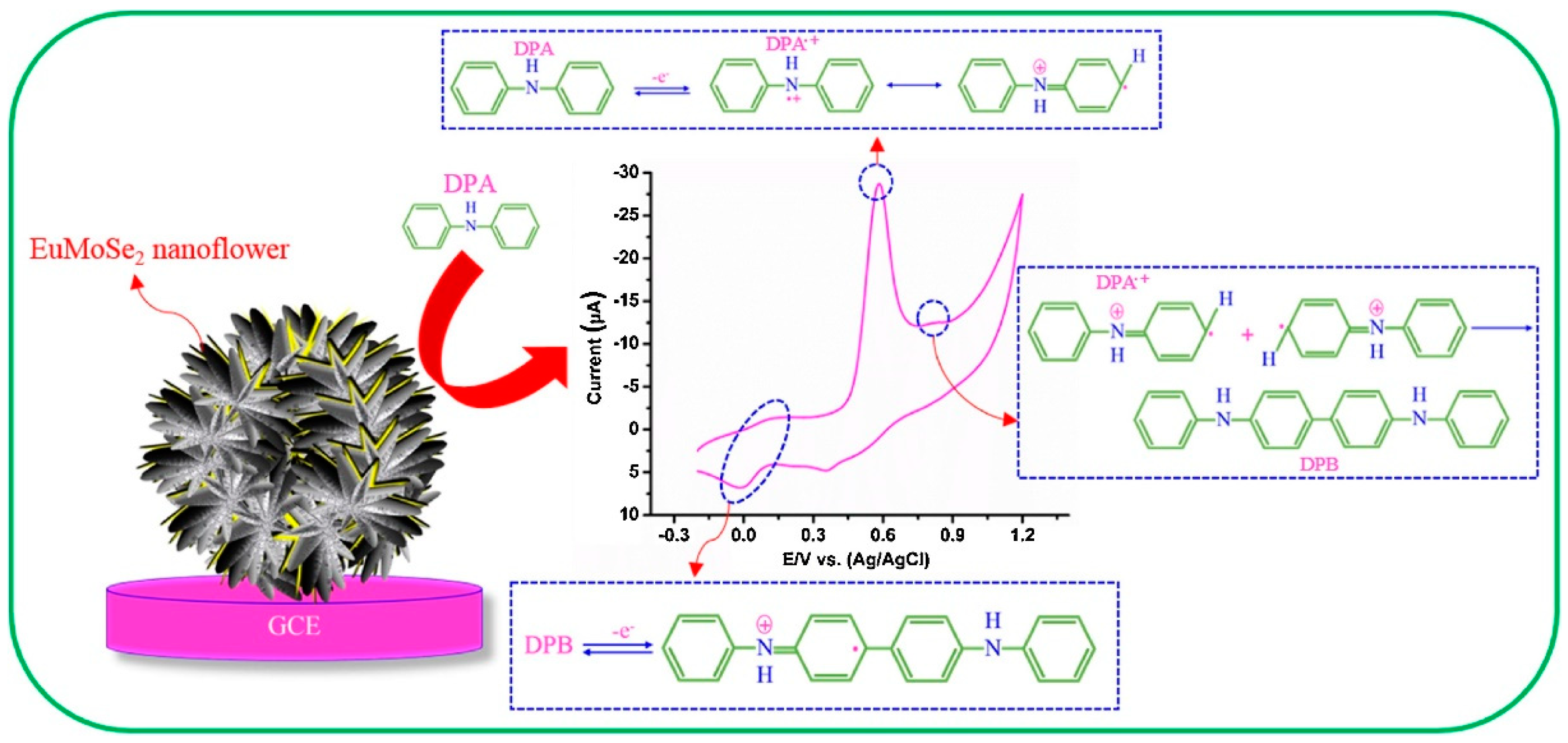
- Sakthivel, M.; Sukanya, R.; Chen, S.-M. Fabrication of europium doped molybdenum diselenide nanoflower based electrochemical sensor for sensitive detection of diphenylamine in apple juice. Sens. Actuators B Chem. 2018, 273, 616–626. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Jia, Y.; Zhang, X.; Dong, Y.; Li, X.; Liu, Q.; Li, Y.; Zhao, Z. Sandwich-type electrochemical immunosensor based on Au@Pt DNRs/NH2-MoSe2 NSs nanocomposite as signal amplifiers for the sensitive detection of alpha-fetoprotein. Bioelectrochemistry 2019, 128, 140–147. [Google Scholar] [CrossRef]
- Zang, Y.-J.; Nie, J.; He, B.; Yin, W.; Zheng, J.; Hou, C.-J.; Huo, D.-Q.; Yang, M.; Liu, F.-M.; Sun, Q.-Q.; et al. Fabrication of S-MoSe2/NSG/Au/MIPs imprinted composites for electrochemical detection of dopamine based on synergistic effect. Microchem. J. 2020, 156, 104845. [Google Scholar] [CrossRef]
- Ganguly, A.; Hwa, K.-Y.; Santhan, A.; Sharma, T.S.K. Strategic orchestration of MoSe2 microspheres on β-cd functionalized rGO: A sustainable electrocatalyst for detection of rifampicin in real samples. Chemosphere 2022, 307, 135373. [Google Scholar] [CrossRef]
- Pothipor, C.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A gold nanoparticle-dye/poly(3-aminobenzylamine)/two dimensional MoSe2/graphene oxide electrode towards label-free electrochemical biosensor for simultaneous dual-mode detection of cancer antigen 15-3 and microRNA-21. Colloids Surf. B Biointerfaces 2022, 210, 112260. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Lu, X.; Xu, L.; Zhu, Y.; Xue, T.; Ge, Y.; Duan, Z.; Duan, X.; Wen, Y.; Xu, J. Green synthesis of kudzu vine biochar decorated graphene-like MoSe2 with the oxidase-like activity as intelligent nanozyme sensing platform for hesperetin. Chemosphere 2022, 289, 133116. [Google Scholar] [CrossRef]
- Shah, M.; Kolhe, P.; Gandhi, S. Two-dimensional layered MoSe2/graphene oxide (GO) nanohybrid coupled with the specific immune-recognition element for rapid detection of endosulfan. Environ. Res. 2023, 238, 117127. [Google Scholar] [CrossRef]
- Yan, J.; Wang, K.; Liu, H.; Wang, L.; Li, Y.; Zhang, G.; Deng, L. Construction of electrochemical biosensors based on MoSe2@1T-MoS2 heterojunction for the sensitive and rapid detection of miRNA-155 biomarker in breast cancer. Bioelectrochemistry 2023, 154, 108541. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Khanuja, M. Superior photocatalytic and electrochemical activity of the MoSe2 modified ZIF-67 for the reduction and detection of Cr (VI). J. Environ. Chem. Eng. 2023, 11, 111442. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Li, P.; You, S.; Ye, J.; Bao, P.; Chen, X.; Tong, H.; Wang, S. Non-enzymatic electrochemical sensor based on Pt/MoSe2 nanomesh for the detection of hydrogen peroxide. Mater. Chem. Phys. 2023, 310, 128496. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Mohammadi, A.; Han, S.; Tiwari, J.N.; Kumar, K.; Kumar, A.S.; Saravanan, A.; Huh, Y.S.; Han, Y.-K. Gold nanoclusters supported Molybdenum diselenide-porous carbon composite as an efficient electrocatalyst for selective ultrafast probing of chlorpyrifos-pesticide. Chem. Eng. J. 2023, 472, 145048. [Google Scholar] [CrossRef]
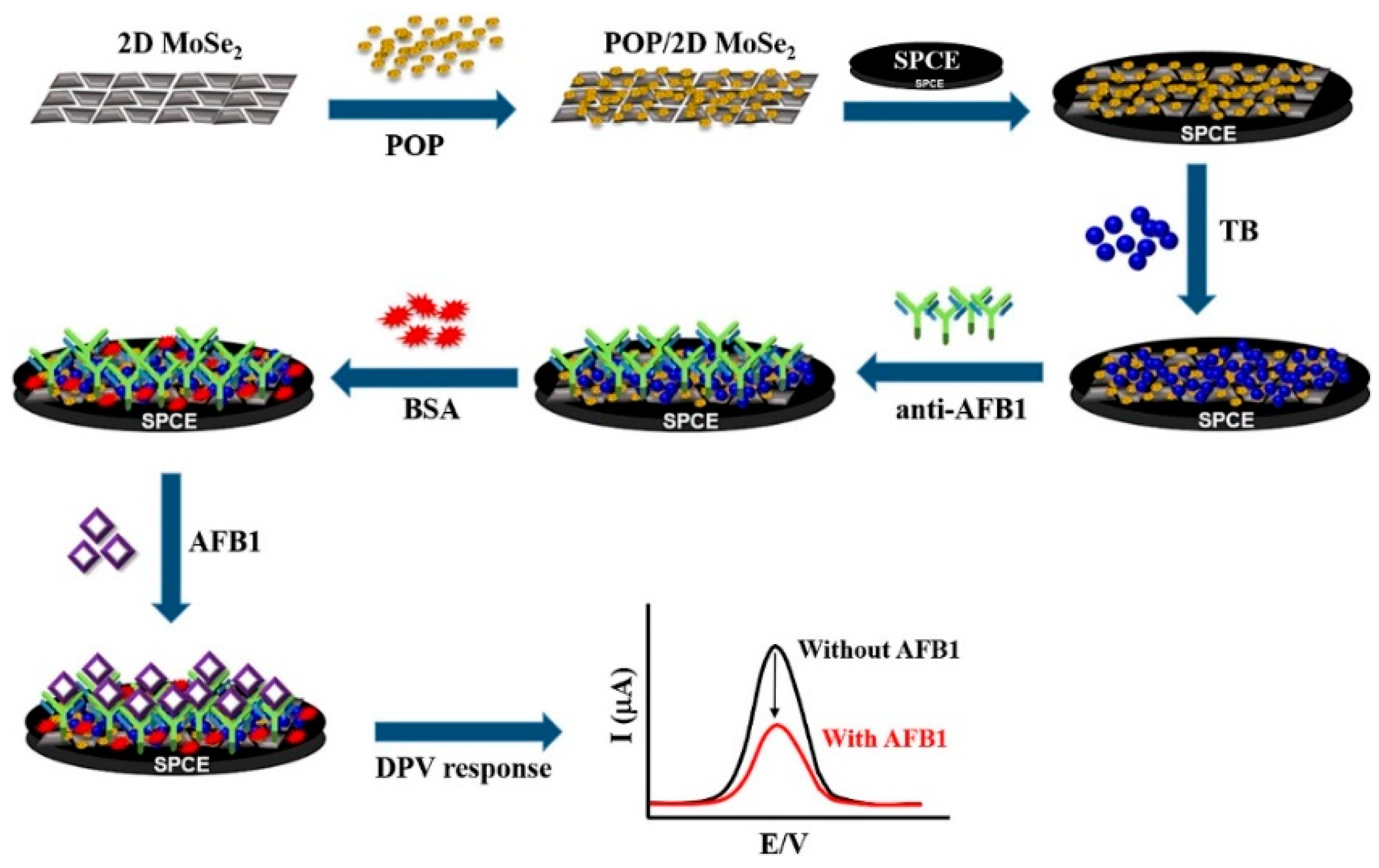
- Yaiwong, P.; Iamsawat, K.; Wiratchan, S.; Jumpathong, W.; Semakul, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A toluidine blue/porous organic polymer/2D MoSe2 nanocomposite as an electrochemical signaling platform for a sensitive label-free aflatoxin B1 bioassay in some crops. Food Chem. 2024, 439, 138147. [Google Scholar] [CrossRef] [PubMed]
- Abid, K.; Foti, A.; Khaskhoussi, A.; Celesti, C.; D’Andrea, C.; Polykretis, P.; Matteini, P.; Iannazzo, D.; Maalej, R.; Gucciardi, P.G.; et al. A study of screen-printed electrodes modified with MoSe2 and AuNPs-MoSe2 nanosheets for dopamine sensing. Electrochim. Acta 2024, 475, 143371. [Google Scholar] [CrossRef]
- Xia, X.; Xu, H.; Ye, C.-J.; Liu, Z.; Wang, Q.-Y.; Li, S.-S. Hypersensitized electrochemical sensing of Hg(II) based on phase engineering of Co doped MoSe2 nanosheets: Insight in dynamic phase change induced by the chemical interaction between Se and Hg(II). Sens. Actuators B Chem. 2024, 410, 135661. [Google Scholar] [CrossRef]
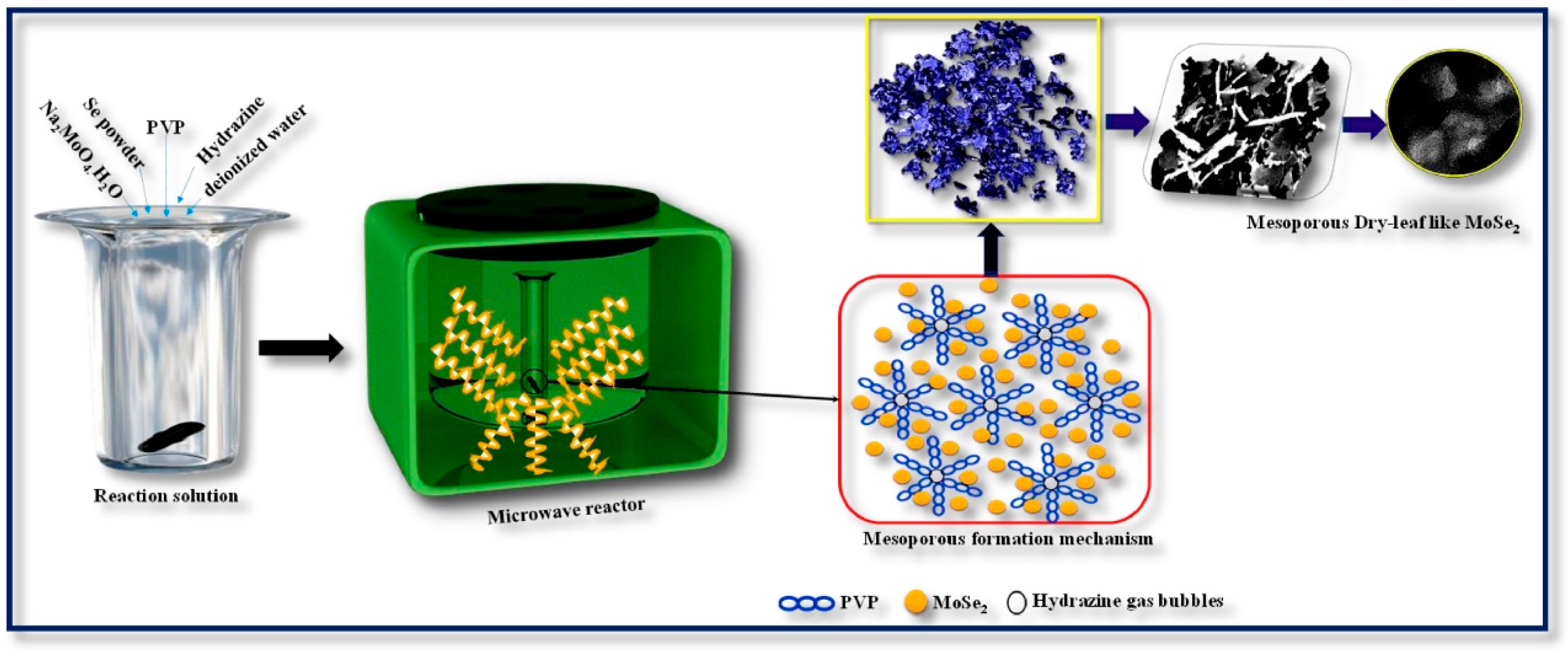



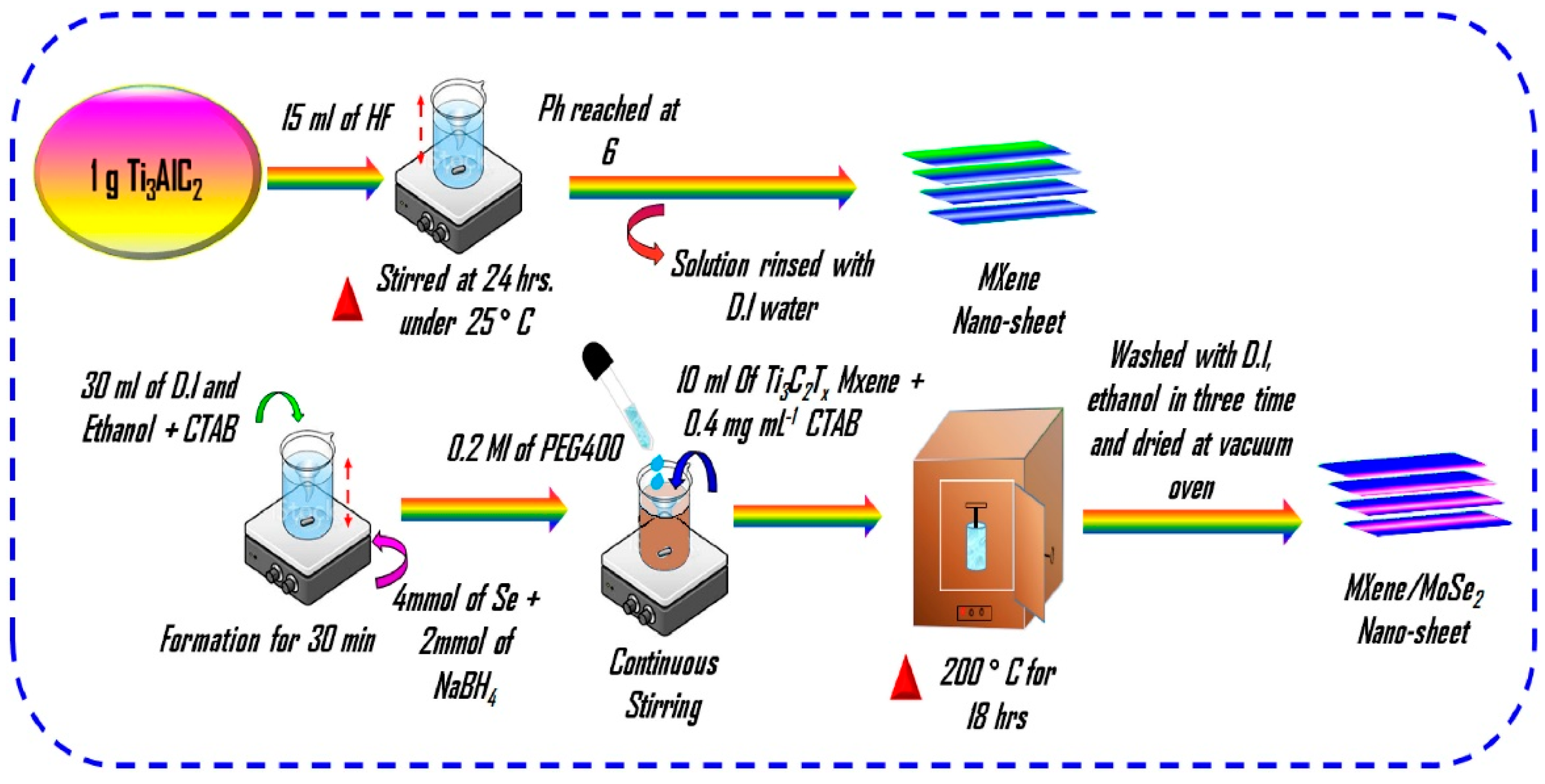

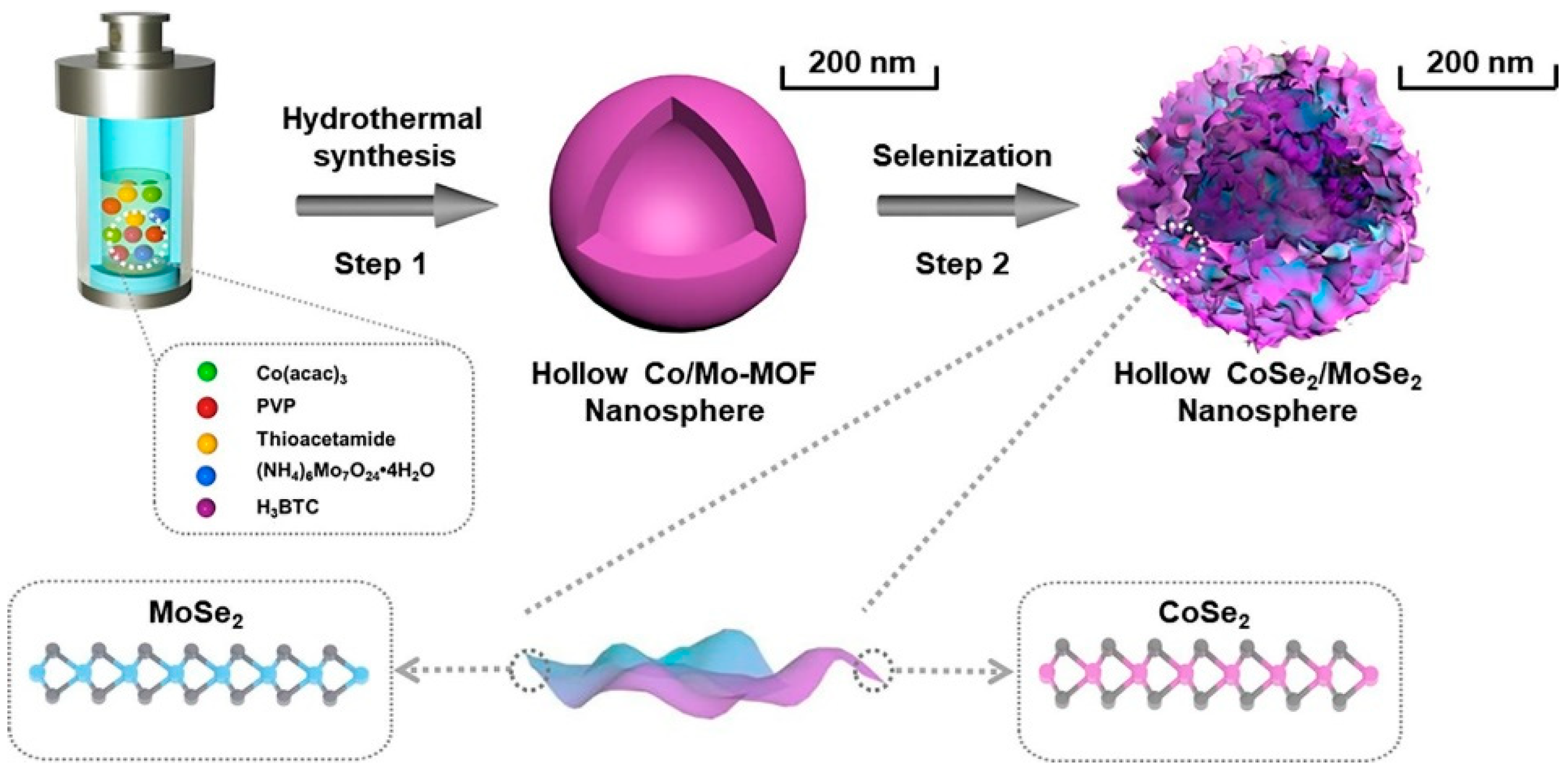


| Material | Synthesis Method | Electrolyte | Specific Capacitance (F/g) | Current Density (A/g) | Stability | References |
|---|---|---|---|---|---|---|
| MoSe2 | Microwave | 0.5 H2SO4 | 257.38 | 1 | 95% for 5000 cycles | [31] |
| NiSe/MoSe2/MoO2 | Hydrothermal | 1061 | 2 | 93.9% for 10,000 cycles | [32] | |
| Co(OH)2-MoSe2 | Hydrothermal | 6M KOH | 541.55 | 1 | 91.4% after 3000-cycles | [33] |
| 2H-MoSe2 | In situ selenization | 2M KOH | 46.22 mAh.g−1 | 2 | 100% for 10,000 cycles | [35] |
| MoSSe/rGO | Hydrothermal | 6 M KOH | 373 | 1 | 89.5% for 4600 cycle | [36] |
| MoSe2 | Liquid exfoliation | 15 | 0.1 | 95% for 12 cycles | [37] | |
| MoSe2/FeOOH | Hydrothermal-assisted chemical blending | 132 | 1 | 100% for 3000 cycles | [38] | |
| MoSe2/AC (Activated carbon) | Facile hydrothermal | 6M KOH | 394 | 1 | 105.1% for 15,000 cycles | [39] |
| Ni0.85Se/N-MoSe2 | Hydrothermal | 4 M KOH | 276.5 C·g−1 | 0.25 | [40] | |
| MoSe2-MXene | Hydrothermal | 3M KOH | 1531.2 | 1 | 96% for 10,000 cycles | [42] |
| MoSe2-Au | Hydrothermal | 1338 | 1 | 95% for 500 cycles | [44] | |
| Ti/(TiO2) nanotubes/MoSe2 | Hydrothermal | 0.5 M K2SO4 | 239 mF cm−2 | 1 mA cm−2 | 127% for 1000 cycles | [49] |
| Material | Synthesis Method | Electrolyte | Tafel Slope (mV.dec−1) | Overpotential mV (at 10 mA cm−2) | References |
|---|---|---|---|---|---|
| MoSe2 | Microwave | 0.5 M H2SO4 | 58 | 110 | [31] |
| MoSSe/rGO | hydrothermal | 1 M H2SO4 | 98 | 285 | [36] |
| MoSe2/Mo2CTx@C | Hydrothermal | 0.5 M H2SO4 | 70.7 | 108.3 | [56] |
| MoSe2 | CVD | 0.5 M H2SO4 | 218 | [57] | |
| Ag/MoSe2-5 | Self-assemble | 0.5 M H2SO4 | 80.3 | 187 | [58] |
| MoSe2-CTAB@F68 | Hydrothermal | 0.5 M H2SO4 | 62 | 189 | [59] |
| MoSe2 nanosheets | Hydrothermal | 0.5 M H2SO4 | 55.2 | 165 | [60] |
| 2 H-MoSe2 | Chemical vapor transport | 0.5 M H2SO4 | 60 | 170 | [61] |
| H2O2 assisted MoSe2/WSe2 | Liquid exfoliation | 0.5 M H2So4 | 80 | 275 | [62] |
| MoSe2–Ni(OH)2 | Hydrothermal technique | 0.5 M H2So4 | 54 | 230 | [63] |
| 1T@2H–MoSe2 | Hydrothermal | 0.5 M H2So4 | 65.8 | 118.75 | [64] |
| MoSe2-2xS2x | Hydrothermal | 0.5 M H2So4 | 54 | 167 | [71] |
| Co-MoSe2-GNS@CNT | Solvothermal | 0.5 M H2So4 | 61 | 151 | [74] |
| MoSe2/ZnO | Hydrothermal | 0.5 M H2So4 | 87 | 250 | [78] |
| gMoSe2/Gr(2h) | Ball mill | 0.5 M H2SO4 | 40 | 75 | [84] |
| Material | Synthesis Method | LOD (µM) | Linear Range (µM) | Sensing Analyte | Sensing Technique | References |
|---|---|---|---|---|---|---|
| MoSe2-CA hybrids | Hydrothermal | 2.0 × 10−13 M | 5.0 × 10−12–5.0 × 10−9 M | 17β-estradiol | DPV | [90] |
| EuMoSe2 | Hydrothermal | 0.008 | 0.01–243.17 | diphenylamine (DPA) | DPV | [91] |
| S-MoSe2/NSG/Au/MIPs | Hydrothermal | 0.02 | 0.05–1000 | dopamine (DA) | DPV | [93] |
| MoSe2/rGO/β-cd | Hydrothermal | 0.0028 | 0.019–374.5 | Rifampicin (RIF) | DPV | [94] |
| MoSe2@1T-MoS2 | Hydrothermal | 3.4 × 10−10 M | 1 × 10−9–1 × 10−3 M | miRNA-155 | DPV | [98] |
| MoSe2 @ZIF-67 | Stirring at RT | 0.01 | 0.01–500 | hexavalent chromium (Cr (VI) | LSV | [99] |
| Pt/MoSe2 nanomesh | Template and in situ modification | 2.56 | H2O2 | CV | [100] | |
| Au-MoSe2-PC-GCE | Hydrothermal | 1.5 × 10−4 M | 5 × 10−3 to 185 × 10−1 M | Chlorpyrifos (CPS) | Amperometry | [101] |
| AuNps@MoSe2/SPCE | Liquid-phase exfoliation | 0.21 | 3–20 | DA | LSV | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignesh, S.; Ramkumar, R.; Suganthi, S.; Kumar, P.; Ahmad, K.; Kim, W.K.; Oh, T.H. MoSe2 as Electrode Material for Super-Capacitor, Hydrogen Evolution, and Electrochemical Sensing Applications: A Review. Crystals 2025, 15, 238. https://doi.org/10.3390/cryst15030238
Vignesh S, Ramkumar R, Suganthi S, Kumar P, Ahmad K, Kim WK, Oh TH. MoSe2 as Electrode Material for Super-Capacitor, Hydrogen Evolution, and Electrochemical Sensing Applications: A Review. Crystals. 2025; 15(3):238. https://doi.org/10.3390/cryst15030238
Chicago/Turabian StyleVignesh, Shanmugam, Ramya Ramkumar, Sanjeevamuthu Suganthi, Praveen Kumar, Khursheed Ahmad, Woo Kyoung Kim, and Tae Hwan Oh. 2025. "MoSe2 as Electrode Material for Super-Capacitor, Hydrogen Evolution, and Electrochemical Sensing Applications: A Review" Crystals 15, no. 3: 238. https://doi.org/10.3390/cryst15030238
APA StyleVignesh, S., Ramkumar, R., Suganthi, S., Kumar, P., Ahmad, K., Kim, W. K., & Oh, T. H. (2025). MoSe2 as Electrode Material for Super-Capacitor, Hydrogen Evolution, and Electrochemical Sensing Applications: A Review. Crystals, 15(3), 238. https://doi.org/10.3390/cryst15030238






