Rapid Colorimetric pH-Responsive Gold Nanocomposite Hydrogels for Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
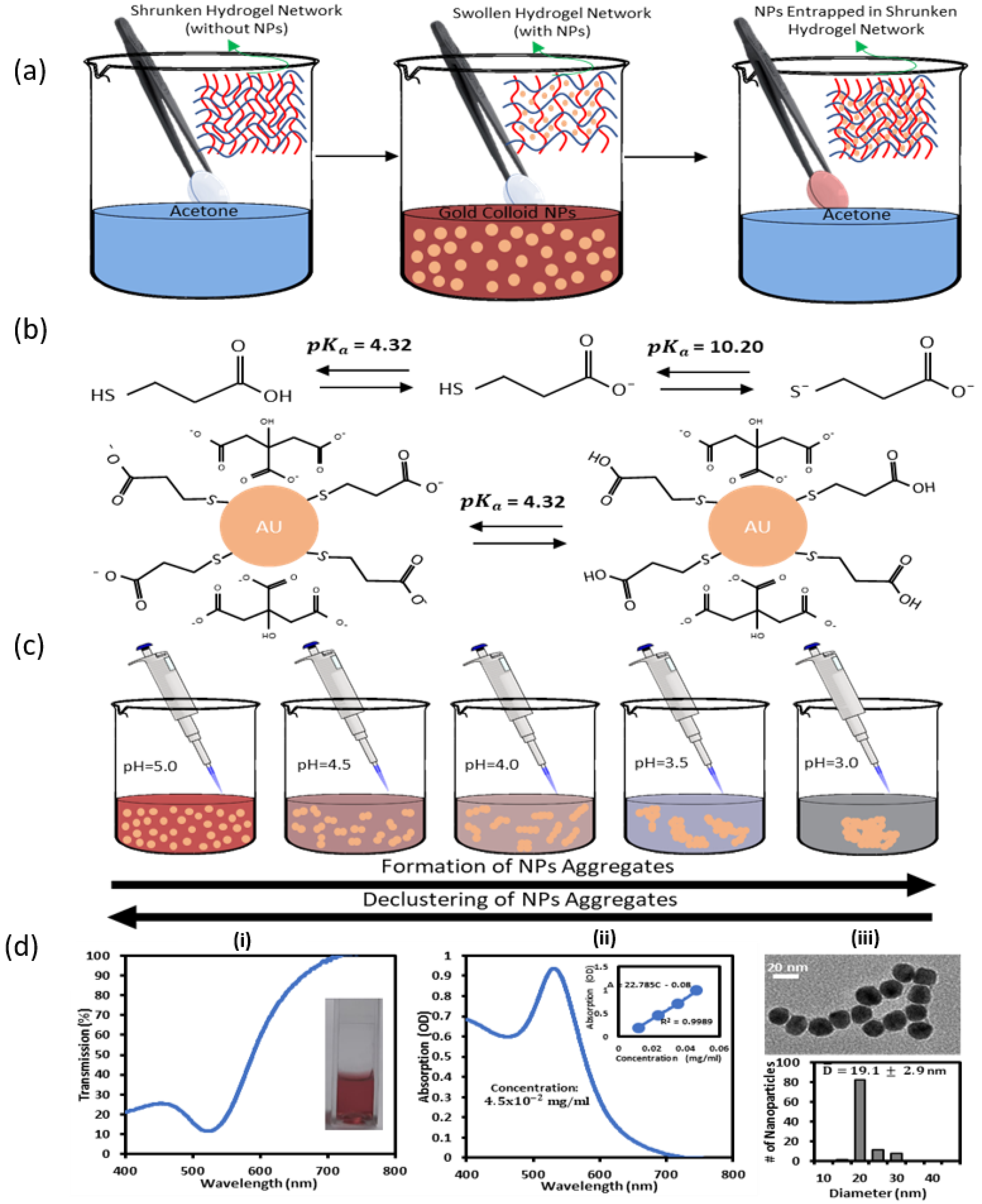
2.2. Preparation and Fabrication of Gold Nanoparticles Protected by MPA
2.3. Breathing of GNPs-MPA into Hydrogel Matrix
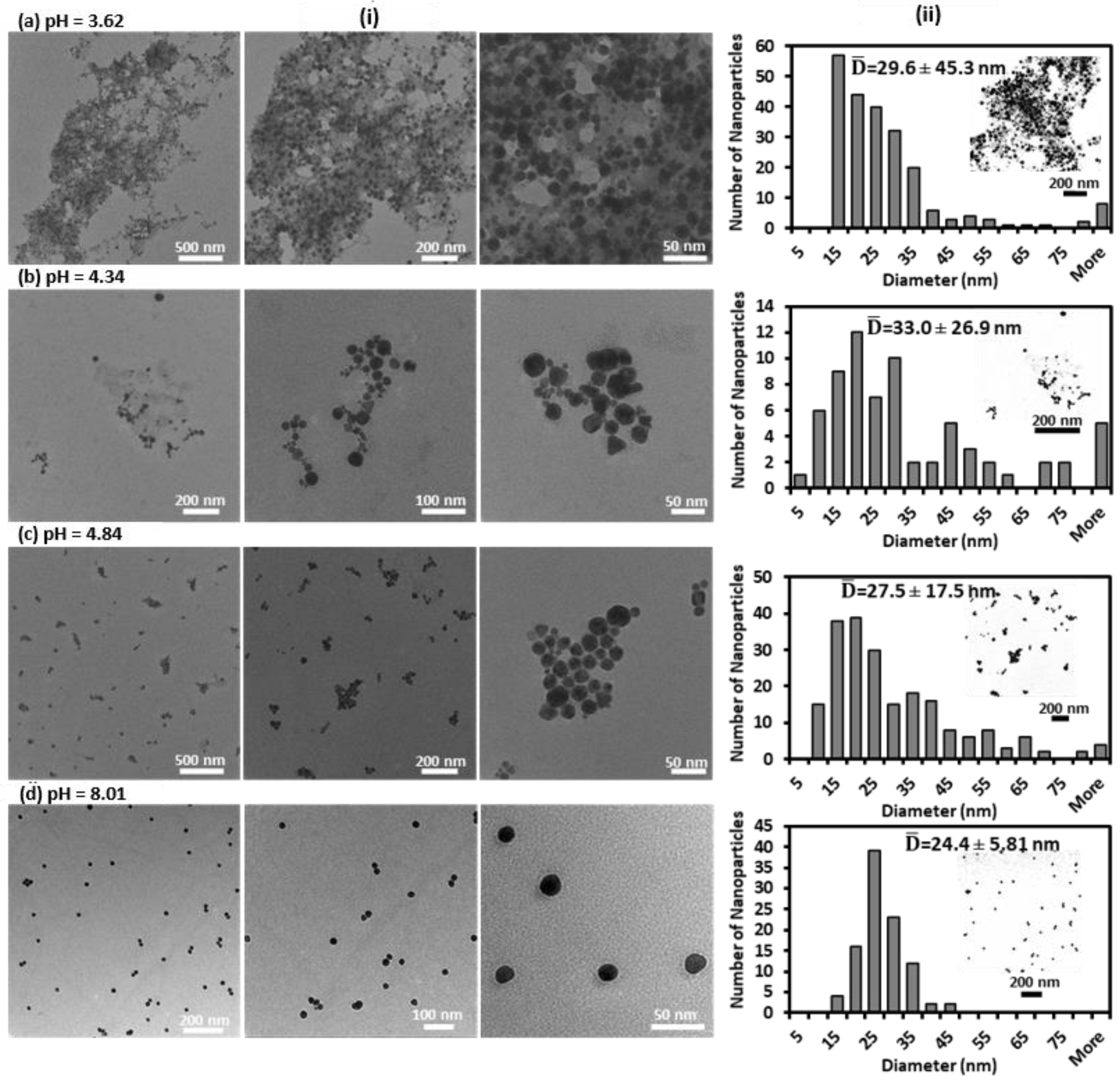
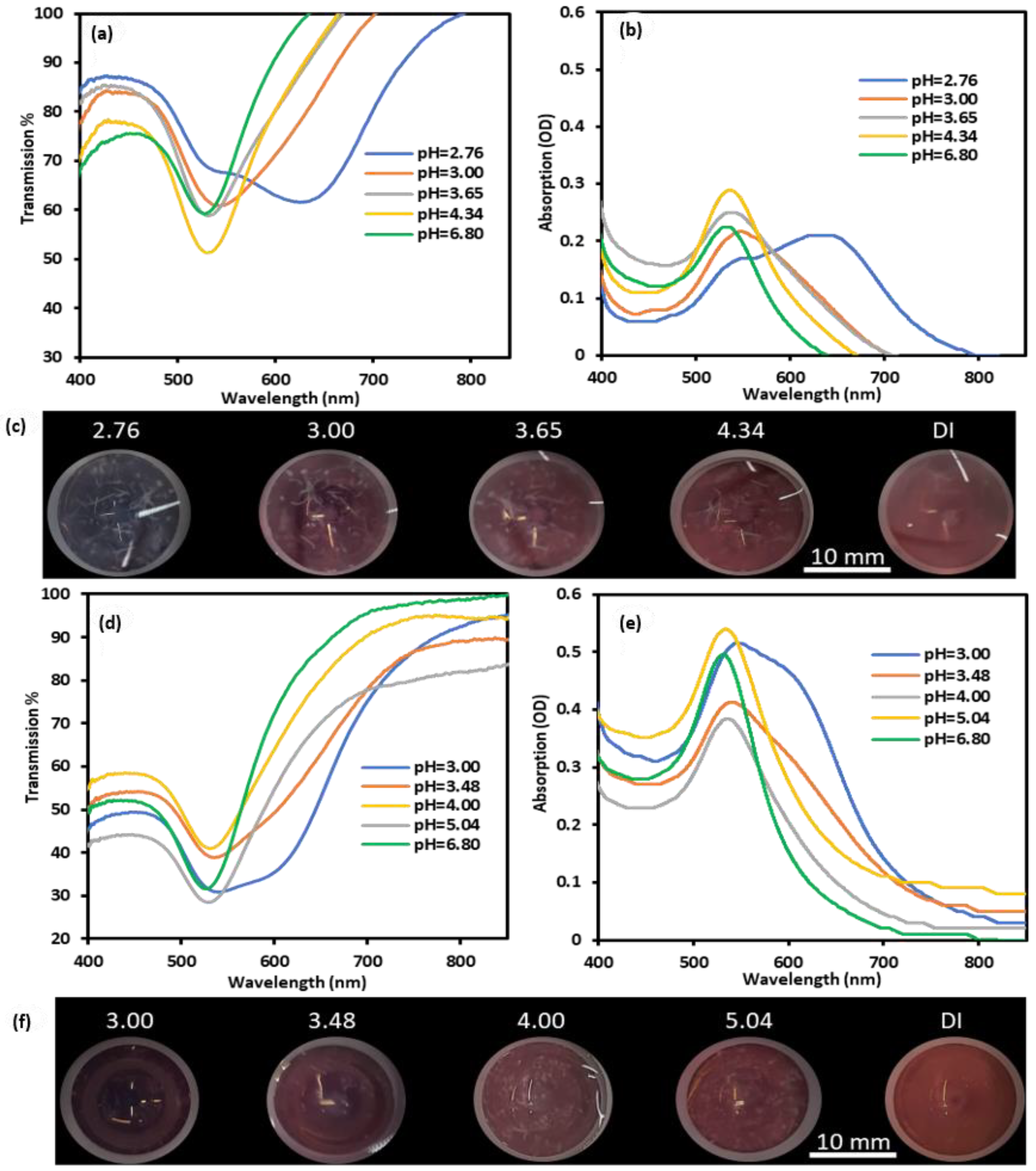
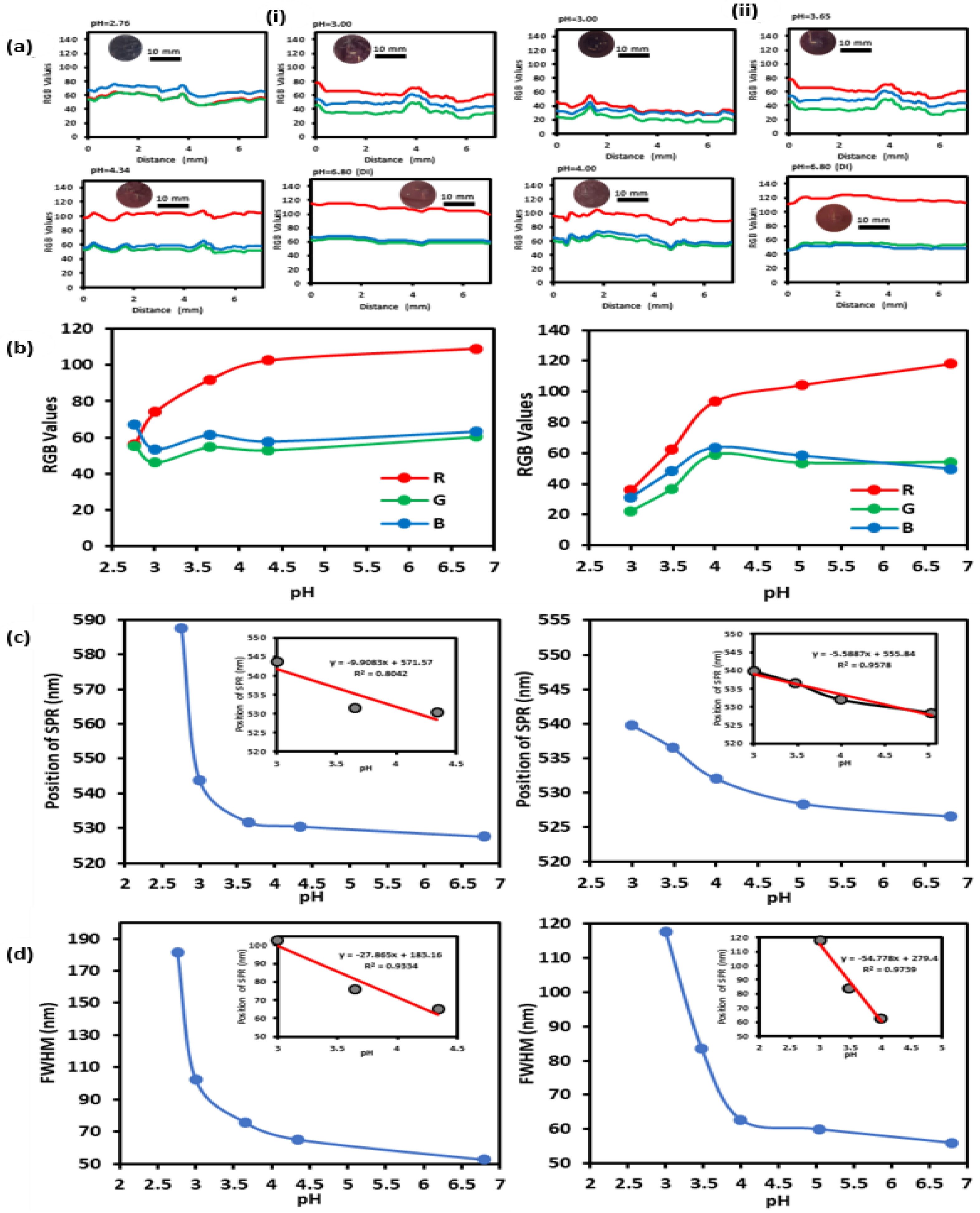
2.4. Preparations and Testing of GNPs-MPA Solutions and GELs at Different pHs
2.5. Characterization of pH-Responsive GNPs-MPA Solutions and Gels
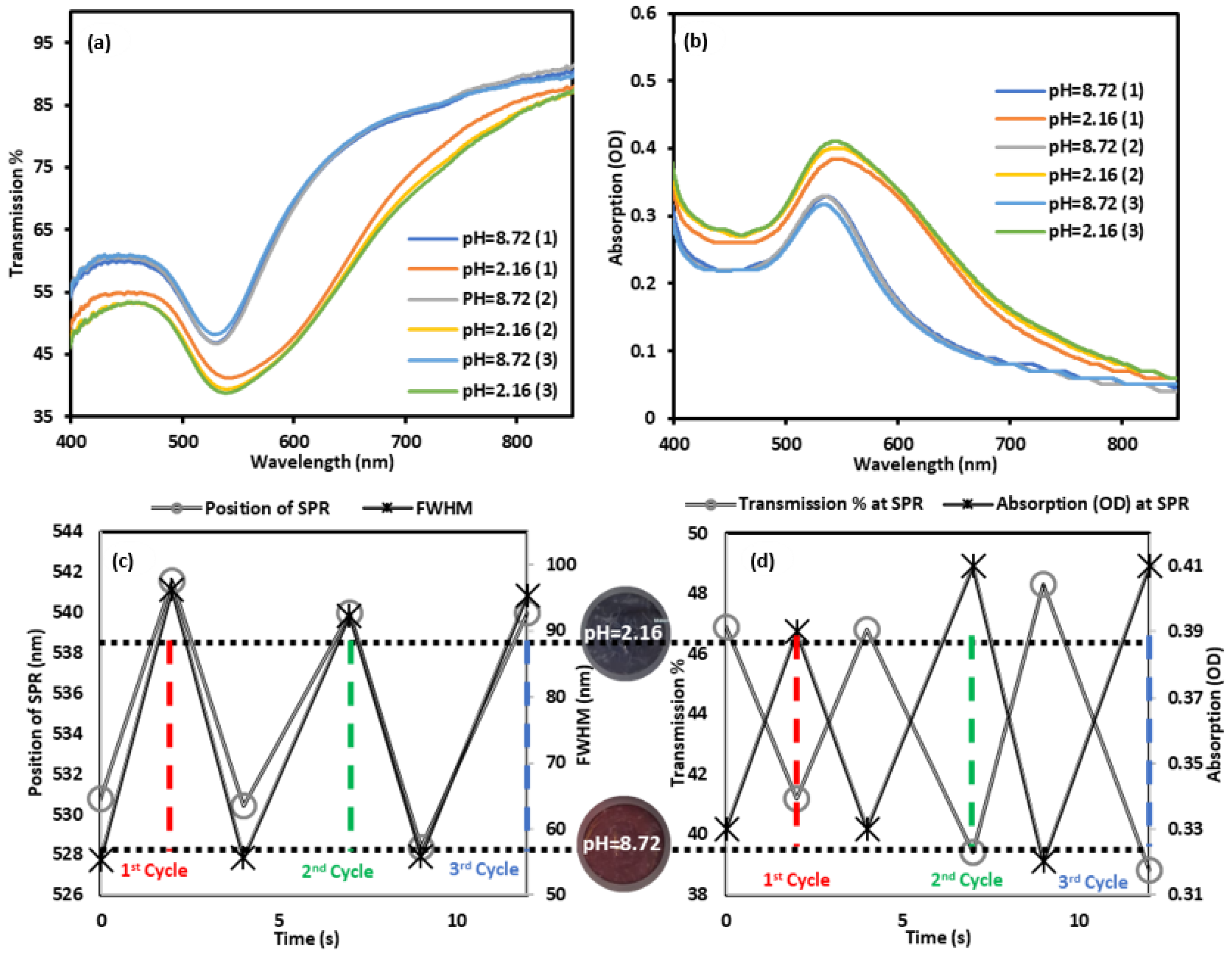
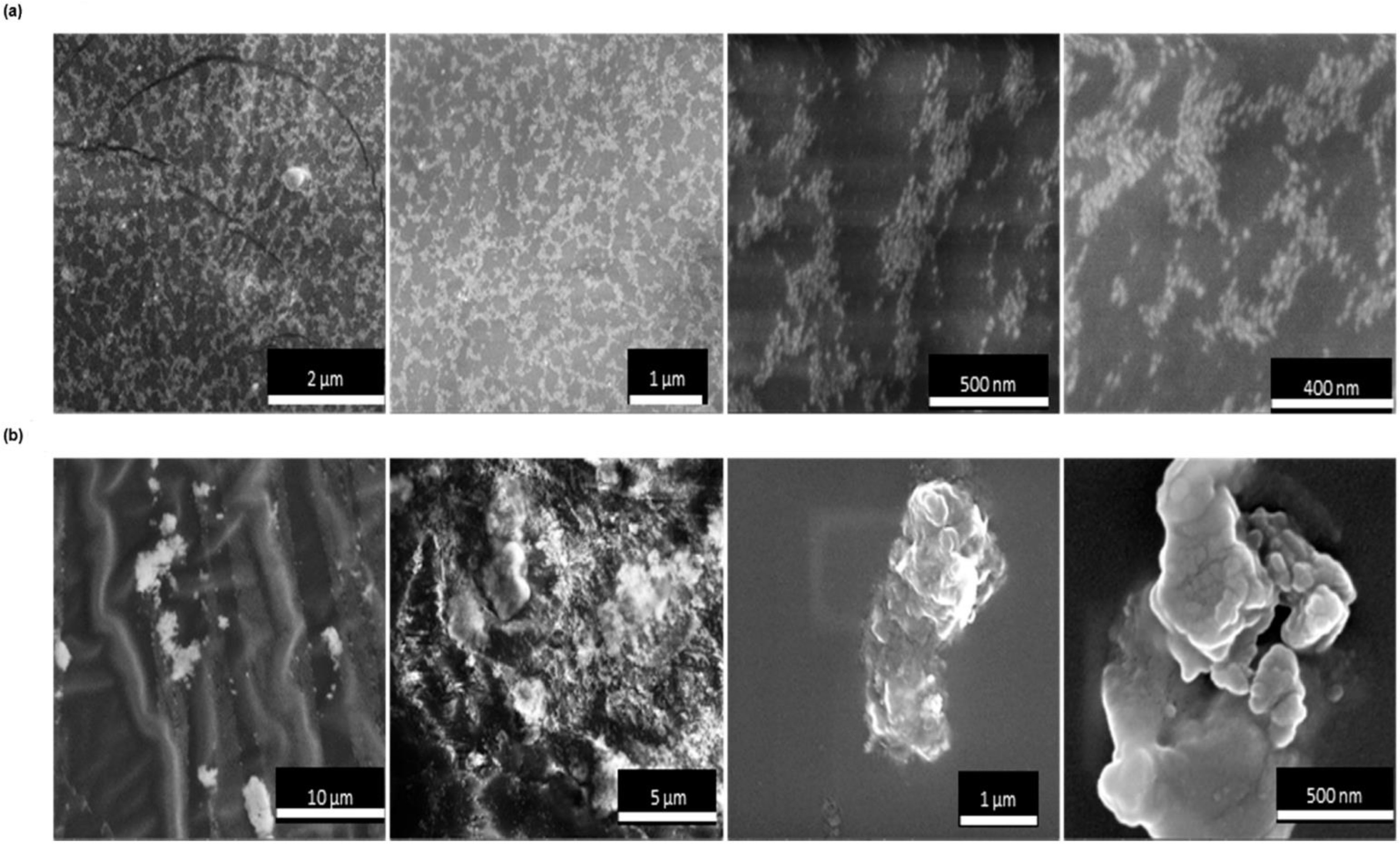
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter Inst. Phys. J. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold Nanoparticles: Past, Present, and Future. Langmuir ACS J. Surf. Colloids 2009, 25, 13840–13851. [Google Scholar] [CrossRef]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahra, Q.U.A.; Luo, Z.; Ali, R.; Khan, M.I.; Li, F.; Qiu, B. Advances in gold nanoparticles-based colorimetric aptasensors for the detection of antibiotics: An overview of the past decade. Nanomaterials 2021, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Ansar, S.M.; Chakraborty, S.; Kitchens, C.L. pH-Responsive Mercaptoundecanoic Acid Functionalized Gold Nanoparticles and Applications in Catalysis. Nanomaterials 2018, 8, 339. [Google Scholar] [CrossRef] [Green Version]
- Ansar, S.M.; Fellows, B.; Mispireta, P.; Mefford, O.T.; Kitchens, C.L. pH Triggered Recovery and Reuse of Thiolated Poly(acrylic acid) Functionalized Gold Nanoparticles with Applications in Colloidal Catalysis. Langmuir ACS J. Surf. Colloids 2017, 33, 7642–7648. [Google Scholar] [CrossRef]
- Rostek, A.; Mahl, D.; Epple, M. Chemical composition of surface-functionalized gold nanoparticles. J. Nanopart. Res. 2011, 13, 4809–4814. [Google Scholar] [CrossRef]
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold nanoparticle surface functionalization: A necessary requirement in the development of novel nanotherapeutics. Nanomedicine 2015, 10, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Won, N.; Jin, H.; Chung, H.; Kim, S. pH-Induced Aggregation of Gold Nanoparticles for Photothermal Cancer Therapy. J. Am. Chem. Soc. 2009, 131, 13639–13645. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Bachelet, M. Design of pH-responsive gold nanoparticles in oncology. Mater. Sci. Technol. 2016, 32, 794–804. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, T.R. Thermo-and pH-responsive hydrogel-coated gold nanoparticles. Chem. Mater. 2004, 16, 3647–3651. [Google Scholar] [CrossRef]
- Laaksonen, T.; Ahonen, P.; Johans, C.; Kontturi, K. Stability and Electrostatics of Mercaptoundecanoic Acid-Capped Gold Nanoparticles with Varying Counterion Size. ChemPhysChem 2006, 7, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, W.J.; Park, S.; Choi, D.; Kim, S.; Park, N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci. Rep. 2019, 9, 20180. [Google Scholar] [CrossRef]
- Pillai, P.P.; Huda, S.; Kowalczyk, B.; Grzybowski, B.A. Controlled pH Stability and Adjustable Cellular Uptake of Mixed-Charge Nanoparticles. J. Am. Chem. Soc. 2013, 135, 6392–6395. [Google Scholar] [CrossRef]
- Su, C.-H.; Wu, P.-L.; Yeh, C.-S. pH Dependence of Interparticle Coupling for Gold Nanoparticle Assemblies Formation: Electrostatic Attraction and Hydrogen Bonding. Bull. Chem. Soc. Jpn. 2004, 77, 189–193. [Google Scholar] [CrossRef]
- Taladriz-Blanco, P.; Buurma, N.J.; Rodríguez-Lorenzo, L.; Pérez-Juste, J.; Liz-Marzán, L.M.; Hervés, P. Reversible assembly of metal nanoparticles induced by penicillamine. Dynamic formation of SERS hot spots. J. Mater. Chem. 2011, 21, 16880–16887. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Bian, T.; Shang, L.; Shi, R.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. pH-Responsive reversible self-assembly of gold nanoparticles into nanovesicles. Nanoscale 2016, 8, 3923–3925. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Arakawa, D.; Toshima, N. pH-dependent color change of colloidal dispersions of gold nanoclusters: Effect of stabilizer. Eur. Phys. J. E Soft Matter 2002, 8, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Dennyson Savariraj, A.; Salih, A.; Alam, F.; Elsherif, M.; AlQattan, B.; Khan, A.A.; Yetisen, A.K.; Butt, H. Ophthalmic Sensors and Drug Delivery. ACS Sens. 2021, 6, 2046–2076. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, M.; Alam, F.; Salih, A.E.; AlQattan, B.; Yetisen, A.K.; Butt, H. Wearable Bifocal Contact Lens for Continual Glucose Monitoring Integrated with Smartphone Readers. Small 2021, 17, 2102876. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable Contact Lens Biosensors for Continuous Glucose Monitoring Using Smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Glucose Sensing with Phenylboronic Acid Functionalized Hydrogel-Based Optical Diffusers. ACS Nano 2018, 12, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Salih, A.E.; Elsherif, M.; Yetisen, A.K.; Butt, H. 3D printed contact lenses for the management of color blindness. Addit. Manuf. 2021, 49, 102464. [Google Scholar] [CrossRef]
- Elsherif, M.; Salih, A.E.; Yetisen, A.K.; Butt, H. Contact Lenses for Color Vision Deficiency. Adv. Mater. Technol. 2021, 6, 2000797. [Google Scholar] [CrossRef]
- Salih, A.E.; Elsherif, M.; Ali, M.; Vahdati, N.; Yetisen, A.K.; Butt, H. Ophthalmic Wearable Devices for Color Blindness Management. Adv. Mater. Technol. 2020, 5, 1901134. [Google Scholar] [CrossRef]
- Salih, A.E.; Elsherif, M.; Alam, F.; Yetisen, A.K.; Butt, H. Gold Nanocomposite Contact Lenses for Color Blindness Management. ACS Nano 2021, 15, 4870–4880. [Google Scholar] [CrossRef]
- Salih, A.E.; Shanti, A.; Elsherif, M.; Alam, F.; Lee, S.; Polychronopoulou, K.; Almaskari, F.; Al Safar, H.; Yetisen, A.K.; Butt, H. Silver Nanoparticle-Loaded Contact Lenses for Blue-Yellow Color Vision Deficiency. Phys. Status Solidi (A) 2022, 219, 2100294. [Google Scholar] [CrossRef]
- Sheeney-Haj-Ichia, L.; Sharabi, G.; Willner, I. Control of the Electronic Properties of Thermosensitive Poly(N-isopropylacrylamide) and Au-Nano-particle/Poly(N-isopropylacrylamide) Composite Hydrogels upon Phase Transition. Adv. Funct. Mater. 2002, 12, 27–32. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S. Breathing-in/breathing-out approach to preparing nanosilver-loaded hydrogels: Highly efficient antibacterial nanocomposites. J. Appl. Polym. Sci. 2009, 111, 934–944. [Google Scholar] [CrossRef]
- Guo, Y.G.; Hu, J.-S.; Liang, H.-P.; Wan, L.-J.; Bai, C.-L. Highly Dispersed Metal Nanoparticles in Porous Anodic Alumina Films Prepared by a Breathing Process of Polyacrylamide Hydrogel. Chem. Mater. 2003, 15, 4332–4336. [Google Scholar] [CrossRef]
- Salih, A.E.; Elsherif, M.; Alam, F.; Alqattan, B.; Yetisen, A.K.; Butt, H. Syntheses of Gold and Silver Nanocomposite Contact Lenses via Chemical Volumetric Modulation of Hydrogels. ACS Biomater. Sci. Eng. 2022, in press.
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Kunitake, T. Practical preparation of anionic mercapto ligand-stabilized gold nanoparticles and their immobilization. Colloids Surf. A Physicochem. Eng. Asp. 1999, 149, 193–199. [Google Scholar] [CrossRef]
- Simard, J.; Briggs, C.; Boal, A.K.; Rotello, V.M. Formation and pH-controlled assembly of amphiphilic gold nanoparticles. Chem. Commun. 2000, 1943–1944. [Google Scholar] [CrossRef]
- Pardo-Yissar, V.; Gabai, R.; Shipway, A.N.; Bourenko, T.; Willner, I. Gold Nanoparticle/Hydrogel Composites with Solvent-Switchable Electronic Properties. Adv. Mater. 2001, 13, 1320–1323. [Google Scholar] [CrossRef]
- Dent, J. Roles of gastric acid and pH in the pathogenesis of gastro-oesophageal reflux disease. Scand. J. Gastroenterol. Suppl. 1994, 201, 55–61. [Google Scholar] [CrossRef]
- Christiansen, P.M. The Incidence of Achlorhydria and Hypochlorhydria in Healthy Subjects and Patients with Gastrointestinal Diseases. Scand. J. Gastroenterol. 1968, 3, 497–508. [Google Scholar] [CrossRef]
- Negrini, R.; Mezzenga, R. pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir ACS J. Surf. Colloids 2011, 27, 5296–5303. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, W.; Oscai, M.; Ziaie, B. A Smart Capsule with a Hydrogel-Based pH-Triggered Release Switch for GI-Tract Site-Specific Drug Delivery. IEEE Trans. Biomed. Eng. 2018, 65, 2808–2813. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, A.E.; Elsherif, M.; Alam, F.; Chiesa, M.; Butt, H. Rapid Colorimetric pH-Responsive Gold Nanocomposite Hydrogels for Sensing Applications. Nanomaterials 2022, 12, 1486. https://doi.org/10.3390/nano12091486
Salih AE, Elsherif M, Alam F, Chiesa M, Butt H. Rapid Colorimetric pH-Responsive Gold Nanocomposite Hydrogels for Sensing Applications. Nanomaterials. 2022; 12(9):1486. https://doi.org/10.3390/nano12091486
Chicago/Turabian StyleSalih, Ahmed E., Mohamed Elsherif, Fahad Alam, Matteo Chiesa, and Haider Butt. 2022. "Rapid Colorimetric pH-Responsive Gold Nanocomposite Hydrogels for Sensing Applications" Nanomaterials 12, no. 9: 1486. https://doi.org/10.3390/nano12091486
APA StyleSalih, A. E., Elsherif, M., Alam, F., Chiesa, M., & Butt, H. (2022). Rapid Colorimetric pH-Responsive Gold Nanocomposite Hydrogels for Sensing Applications. Nanomaterials, 12(9), 1486. https://doi.org/10.3390/nano12091486







