Multimodality Imaging to Detect Rejection, and Cardiac Allograft Vasculopathy in Pediatric Heart Transplant Recipients—An Illustrative Review
Abstract
:1. Introduction
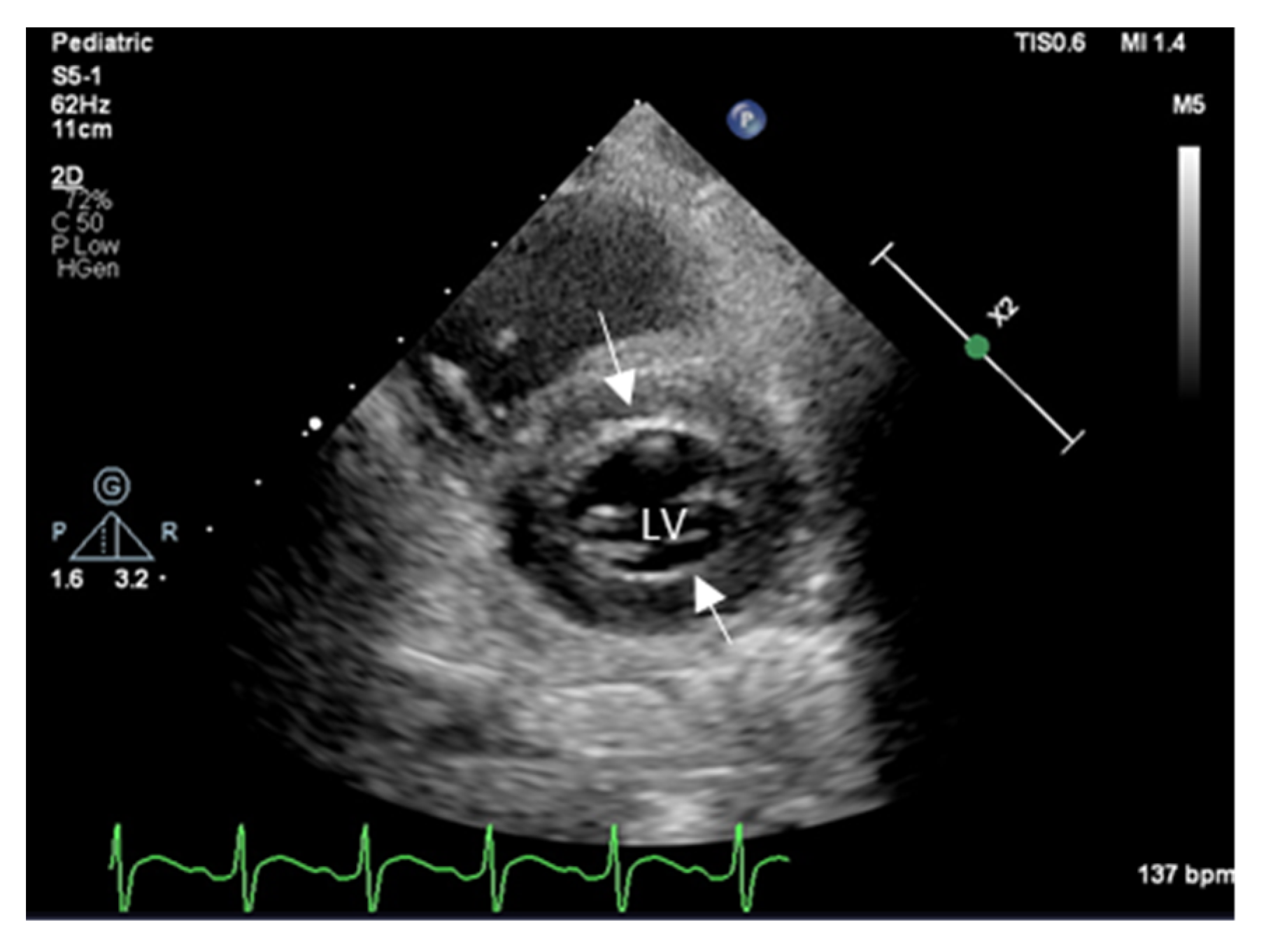
2. Echocardiography
3. Myocardial Deformation Imaging
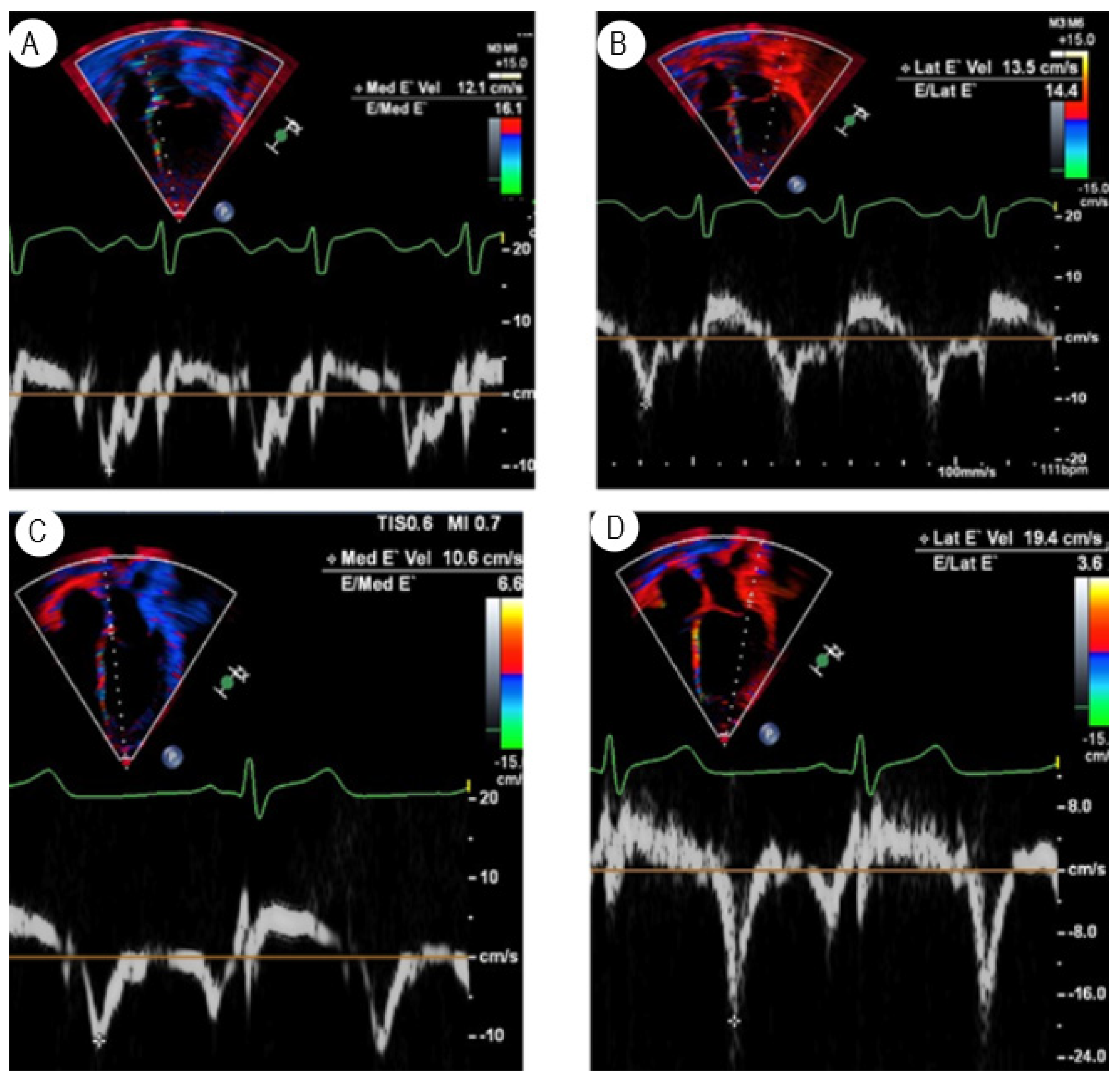
3.1. Tissue Doppler Imaging
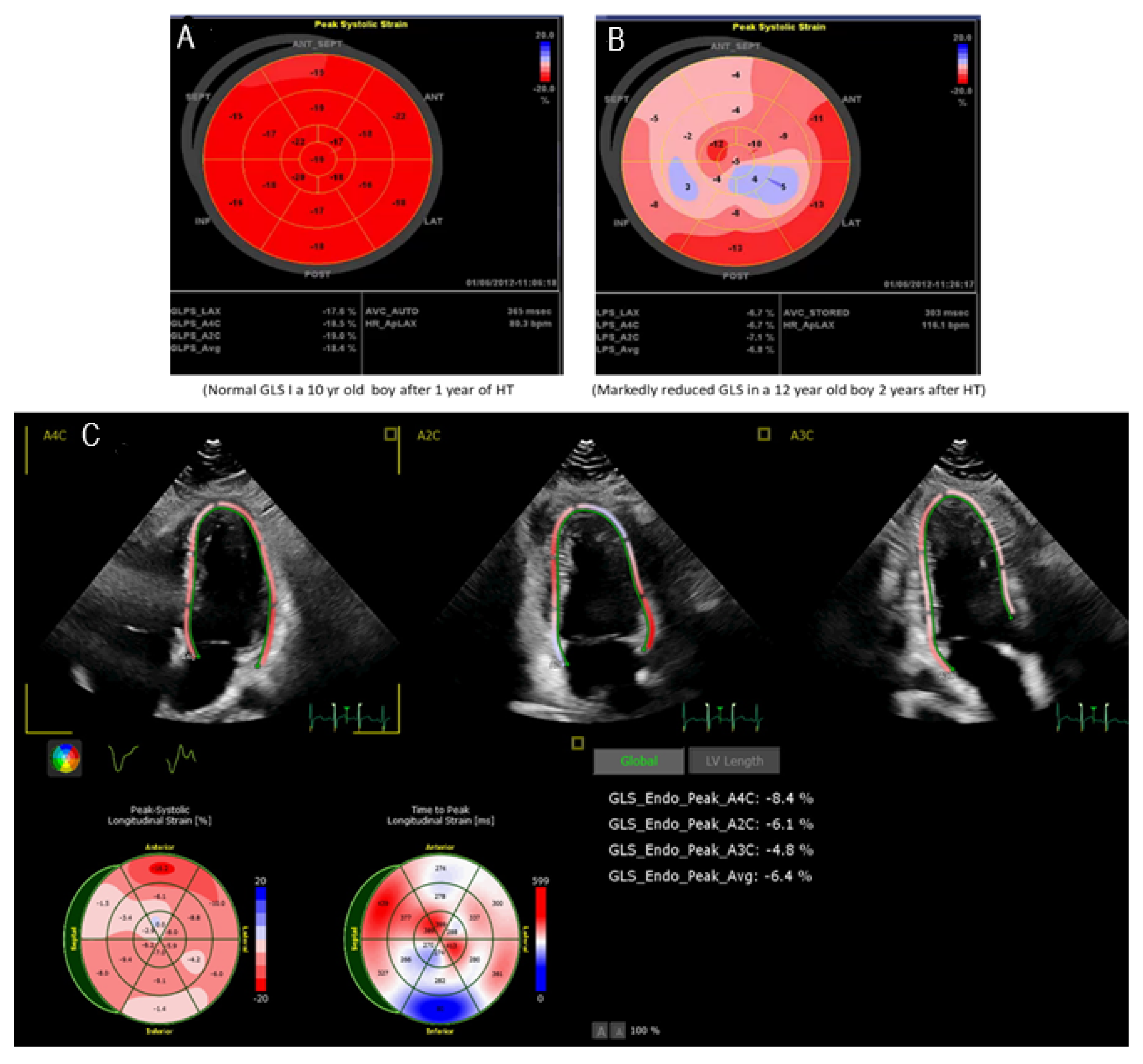
3.2. Speckle Tracking Echocardiography
3.3. Stress Echocardiography
4. Computed Tomography Coronary Angiogram
5. CMR Imaging
Adenosine Stress Perfusion Cardiac Magnetic Resonance Imaging
6. Intracoronary Imaging
6.1. Intravascular Ultrasound
6.2. Coronary Flow Reserve
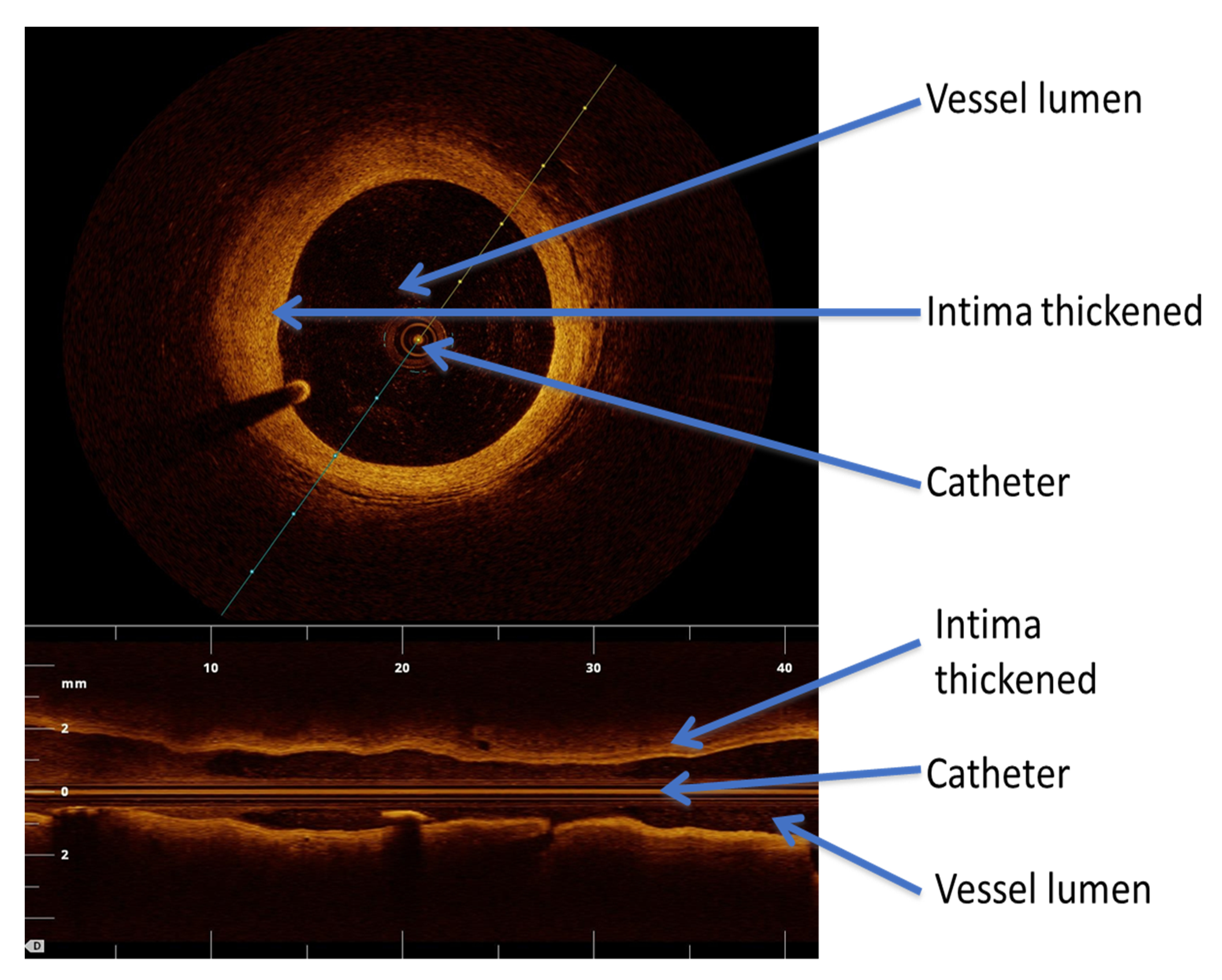
6.3. Optical Coherence Tomography
7. Single-Photon Emission Computed Tomography
8. Positron Emission Tomography
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanderlaan, R.D.; Manlhiot, C.; Edwards, L.B.; Conway, J.; McCrindle, B.W.; Dipchand, A.I. Risk factors for specific causes of death following pediatric heart transplant: An analysis of the registry of the International Society of Heart and Lung Transplantation. Pediatr. Transpl. 2015, 19, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Almond, C.S.; Hoen, H.; Rossano, J.W.; Castleberry, C.; Auerbach, S.R.; Yang, L.; Lal, A.K.; Everitt, M.D.; Fenton, M.; Hollander, S.A.; et al. Development and validation of a major adverse transplant event (MATE) score to predict late graft loss in pediatric heart transplantation. J. Heart Lung Transpl. 2018, 37, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J. Heart Lung Transpl. 2010, 29, 914–956. [Google Scholar] [CrossRef] [PubMed]
- Pophal, S.G.; Sigfusson, G.; Booth, K.L.; Bacanu, S.A.; Webber, S.A.; Ettedgui, J.A.; Neches, W.H.; Park, S.C. Complications of endomyocardial biopsy in children. J. Am. Coll Cardiol. 1999, 34, 2105–2110. [Google Scholar] [CrossRef] [Green Version]
- Fyfe, D.A.; Ketchum, D.; Lewis, R.; Sabatier, J.; Kanter, K.; Mahle, W.; Vincent, R. Tissue Doppler imaging detects severely abnormal myocardial velocities that identify children with pre-terminal cardiac graft failure after heart transplantation. J. Heart Lung Transpl. 2006, 25, 510–517. [Google Scholar] [CrossRef]
- Godown, J.; McEachern, W.A.; Dodd, D.A.; Stanley, M.; Havens, C.; Xu, M.; Slaughter, J.C.; Bearl, D.W.; Soslow, J.H. Temporal changes in left ventricular strain with the development of rejection in paediatric heart transplant recipients. Cardiol. Young 2019, 29, 954–959. [Google Scholar] [CrossRef]
- Godown, J.; Cantor, R.; Koehl, D.; Cummings, E.; Vo, J.B.; Dodd, D.A.; Lytrivi, I.; Boyle, G.J.; Sutcliffe, D.L.; Kleinmahon, J.A.; et al. Practice variation in the diagnosis of acute rejection among pediatric heart transplant centers: An analysis of the pediatric heart transplant society (PHTS) registry. J. Heart Lung Transpl. 2021, 40, 1550–1559. [Google Scholar] [CrossRef]
- Asante-Korang, A.; Fickey, M.; Boucek, M.M.; Boucek, R.J., Jr. Diastolic performance assessed by tissue Doppler after pediatric heart transplantation. J. Heart Lung Transpl. 2004, 23, 865–872. [Google Scholar] [CrossRef]
- Giacomin, E.; Gasperini, S.; Zaca, V.; Ballo, P.; Diciolla, F.; Bernazzali, S.; Maccherini, M.; Galderisi, M.; Chiavarelli, M.; Mondillo, S. Relationship between coronary microcirculatory dysfunction and left ventricular long-axis function in heart transplant recipients. J. Heart Lung Transpl. 2007, 26, 1349–1350. [Google Scholar] [CrossRef]
- Mondillo, S.; Maccherini, M.; Galderisi, M. Usefulness and limitations of transthoracic echocardiography in heart transplantation recipients. Cardiovasc. Ultrasound 2008, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Badano, L.P.; Miglioranza, M.H.; Edvardsen, T.; Colafranceschi, A.S.; Muraru, D.; Bacal, F.; Nieman, K.; Zoppellaro, G.; Marcondes Braga, F.G.; Binder, T.; et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur. Heart J. Cardiovasc. Imag. 2015, 16, 919–948. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Butts, R.J.; Hlavacek, A.M.; Taylor, C.L.; Chessa, K.S.; Bandisode, V.M.; Shirali, G.S.; Nutting, A.; Baker, G.H. Echocardiographic Detection of Increased Ventricular Diastolic Stiffness in Pediatric Heart Transplant Recipients: A Pilot Study. J. Am. Soc. Echocardiogr. 2018, 31, 342–348.e1. [Google Scholar] [CrossRef] [PubMed]
- Eun, L.Y.; Gajarski, R.J.; Graziano, J.N.; Ensing, G.J. Relation of left ventricular diastolic function as measured by echocardiography and pulmonary capillary wedge pressure to rejection in young patients (< or = 31 years) after heart transplantation. Am. J. Cardiol. 2005, 96, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Pauliks, L.B.; Pietra, B.A.; DeGroff, C.G.; Kirby, K.S.; Knudson, O.A.; Logan, L.; Boucek, M.M.; Valdes-Cruz, L.M. Non-invasive detection of acute allograft rejection in children by tissue Doppler imaging: Myocardial velocities and myocardial acceleration during isovolumic contraction. J. Heart Lung Transpl. 2005, 24, S239–S248. [Google Scholar] [CrossRef] [PubMed]
- Eidem, B.W.; McMahon, C.J.; Cohen, R.R.; Wu, J.; Finkelshteyn, I.; Kovalchin, J.P.; Ayres, N.A.; Bezold, L.I.; O’Brian Smith, E.; Pignatelli, R.H. Impact of cardiac growth on Doppler tissue imaging velocities: A study in healthy children. J. Am. Soc. Echocardiogr. 2004, 17, 212–221. [Google Scholar] [CrossRef]
- Behera, S.K.; Trang, J.; Feeley, B.T.; Levi, D.S.; Alejos, J.C.; Drant, S. The use of Doppler tissue imaging to predict cellular and antibody-mediated rejection in pediatric heart transplant recipients. Pediatr. Transpl. 2008, 12, 207–214. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Quartermain, M.D.; Glatz, A.C.; Hall, E.K.; Davis, E.; Kren, S.A.; Hanna, B.D.; Cohen, M.S. Doppler tissue imaging in children following cardiac transplantation: A comparison to catheter derived hemodynamics. Pediatr. Transpl. 2011, 15, 488–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdeva, R.; Malik, S.; Seib, P.M.; Frazier, E.A.; Cleves, M.A. Doppler tissue imaging and catheter-derived measures are not independent predictors of rejection in pediatric heart transplant recipients. Int. J. Cardiovasc. Imag. 2011, 27, 947–954. [Google Scholar] [CrossRef]
- Lunze, F.I.; Singh, T.P.; Gauvreau, K.; Molloy, M.A.; Blume, E.D.; Berger, F.; Colan, S.D. Comparison of tissue Doppler imaging and conventional echocardiography to discriminate rejection from non-rejection after pediatric heart transplantation. Pediatr. Transpl. 2020, 24, e13738. [Google Scholar] [CrossRef]
- Hernandez, L.E.; Shepard, C.W.; Menk, J.; Lilliam, V.C.; Ameduri, R.K. Global left ventricular relaxation: A novel tissue Doppler index of acute rejection in pediatric heart transplantation. J. Heart Lung Transpl. 2015, 34, 1190–1197. [Google Scholar] [CrossRef]
- Hernandez, L.E.; Chrisant, M.K.; Valdes-Cruz, L.M. Global Left Ventricular Relaxation: A Useful Echocardiographic Marker of Heart Transplant Rejection and Recovery in Children. J. Am. Soc. Echocardiogr. 2019, 32, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Myers, C.; Negishi, K.; Singh, G.K.; Anwar, S. Two-Dimensional Strain is more Precise than Conventional Measures of Left Ventricular Systolic Function in Pediatric Patients. Pediatr. Cardiol. 2020, 41, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Butts, R.J.; Taylor, C.L.; Bandisode, V.M.; Chessa, K.S.; Hlavacek, A.M.; Nutting, A.; Shirali, G.S.; Baker, G.H. Longitudinal measures of deformation are associated with a composite measure of contractility derived from pressure-volume loop analysis in children. Eur. Heart J. Cardiovasc. Imag. 2018, 19, 562–568. [Google Scholar] [CrossRef]
- Goudar, S.P.; Baker, G.H.; Chowdhury, S.M.; Reid, K.J.; Shirali, G.; Scheurer, M.A. Interpreting measurements of cardiac function using vendor-independent speckle tracking echocardiography in children: A prospective, blinded comparison with catheter-derived measurements. Echocardiography 2016, 33, 1903–1910. [Google Scholar] [CrossRef] [Green Version]
- Parthiban, A.; Jani, V.; Zhang, J.; Li, L.; Craft, M.; Barnes, A.; Ballweg, J.A.; Schuster, A.; Danford, D.A.; Kutty, S. Altered Biatrial Phasic Function after Heart Transplantation in Children. J. Am. Soc. Echocardiogr. 2020, 33, 1132–1140.e1132. [Google Scholar] [CrossRef]
- Engelhardt, K.; Das, B.; Sorensen, M.; Malik, S.; Zellers, T.; Lemler, M. Two-dimensional systolic speckle tracking echocardiography provides a noninvasive aid in the identification of acute pediatric heart transplant rejection. Echocardiography 2019, 36, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Boucek, K.; Burnette, A.; Henderson, H.; Savage, A.; Chowdhury, S.M. Changes in circumferential strain can differentiate pediatric heart transplant recipients with and without graft rejection. Pediatr. Transpl. 2022, 26, e14195. [Google Scholar] [CrossRef] [PubMed]
- Wisotzkey, B.L.; Jorgensen, N.W.; Albers, E.L.; Kemna, M.S.; Boucek, R.J.; Kronmal, R.A.; Law, Y.M.; Bhat, A.H. Feasibility and interpretation of global longitudinal strain imaging in pediatric heart transplant recipients. Pediatr. Transpl. 2017, 21, 12909. [Google Scholar] [CrossRef]
- Antonczyk, K.; Niklewski, T.; Antonczyk, R.; Zakliczynski, M.; Zembala, M.; Kukulski, T. Speckle-Tracking Echocardiography for Monitoring Acute Rejection in Transplanted Heart. Transpl. Proc. 2018, 50, 2090–2094. [Google Scholar] [CrossRef]
- Gursu, H.A.; Varan, B.; Sade, E.; Erdogan, I.; Sezgin, A.; Aslamaci, S. Evaluation of Acute Rejection by Measuring Strain and Strain Rate in Children With Heart Transplant: A Preliminary Report. Exp. Clin. Transpl. 2017, 15, 561–566. [Google Scholar] [CrossRef]
- Buddhe, S.; Richmond, M.E.; Gilbreth, J.; Lai, W.W. Longitudinal Strain by Speckle Tracking Echocardiography in Pediatric Heart Transplant Recipients. Congenit. Heart Dis. 2015, 10, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.; Aiyagari, R.; Gajarski, R.J.; Zamberlan, M.C.; Lu, J.C. Left atrial deformation predicts pulmonary capillary wedge pressure in pediatric heart transplant recipients. Echocardiography 2015, 32, 535–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boruta, R.J.; Miyamoto, S.D.; Younoszai, A.K.; Patel, S.S.; Landeck, B.F., 2nd. Worsening in Longitudinal Strain and Strain Rate Anticipates Development of Pediatric Transplant Coronary Artery Vasculopathy as Soon as One Year Following Transplant. Pediatr. Cardiol. 2018, 39, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.P.; Human, D.G.; Sandor, G.G.; De Souza, A.M.; Potts, J.E. Serial measurements of exercise performance in pediatric heart transplant patients using stress echocardiography. Pediatr. Transpl. 2011, 15, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Pahl, E.; Crawford, S.E.; Swenson, J.M.; Duffy, C.E.; Fricker, F.J.; Backer, C.L.; Mavroudis, C.; Chaudhry, F.A. Dobutamine stress echocardiography: Experience in pediatric heart transplant recipients. J. Heart Lung Transpl. 1999, 18, 725–732. [Google Scholar] [CrossRef]
- Dipchand, A.I.; Bharat, W.; Manlhiot, C.; Safi, M.; Lobach, N.E.; McCrindle, B.W. A prospective study of dobutamine stress echocardiography for the assessment of cardiac allograft vasculopathy in pediatric heart transplant recipients. Pediatr. Transpl. 2008, 12, 570–576. [Google Scholar] [CrossRef]
- Fine, N.M.; Greenway, S.C.; Mulvagh, S.L.; Huang, R.Q.; Maxon, S.A.; Hepinstall, M.J.; Anderson, J.H.; Johnson, J.N. Feasibility of Real-Time Myocardial Contrast Echocardiography to Detect Cardiac Allograft Vasculopathy in Pediatric Heart Transplant Recipients. J. Am. Soc. Echocardiog. 2021, 34, 503–510. [Google Scholar] [CrossRef]
- Larsen, R.L.; Applegate, P.M.; Dyar, D.A.; Ribeiro, P.A.; Fritzsche, S.D.; Mulla, N.F.; Shirali, G.S.; Kuhn, M.A.; Chinnock, R.E.; Shah, P.M. Dobutamine stress echocardiography for assessing coronary artery disease after transplantation in children. J. Am. Coll Cardiol. 1998, 32, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Di Filippo, S.; Semiond, B.; Roriz, R.; Sassolas, F.; Raboisson, M.J.; Bozio, A. Non-invasive detection of coronary artery disease by dobutamine-stress echocardiography in children after heart transplantation. J. Heart Lung Transpl. 2003, 22, 876–882. [Google Scholar] [CrossRef]
- Cifra, B.; Morgan, C.T.; Dragulescu, A.; Guerra, V.C.; Slorach, C.; Friedberg, M.K.; Manlhiot, C.; McCrindle, B.W.; Dipchand, A.I.; Mertens, L. Right ventricular function during exercise in children after heart transplantation. Eur. Heart J. Cardiovasc. Imag. 2018, 19, 647–653. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Eiskjaer, H.; Logstrup, B.B.; Tolbod, L.P.; Harms, H.J.; Bouchelouche, K.; Hoff, C.; Frokiaer, J.; Poulsen, S.H. Noninvasive Detection of Cardiac Allograft Vasculopathy by Stress Exercise Echocardiographic Assessment of Myocardial Deformation. J. Am. Soc. Echocardiogr. 2016, 29, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Nous, F.M.A.; Roest, S.; van Dijkman, E.D.; Attrach, M.; Caliskan, K.; Brugts, J.J.; Nieman, K.; Hirsch, A.; Constantinescu, A.A.; Manintveld, O.C.; et al. Clinical implementation of coronary computed tomography angiography for routine detection of cardiac allograft vasculopathy in heart transplant patients. Transpl. Int. 2021, 34, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- Vermes, E.; Pantaleon, C.; Auvet, A.; Cazeneuve, N.; Machet, M.C.; Delhommais, A.; Bourguignon, T.; Aupart, M.; Brunereau, L. Cardiovascular magnetic resonance in heart transplant patients: Diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J. Cardiovasc. Magn. Reson. 2018, 20, 59. [Google Scholar] [CrossRef] [Green Version]
- Ide, S.; Riesenkampff, E.; Chiasson, D.A.; Dipchand, A.I.; Kantor, P.F.; Chaturvedi, R.R.; Yoo, S.J.; Grosse-Wortmann, L. Histological validation of cardiovascular magnetic resonance T1 mapping markers of myocardial fibrosis in paediatric heart transplant recipients. J. Cardiovasc. Magn. Reson. 2017, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aherne, T.; Tscholakoff, D.; Finkbeiner, W.; Sechtem, U.; Derugin, N.; Yee, E.; Higgins, C.B. Magnetic resonance imaging of cardiac transplants: The evaluation of rejection of cardiac allografts with and without immunosuppression. Circulation 1986, 74, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Sada, M.; Nishimura, T.; Yutani, C.; Nakatani, H.; Yaku, H.; Yamaguchi, T.; Kawazoe, K.; Amemiya, H.; Fujita, T. The expanded scope of effectiveness of nuclear magnetic resonance imaging to determine cardiac allograft rejection. Transpl. Proc. 1987, 19, 1062–1064. [Google Scholar]
- Wisenberg, G.; Pflugfelder, P.W.; Kostuk, W.J.; McKenzie, F.N.; Prato, F.S. Diagnostic applicability of magnetic resonance imaging in assessing human cardiac allograft rejection. Am. J. Cardiol. 1987, 60, 130–136. [Google Scholar] [CrossRef]
- Dedieu, N.; Silva Vieira, M.; Fenton, M.; Wong, J.; Botnar, R.; Burch, M.; Greil, G.; Hussain, T. The importance of qualitative and quantitative regional wall motion abnormality assessment at rest in pediatric coronary allograft vasculopathy. Pediatr. Transpl. 2018, 22, e13208. [Google Scholar] [CrossRef]
- Feingold, B.; Salgado, C.M.; Reyes-Mugica, M.; Drant, S.E.; Miller, S.A.; Kennedy, M.; Kellman, P.; Schelbert, E.B.; Wong, T.C. Diffuse myocardial fibrosis among healthy pediatric heart transplant recipients: Correlation of histology, cardiovascular magnetic resonance, and clinical phenotype. Pediatr. Transpl. 2017, 21, 12986. [Google Scholar] [CrossRef]
- Husain, N.; Watanabe, K.; Berhane, H.; Gupta, A.; Markl, M.; Rigsby, C.K.; Robinson, J.D. Multi-parametric cardiovascular magnetic resonance with regadenoson stress perfusion is safe following pediatric heart transplantation and identifies history of rejection and cardiac allograft vasculopathy. J. Cardiovasc. Magn. Reson. 2021, 23, 135. [Google Scholar] [CrossRef] [PubMed]
- Simsek, E.; Nalbantgil, S.; Ceylan, N.; Zoghi, M.; Kemal, H.S.; Engin, C.; Yagdi, T.; Ozbaran, M. Diagnostic performance of late gadolinium enhancement in the assessment of acute cellular rejection after heart transplantation. Anatol. J. Cardiol. 2016, 16, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.S.; Rahsepar, A.A.; Blaisdell, J.; Suwa, K.; Ghafourian, K.; Wilcox, J.E.; Khan, S.S.; Vorovich, E.E.; Rich, J.D.; Anderson, A.S.; et al. Multiparametric Cardiac Magnetic Resonance Imaging Can Detect Acute Cardiac Allograft Rejection After Heart Transplantation. JACC Cardiovasc. Imag. 2019, 12, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.; Doshi, A.; Doshi, T.; Cross, R.; Cronin, I.; Amin, E.; Kanter, J.; Scheel, J.; Khan, S.; Campbell-Washburn, A.; et al. Quantitative cardiac magnetic resonance T2 imaging offers ability to non-invasively predict acute allograft rejection in children. Cardiol. Young 2020, 30, 852–859. [Google Scholar] [CrossRef]
- Greenway, S.C.; Dallaire, F.; Kantor, P.F.; Dipchand, A.I.; Chaturvedi, R.R.; Warade, M.; Riesenkampff, E.; Yoo, S.J.; Grosse-Wortmann, L. Magnetic resonance imaging of the transplanted pediatric heart as a potential predictor of rejection. World J. Transpl. 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Scannell, C.M.; Hasaneen, H.; Greil, G.; Hussain, T.; Razavi, R.; Lee, J.; Pushparajah, K.; Duong, P.; Chiribiri, A. Automated Quantitative Stress Perfusion Cardiac Magnetic Resonance in Pediatric Patients. Front. Pediatr. 2021, 9, 699497. [Google Scholar] [CrossRef]
- Miller, C.A.; Sarma, J.; Naish, J.H.; Yonan, N.; Williams, S.G.; Shaw, S.M.; Clark, D.; Pearce, K.; Stout, M.; Potluri, R.; et al. Multiparametric cardiovascular magnetic resonance assessment of cardiac allograft vasculopathy. J. Am. Coll Cardiol. 2014, 63, 799–808. [Google Scholar] [CrossRef]
- Duran, S.R.; Huffaker, T.; Dixon, B.; Gooty, V.; Abou Zahr, R.; Arar, Y.; Greer, J.S.; Butts, R.J.; Hussain, M.T. Feasibility and safety of quantitative adenosine stress perfusion cardiac magnetic resonance imaging in pediatric heart transplant patients with and without coronary allograft vasculopathy. Pediatr. Radiol. 2021, 51, 1311–1321. [Google Scholar] [CrossRef]
- Rickenbacher, P.R.; Pinto, F.J.; Chenzbraun, A.; Botas, J.; Lewis, N.P.; Alderman, E.L.; Valantine, H.A.; Hunt, S.A.; Schroeder, J.S.; Popp, R.L.; et al. Incidence and severity of transplant coronary artery disease early and up to 15 years after transplantation as detected by intravascular ultrasound. J. Am. Coll Cardiol. 1995, 25, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Kobashigawa, J.A.; Tobis, J.M.; Starling, R.C.; Tuzcu, E.M.; Smith, A.L.; Valantine, H.A.; Yeung, A.C.; Mehra, M.R.; Anzai, H.; Oeser, B.T.; et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: Outcomes after five years. J. Am. Coll Cardiol. 2005, 45, 1532–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potena, L.; Masetti, M.; Sabatino, M.; Bacchi-Reggiani, M.L.; Pece, V.; Prestinenzi, P.; Dall’Ara, G.; Taglieri, N.; Saia, F.; Fallani, F.; et al. Interplay of coronary angiography and intravascular ultrasound in predicting long-term outcomes after heart transplantation. J. Heart Lung Transpl. 2015, 34, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Kindel, S.J.; Pahl, E. Current therapies for cardiac allograft vasculopathy in children. Congenit. Heart Dis. 2012, 7, 324–335. [Google Scholar] [CrossRef]
- Nicolas, R.T.; Kort, H.W.; Balzer, D.T.; Trinkaus, K.; Dent, C.L.; Hirsch, R.; Canter, C.E. Surveillance for transplant coronary artery disease in infant, child and adolescent heart transplant recipients: An intravascular ultrasound study. J. Heart Lung Transpl. 2006, 25, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.A.; Jutzy, K.R.; Deming, D.D.; Cephus, C.E.; Chinnock, R.E.; Johnston, J.; Bailey, L.L.; Larsen, R.L. The medium-term findings in coronary arteries by intravascular ultrasound in infants and children after heart transplantation. J. Am. Coll Cardiol. 2000, 36, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Spaan, J.A.; Piek, J.J.; Hoffman, J.I.; Siebes, M. Physiological basis of clinically used coronary hemodynamic indices. Circulation 2006, 113, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Abdul-Khaliq, H.; Wellnhofer, E.; Hiemann, N.E.; Ewert, P.; Lehmkuhl, H.B.; Meyer, R.; Miera, O.; Peters, B.; Hetzer, R.; et al. Coronary flow reserve measurement detects transplant coronary artery disease in pediatric heart transplant patients. J. Heart Lung Transpl. 2008, 27, 514–521. [Google Scholar] [CrossRef]
- Hiraishi, S.; Hirota, H.; Horiguchi, Y.; Takeda, N.; Fujino, N.; Ogawa, N.; Nakahata, Y. Transthoracic Doppler assessment of coronary flow velocity reserve in children with Kawasaki disease: Comparison with coronary angiography and thallium-201 imaging. J. Am. Coll Cardiol. 2002, 40, 1816–1824. [Google Scholar] [CrossRef] [Green Version]
- Pinto, T.L.; Waksman, R. Clinical applications of optical coherence tomography. J. Interv. Cardiol. 2006, 19, 566–573. [Google Scholar] [CrossRef]
- McGovern, E.; Hosking, M.C.K.; Balbacid, E.; Voss, C.; Berger, F.; Schubert, S.; Harris, K.C. Optical Coherence Tomography for the Early Detection of Coronary Vascular Changes in Children and Adolescents After Cardiac Transplantation: Findings From the International Pediatric OCT Registry. JACC Cardiovasc. Imag. 2019, 12, 2492–2501. [Google Scholar] [CrossRef]
- Lague, S.L.; Bone, J.N.; Samuel, R.; Voss, C.; Balbacid, E.; Hosking, M.C.K.; Schubert, S.; Harris, K.C. Patterns of Early Coronary Artery Changes in Pediatric Heart Transplant Recipients Detected Using Optical Coherence Tomography. Circ Cardiovasc. Imag. 2022, 15, e012486. [Google Scholar] [CrossRef]
- Tomai, F.; De Luca, L.; Petrolini, A.; Di Vito, L.; Ghini, A.S.; Corvo, P.; De Persio, G.; Parisi, F.; Pongiglione, G.; Giulia Gagliardi, M.; et al. Optical coherence tomography for characterization of cardiac allograft vasculopathy in late survivors of pediatric heart transplantation. J. Heart Lung Transpl. 2016, 35, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Dedieu, N.; Greil, G.; Wong, J.; Fenton, M.; Burch, M.; Hussain, T. Diagnosis and management of coronary allograft vasculopaathy in children and adolescents. World J. Transpl. 2014, 4, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.; Hayes, S.W.; Hartigan, P.M.; Weintraub, W.S.; O’Rourke, R.A.; Dada, M.; Spertus, J.A.; et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008, 117, 1283–1291. [Google Scholar] [CrossRef] [Green Version]
- Klocke, F.J.; Baird, M.G.; Lorell, B.H.; Bateman, T.M.; Messer, J.V.; Berman, D.S.; O’Gara, P.T.; Carabello, B.A.; Russell, R.O., Jr.; Cerqueira, M.D.; et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 2003, 108, 1404–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, J.; Miller, R.J.H.; Otaki, Y.; Tamarappoo, B.; Hayes, S.; Friedman, J.; Slomka, P.J.; Thomson, L.E.J.; Kittleson, M.; Patel, J.K.; et al. Clinical Utility of SPECT in the Heart Transplant Population: Analysis From a Single Large-volume Center. Transplantation 2022, 106, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.S.; Sayed, A.O.; Al Said, Y.M. Assessment of coronary ischaemia by myocardial perfusion dipyridamole stress technetium-99 m tetrofosmin, single-photon emission computed tomography, and coronary angiography in children with Kawasaki disease: Pre- and post-coronary bypass grafting. Cardiol. Young 2015, 25, 927–934. [Google Scholar] [CrossRef]
- Maiers, J.; Hurwitz, R. Identification of coronary artery disease in the pediatric cardiac transplant patient. Pediatr. Cardiol. 2008, 29, 19–23. [Google Scholar] [CrossRef]
- Singh, T.P.; Muzik, O.; Forbes, T.F.; Di Carli, M.F. Positron emission tomography myocardial perfusion imaging in children with suspected coronary abnormalities. Pediatr. Cardiol. 2003, 24, 138–144. [Google Scholar] [CrossRef]
- Daly, K.P.; Dearling, J.L.; Seto, T.; Dunning, P.; Fahey, F.; Packard, A.B.; Briscoe, D.M. Use of [18F]FDG Positron Emission Tomography to Monitor the Development of Cardiac Allograft Rejection. Transplantation 2015, 99, e132–e139. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.B.; Deshpande, S.; Hussain, T. Multimodality Imaging to Detect Rejection, and Cardiac Allograft Vasculopathy in Pediatric Heart Transplant Recipients—An Illustrative Review. Transplantology 2022, 3, 241-256. https://doi.org/10.3390/transplantology3030025
Das BB, Deshpande S, Hussain T. Multimodality Imaging to Detect Rejection, and Cardiac Allograft Vasculopathy in Pediatric Heart Transplant Recipients—An Illustrative Review. Transplantology. 2022; 3(3):241-256. https://doi.org/10.3390/transplantology3030025
Chicago/Turabian StyleDas, Bibhuti B., Shriprasad Deshpande, and Tarique Hussain. 2022. "Multimodality Imaging to Detect Rejection, and Cardiac Allograft Vasculopathy in Pediatric Heart Transplant Recipients—An Illustrative Review" Transplantology 3, no. 3: 241-256. https://doi.org/10.3390/transplantology3030025
APA StyleDas, B. B., Deshpande, S., & Hussain, T. (2022). Multimodality Imaging to Detect Rejection, and Cardiac Allograft Vasculopathy in Pediatric Heart Transplant Recipients—An Illustrative Review. Transplantology, 3(3), 241-256. https://doi.org/10.3390/transplantology3030025






