Application of Pulsed Electric Fields for Obtaining Antioxidant Extracts from Fish Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Extraction Procedures
2.2.1. Extraction with Solvents (Water and Methanol) without PEF
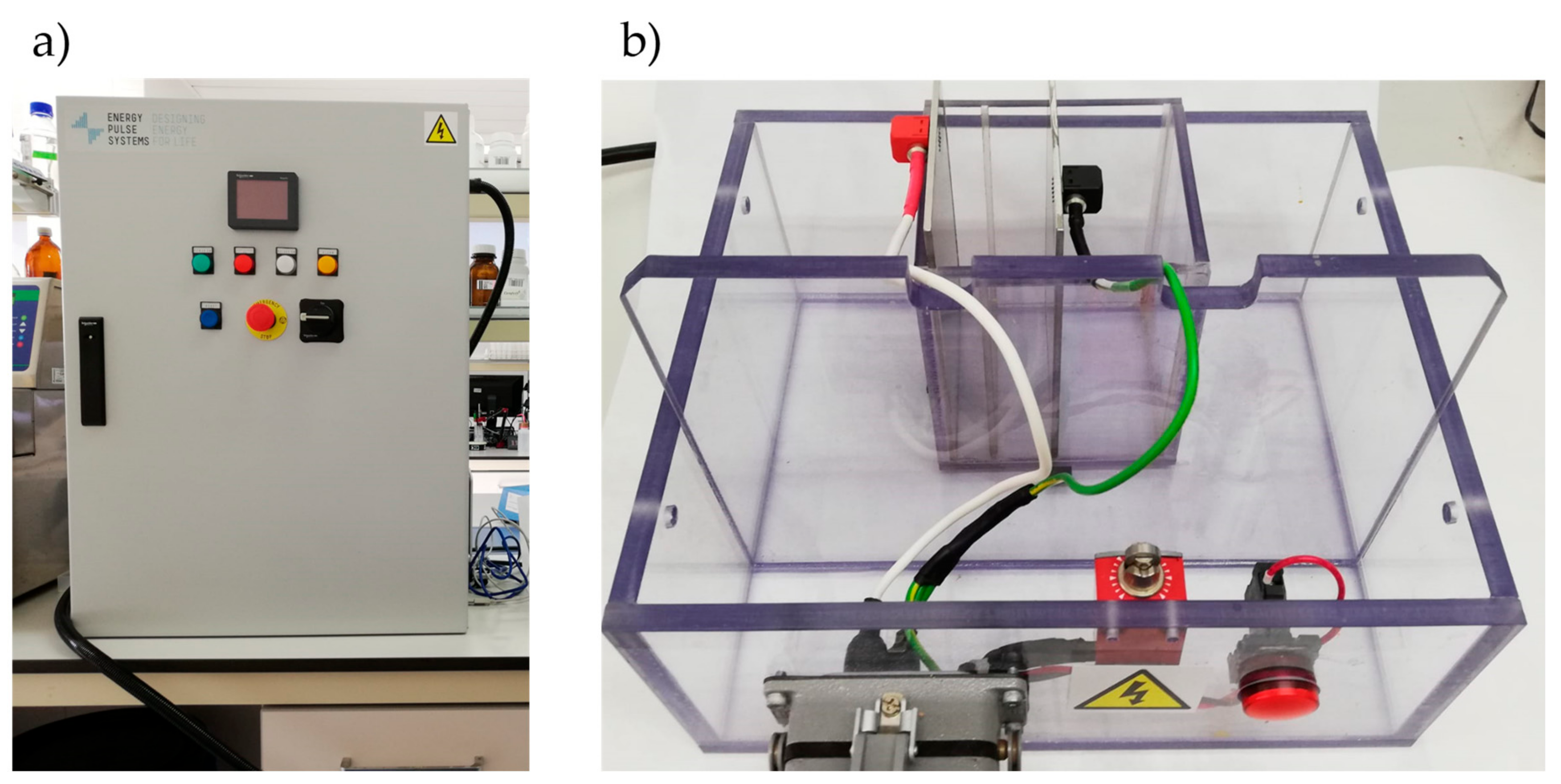
2.2.2. Extraction with Pulsed Electric Fields (PEF)
2.3. Analytical Determinations
2.3.1. Chemical Composition, Fatty Acid, Amino Acid, and Mineral Profile
2.3.2. Determination of Antioxidant Capacity
DPPH Radical Scavenging Assay
ABTS Radical Cation Decolorization Assay
Ferric-Reducing Antioxidant Power (FRAP) Assay
Oxygen Radical Absorbance Capacity (ORAC) Assay
2.4. Statistical Analysis
3. Results
3.1. Chemical, Mineral, and Amino Acids Composition of Fish Residues
3.2. Antioxidant Activity of EXTRACTs from Residues
4. Discussion
4.1. Chemical, Mineral, and Amino Acids Composition of Fish Residues
4.2. Antioxidant Activity of Extracts from Residues
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janssen, K.; Chavanne, H.; Berentsen, P.; Komen, H. Impact of selective breeding on European aquaculture. Aquaculture 2017, 472, 8–16. [Google Scholar] [CrossRef]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M.L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2233. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of bioprocesses for the integral valorisation of fish residues. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Zapata, C.; Franco, D. Proteins and amino acids. In Innovative Thermal and Non-Thermal Processing; Barba, F.J., Saraiba, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Elsevier Inc.: Duxford, UK, 2019; pp. 139–168. ISBN 9781469816593. [Google Scholar]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Ghosh, S.; Gillis, A.; Sheviryov, J.; Levkov, K.; Golberg, A. Towards waste meat biorefinery: Extraction of proteins from waste chicken meat with non-thermal pulsed electric fields and mechanical pressing. J. Clean. Prod. 2019, 208, 220–231. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry and Reactions of Reactive Oxygen Species in Foods. J. Food Sci. 2005, 70, R142–R159. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moure, A.; Cruz, J.M.; Franco, D.; Manuel Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Carlos Parajó, J. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- ISO 1442 Meat and Meat Products—Determination of Moisture Content; International Organization for Standardization: Geneva, Switzerland, 1997.
- ISO 937 Meat and Meat Products—Determination of Nitrogen Content; International Organization for Standardization: Geneva, Switzerland, 1978.
- ISO 936 Meat and Meat Products—Determination of Ash Content; International Organization for Standardization: Geneva, Switzerland, 1998.
- AOCS AOCS Official Procedure Am 5-04. Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction; AOCS, Ed.; American Oil Chemists Society: Urbana, IL, USA, 2005. [Google Scholar]
- Franco, D.; Lorenzo, J.M. Effect of muscle and intensity of finishing diet on meat quality of foals slaughtered at 15months. Meat Sci. 2014, 96, 327–334. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay Iris. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef]
- Gencbay, G.; Turhan, S. Proximate Composition and Nutritional Profile of the Black Sea Anchovy (Engraulis encrasicholus) Whole Fish, Fillets, and By-Products. J. Aquat. Food Prod. Technol. 2016, 25, 864–874. [Google Scholar] [CrossRef]
- Petricorena, Z. Chemical Composition of Fish and Fishery Products. In Handbook of Food Chemistry; Springer: Berlin, Germany, 2015; pp. 403–435. ISBN 9783642366055. [Google Scholar]
- Martínez-Valverde, I.; Jesús Periago, M.; Santaella, M.; Ros, G. The content and nutritional significance of minerals on fish flesh in the presence and absence of bone. Food Chem. 2000, 71, 503–509. [Google Scholar] [CrossRef]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein hydrolysate from salmon frames: Production, characteristics and antioxidative activity. J. Food Biochem. 2019, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Yildiz, M.; Ofori-Mensah, S. The Effects of Different Commercial Feeds and Seasonal Variation on Fillet Amino Acid Profile of Sea Bream (Sparus aurata) and Sea Bass (Dicentrarchus labrax). Turk. J. Fish. Aquat. Sci. 2017, 17, 1297–1307. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Hasan, F.M.; Udenigwe, C.C.; Gill, T.A.; Aluko, R.E. Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chem. 2015, 173, 652–659. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Xiao, H.; Decker, E.A.; Yongsawatdigul, J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015, 167, 7–15. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulln. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Puértolas, E.; Condón, S.; Raso, J.; Alvarez, I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. J. Food Eng. 2009, 90, 60–66. [Google Scholar] [CrossRef]
- Quagliariello, V.; Iaffaioli, R.V.; Falcone, M.; Ferrari, G.; Pataro, G.; Donsì, F. Effect of pulsed electric fields —assisted extraction on anti-inflammatory and cytotoxic activity of brown rice bioactive compounds. Food Res. Int. 2016, 87, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-compounds from Fermented Grape Pomace. Food Bioprocess. Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldrá, F.; Lorenzo, J.M. Antioxidant and Antimicrobial Activity of Peptides Extracted from Meat By-products: A Review. Food Anal. Methods 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Domínguez, R.; Carballo, J.; Muchenje, V.; Barba, F.J.; Lorenzo, J.M. Phenolic content and antioxidant activity of extracts from bifurcaria bifurcata alga, obtained by diverse extraction conditions using three different techniques (Hydrothermal, ultrasounds and supercritical CO2). Environ. Eng. Manag. J. 2019, 18, 1535–1542. [Google Scholar]
- Secci, G.; Borgogno, M.; Lupi, P.; Rossi, S.; Paci, G.; Mancini, S.; Bonelli, A.; Parisi, G. Effect of mechanical separation process on lipid oxidation in European aquacultured sea bass, gilthead sea bream, and rainbow trout products. Food Control. 2016, 67, 75–81. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess. Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Morales-medina, R.; García-moreno, P.J.; Guadix-escobar, A.; Guadix-escobar, E. Fish. Residues as Source of Health-Promoting Biopeptides; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128114469. [Google Scholar]
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, G.X.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Identification and active evaluation of antioxidant peptides from protein hydrolysates of Skipjack tuna (Katsuwonus pelamis) head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazeer, R.A.; Deeptha, R.; Jaiganesh, R.; Sampathkumar, N.S.; Naqash, S.Y. Radical scavenging activity of seela (Sphyraena barracuda) and ribbon fish (Lepturacanthus savala) backbone protein hydrolysates. Int. J. Pept. Res. Ther. 2011, 17, 209–216. [Google Scholar] [CrossRef]
- Agregán, R.; Lorenzo, J.M.; Munekata, P.E.S.; Dominguez, R.; Carballo, J.; Franco, D. Assessment of the antioxidant activity of Bifurcaria bifurcata aqueous extract on canola oil. Effect of extract concentration on the oxidation stability and volatile compound generation during oil storage. Food Res. Int. 2017, 99, 1095–1102. [Google Scholar] [CrossRef] [PubMed]


| Chemical Composition and Mineral Profile | Sea Bass | Sea Bream | SEM | Species | Residue | Species × Residue | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gills | Heads | Bones | Gills | Heads | Bones | |||||
| Water (%) | 62.37a1 | 58.86b1 | 51.6c1 | 55.23B2 | 52.51C2 | 57.63A2 | 0.29 | <0.001 | <0.001 | <0.001 |
| Fat (%) | 14.00b1 | 13.94b1 | 20.20a1 | 21.55A2 | 22.10A2 | 17.12B2 | 0.21 | <0.001 | 0.201 | <0.001 |
| SFA | 26.27a1 | 21.76b1 | 21.75b1 | 20.15B2 | 20.71A2 | 20.84A2 | 0.037 | <0.0001 | <0.0001 | <0.0001 |
| MUFA | 46.02a1 | 43.45c1 | 44.19b | 47.61A2 | 43.45B2 | 44.19B | 0.045 | <0.0001 | <0.0001 | <0.0001 |
| PUFA | 26.98c1 | 33.72a | 32.96b1 | 31.17B2 | 33.76A | 33.75A2 | 0.067 | <0.0001 | <0.0001 | <0.0001 |
| LCn3 | 11.79a1 | 10.22b1 | 8.87c1 | 7.43C2 | 9.77A2 | 9.34B2 | 0.049 | <0.0001 | <0.0001 | <0.0001 |
| n3 | 14.68a1 | 14.20b1 | 12.92c1 | 11.90C2 | 14.03A2 | 13.58B2 | 0.053 | <0.0001 | <0.0001 | <0.0001 |
| n6/n3 | 0.83c1 | 1.37b1 | 1.55a1 | 1.62A2 | 1.40C2 | 1.48B2 | 0.005 | <0.0001 | <0.0001 | <0.0001 |
| Protein (%) | 16.58a1 | 15.48b1 | 14.24c1 | 13.92B2 | 12.91C2 | 16.39A2 | 0.087 | <0.001 | <0.001 | <0.001 |
| Ash (%) | 5.57c1 | 9.96a | 7.52b | 6.44B2 | 9.14A | 6.23B | 0.15 | 0.180 | <0.001 | 0.005 |
| Ca | 1382.62c1 | 2507.15a | 2093.26b | 1873.24B2 | 2389.24A | 1618.82B | 41.69 | 0.685 | <0.001 | <0.001 |
| Fe | 1.22a1 | 0.28c1 | 0.51b1 | 2.15A2 | 0.44C2 | 0.69B2 | 0.02 | <0.001 | <0.001 | <0.001 |
| K | 180.51b1 | 194.31b | 262.73a1 | 134.94C2 | 184.52B | 300.67A2 | 1.96 | 0.144 | <0.001 | <0.001 |
| Mg | 36.77a1 | 29.04b | 24.98c1 | 47.90A2 | 28.04B | 30.70B2 | 0.44 | <0.001 | <0.001 | <0.001 |
| Mn | 500.58a1 | 266.82b1 | 270.37b1 | 585.07A2 | 211.13B2 | 206.76B2 | 11.39 | 0.612 | <0.001 | 0.006 |
| Na | 250.51a | 162.86b | 96.03c | 258.79A | 159.30B | 98.00C | 2.04 | 0.587 | <0.001 | 0.449 |
| P | 742.60b1 | 1277.00a | 1166.36a | 955.92B2 | 1312.27A | 989.20B | 20.68 | 0.567 | <0.001 | 0.01 |
| Zn | 1.41b1 | 2.12a1 | 1.27c | 2.12A2 | 1.71B2 | 1.39C | 0.02 | <0.001 | <0.001 | <0.001 |
| Cu | 0.09a1 | 0.03b | 0.10a | 0.17A2 | 0.04B | 0.14A | 0.009 | 0.017 | <0.001 | 0.172 |
| Amino Acid | Sea Bass | Sea Bream | SEM | Species | Residue | Species × Residue | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gills | Heads | Bones | Gills | Heads | Bones | |||||
| Asp | 846.50 | 891.531 | 875.141 | 790.94B | 695.72B2 | 1000.97A2 | 11.95 | 0.088 | <0.0001 | <0.0001 |
| Ser | 533.61a1 | 491.36b1 | 440.95c1 | 449.97B2 | 399.96C2 | 501.55A2 | 3.10 | <0.0001 | <0.0001 | <0.0001 |
| Glu | 1321.941 | 1337.361 | 1286.851 | 1270.81B2 | 1073.06C2 | 1446.03A2 | 14.84 | 0.088 | <0.0001 | <0.0001 |
| Gli | 1447.95a | 1171.91b | 956.69c | 1073.14 | 1118.67 | 1110.40 | 31.73 | 0.118 | 0.015 | 0.007 |
| Ala | 896.49a1 | 657.11b1 | 618.39b1 | 702.50A2 | 586.47 2 | 688.02A2 | 7.50 | <0.0001 | <0.0001 | <0.0001 |
| Pro | 949.47a1 | 677.66b | 531.53c1 | 623.982 | 625.12 | 626.282 | 16.83 | 0.008 | <0.0001 | <0.0001 |
| Tyr | 254.75 | 293.601 | 270.311 | 228.99B | 204.54B2 | 306.86A2 | 6.79 | 0.062 | 0.003 | <0.0001 |
| NEAA | 6280.75a1 | 5520.56b1 | 4980.08c1 | 5140.34B2 | 4703.57C2 | 5680.13A2 | 24.12 | <0.0001 | <0.0001 | <0.0001 |
| His | 374.00a | 323.26b1 | 300.67b1 | 311.53B | 276.50C2 | 379.58A2 | 3.82 | 0.194 | <0.0001 | <0.0001 |
| Arg | 185.28c | 844.34a1 | 775.38b1 | 206.41C | 701.37B2 | 870.50A2 | 6.33 | 0.486 | <0.0001 | <0.0001 |
| Thr | 694.30a1 | 453.15b1 | 445.73b1 | 567.39A2 | 386.41C2 | 516.28B2 | 5.80 | 0.001 | <0.0001 | <0.0001 |
| Val | 571.80a | 471.88b1 | 454.74b1 | 494.67A | 367.85B2 | 541.70A2 | 6.46 | 0.02 | <0.0001 | <0.0001 |
| Met | n.d. | 142.20 | 128.461 | n.d. | 126.45 | 162.842 | 6.20 | 0.620 | <0.0001 | <0.0001 |
| Lys | 672.73b | 747.62ab1 | 797.34a1 | 722.25B | 581.78 C2 | 903.19A2 | 15.49 | 0.911 | <0.0001 | 0.077 |
| Iso | 382.16 | 387.511 | 378.431 | 342.11B | 277.68B2 | 450.37A2 | 7.80 | 0.105 | <0.0001 | <0.0001 |
| Leu | 663.83 | 637.021 | 608.121 | 611.99 B | 469.31C2 | 742.38A2 | 11.60 | 0.228 | <0.0001 | <0.0001 |
| Phe | 474.36a | 431.84ab1 | 390.92b1 | 382.93 B | 315.45C2 | 462.85A2 | 6.38 | 0.01 | <0.0001 | <0.0001 |
| EAA | 4018.50b | 4438.86a | 4279.83ab1 | 3639.30 B | 3502.84B2 | 5029.73A2 | 48.25 | 0.058 | <0.0001 | <0.0001 |
| EAA/NEAA | 064b | 0.80a1 | 0.86a | 0.70B | 0.74 B2 | 0.88A | 0.01 | 0.647 | <0.0001 | 0.077 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, D.; Munekata, P.E.S.; Agregán, R.; Bermúdez, R.; López-Pedrouso, M.; Pateiro, M.; Lorenzo, J.M. Application of Pulsed Electric Fields for Obtaining Antioxidant Extracts from Fish Residues. Antioxidants 2020, 9, 90. https://doi.org/10.3390/antiox9020090
Franco D, Munekata PES, Agregán R, Bermúdez R, López-Pedrouso M, Pateiro M, Lorenzo JM. Application of Pulsed Electric Fields for Obtaining Antioxidant Extracts from Fish Residues. Antioxidants. 2020; 9(2):90. https://doi.org/10.3390/antiox9020090
Chicago/Turabian StyleFranco, Daniel, Paulo E. S. Munekata, Rubén Agregán, Roberto Bermúdez, María López-Pedrouso, Mirian Pateiro, and José M. Lorenzo. 2020. "Application of Pulsed Electric Fields for Obtaining Antioxidant Extracts from Fish Residues" Antioxidants 9, no. 2: 90. https://doi.org/10.3390/antiox9020090
APA StyleFranco, D., Munekata, P. E. S., Agregán, R., Bermúdez, R., López-Pedrouso, M., Pateiro, M., & Lorenzo, J. M. (2020). Application of Pulsed Electric Fields for Obtaining Antioxidant Extracts from Fish Residues. Antioxidants, 9(2), 90. https://doi.org/10.3390/antiox9020090










