Sensorineural Hearing Loss Post-COVID-19 Infection: An Update
Abstract
:1. Introduction
2. Materials and Methods
- -
- Established SARS-CoV-2 infection (proved by PCR on rhino-pharyngeal swab or serology).
- -
- Cases of new onset SNHL evaluated by pure tone audiometry.
- -
- Temporal correlation between the two events.
- -
- Exclusion criteria:
- -
- Conductive or mixed hearing loss.
- -
- Non-confirmed SARS-CoV-2 infection
- -
- Unclear temporal link between the two events.
- -
- Studies published not in the English language.
- -
- Studies with duplicated data.
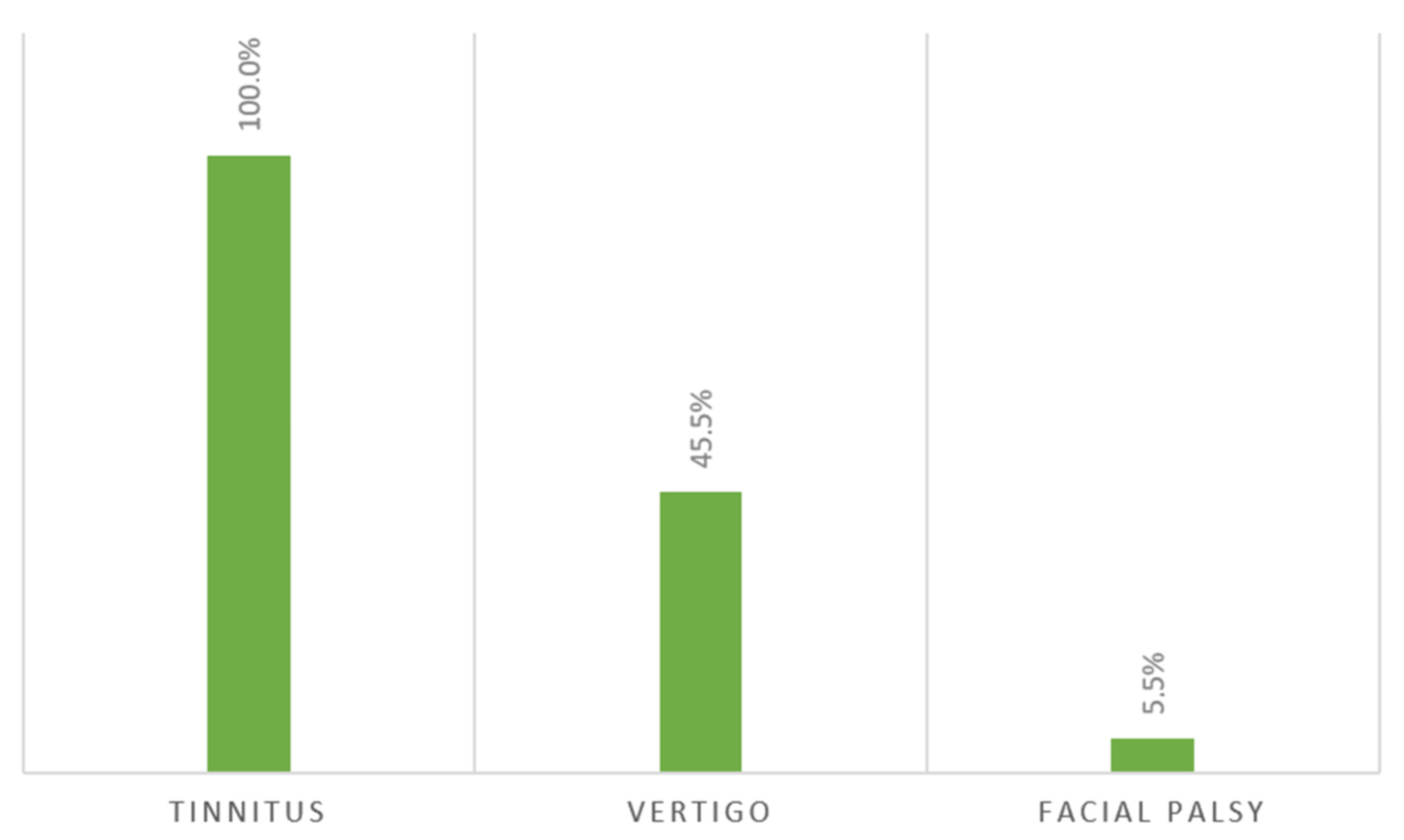
3. Results
- -
- Pronounced contrast enhancement of the cochlea (Dagen, 5) or of the VIII and VII nerves (Ozer, 15 and Jeong, 19);
- -
- Hemorrhagic lesion of cerebral parenchyma (Lamounier, 9);
- -
- Intra-labyrinth micro-hemorrhages (Chern, 11);
- -
- Signs peculiar of a specific disease, such as Susac syndrome (Raymaekers, 23).
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Meziani, F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Sriwijitalai, W.; Wiwanitkit, V. Hearing loss and COVID-19: A note. Am. J. Otolaryngol. 2020, 41, 102473. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Satterfield, L. Clinical practice guideline: Sudden hearing loss (Update). Otolaryngol. Head Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef] [Green Version]
- Kilic, O.; Kalcioglu, M.T.; Cag, Y.; Tuysuz, O.; Pektas, E.; Caskurlu, H.; Cetın, F. Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int. J. Infect. Dis. 2020, 97, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Degen, C.; Lenarz, T.; Willenborg, K. Acute Profound Sensorineural Hearing Loss After COVID-19 Pneumonia. Mayo Clin. Proc. 2020, 95, 1801–1803. [Google Scholar] [CrossRef]
- Abdel Rhman, S.; Abdel Wahid, A. COVID-19 and sudden sensorineural hearing loss, a case report. Otolaryngol. Case Rep. 2020, 16, 100198. [Google Scholar] [CrossRef]
- Lang, B.; Hintze, J.; Conlon, B. Coronavirus disease 2019 and sudden sensorineural hearing loss. J. Laryngol. Otol. 2020, 134, 1026–1028. [Google Scholar] [CrossRef]
- Koumpa, F.S.; Forde, C.T.; Manjaly, J.G. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. 2020, 13, e238419. [Google Scholar] [CrossRef]
- Lamounier, P.; Franco Gonçalves, V.; Ramos, H.V.L.; Gobbo, D.A.; Teixeira, R.P.; Dos Reis, P.C.; Bahmad, F., Jr.; Cândido Costa, C. A 67-Year-Old Woman with Sudden Hearing Loss Associated with SARS-CoV-2 Infection. Am. J. Case Rep. 2020, 21, e927519. [Google Scholar] [CrossRef]
- Karimi-Galougahi, M.; Naeini, A.S.; Raad, N.; Mikaniki, N.; Ghorbani, J. Vertigo and hearing loss during the COVID-19 pandemic—Is there an association? Acta Otorhinolaryngol. Ital. 2020, 40, 463–465. [Google Scholar] [CrossRef]
- Chern, A.; Famuyide, A.O.; Moonis, G.; Lalwani, A.K. Bilateral Sudden Sensorineural Hearing Loss and Intralabyrinthine Hemorrhage in a Patient with COVID-19. Otol. Neurotol. 2020, 42, e10–e14. [Google Scholar] [CrossRef] [PubMed]
- Aasfara, J.; Hajjij, A.; Bensouda, H.; Ouhabi, H.; Benariba, F. A unique association of bifacial weakness, paresthesia and vestibulocochlear neuritis as post COVID 19 manifestation in pregnant women: A case report. Pan Afr. Med. J. 2021, 38, 30. [Google Scholar] [CrossRef] [PubMed]
- Beckers, E.; Chouvel, P.; Cassetto, V.; Mustin, V. Sudden sensorineural hearing loss in COVID-19: A case report and literature review. Clin. Case Rep. 2021, 9, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Muzaffar, J.; Naik, P.; Coulson, C. Catastrophic bilateral sudden sensorineural hearing loss following COVID-19. BMJ Case Rep. 2021, 14, e243157. [Google Scholar] [CrossRef]
- Ozer, F.; Alkan, O. Simultaneous Sudden Hearing Loss and Peripheral Facial Paralysis in a Patient with COVID-19. Ear Nose Throat 2021. [Google Scholar] [CrossRef]
- Ricciardiello, F.; Pisani, D.; Viola, P.; Cristiano, E.; Scarpa, A.; Giannone, A.; Longo, G.; Russo, G.; Bocchetti, M.; Coppola, C.; et al. Sudden Sensorineural Hearing Loss in Mild COVID-19: Case Series and Analysis of the Literature. Audiol. Res. 2021, 11, 313–326. [Google Scholar] [CrossRef]
- Gerstacker, K.; Speck, I.; Riemann, S.; Aschendorff, A.; Knopf, A.; Arndt, S. Deafness after COVID-19? HNO 2021, 69 (Suppl. S2), 92–95. [Google Scholar] [CrossRef]
- Shah, S.; Rocke, J.; France, K.; Izzat, S. Sudden sensorineural hearing loss in COVID-19: A case series from the Wrightington, Wigan and Leigh Teaching Hospitals, United Kingdom. Med. J. Malays. 2021, 76 (Suppl. S4), 55–59. [Google Scholar]
- Jeong, M.; Ocwieja, K.E.; Han, D.; Wackym, P.A.; Zhang, Y.; Brown, A.; Moncada, C.; Vambutas, A.; Kanne, T.; Crain, R.; et al. Direct SARS-CoV-2 infection of the human inner ear may underlie COVID-19-associated audiovestibular dysfunction. Commun. Med. 2021, 1, 44. [Google Scholar] [CrossRef]
- Pokharel, S.; Tamang, S.; Pokharel, S.; Mahaseth, R.K. Sudden sensorineural hearing loss in a post-COVID-19 patient. Clin. Case Rep. 2021, 9, e04956. [Google Scholar] [CrossRef]
- Yaseen, N.K.; Al-Ani, R.M.; Ali Rashid, R. COVID-19-related sudden sensorineural hearing loss. Qatar Med. J. 2021, 2021, 58. [Google Scholar] [CrossRef]
- Asfour, L.; Kay-Rivest, E.; Roland, J.T., Jr. Cochlear implantation for single-sided deafness after COVID-19 hospitalization. Cochlear Implant. Int. 2021, 22, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, V.; D’Hulst, S.; Herijgers, D.; Vercammen, J.; Fabry, A.; Dutoit, J.; D’Heygere, E.; Vancaester, E.; Vanderdonckt, P. Susac syndrome complicating a SARS-CoV-2 infection. J. Neurovirology 2021, 27, 954–959. [Google Scholar] [CrossRef]
- Fancello, V.; Hatzopoulos, S.; Corazzi, V.; Bianchini, C.; Skarżyńska, M.B.; Pelucchi, S.; Skarżyński, P.H.; Ciorba, A. SARS-CoV-2 (COVID-19) and audio-vestibular disorders. Int. J. Immunopathol. Pharmacol. 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Mulvey, J.; Kubiak, J.; Mikhail, S.; Suster, D.; Crowson, A.N.; Laurence, J.; Nuovo, G. Severe COVID-19: A multifaceted viral vasculopathy syndrome. Ann. Diagn. Pathol. 2020, 50, 151645. [Google Scholar] [CrossRef]
- Uranaka, T.; Kashio, A.; Ueha, R.; Sato, T.; Bing, H.; Ying, G.; Kinoshita, M.; Kondo, K.; Yamasoba, T. Expression of ACE2, TMPRSS2, and Furin in Mouse Ear Tissue, and the Implications for SARS-CoV-2 Infection. Laryngoscope 2020, 131, E2013–E2017. [Google Scholar] [CrossRef] [PubMed]
- Celik, T.; Simsek, A.; Koca, C.F.; Aydin, S.; Yasar, S. Evaluation of cochlear functions in infants exposed to SARS-CoV-2 intrauterine. Am. J. Otolaryngol. 2021, 42, 102982. [Google Scholar] [CrossRef] [PubMed]
- Oskovi-Kaplan, Z.A.; Ozgu-Erdinc, A.S.; Buyuk, G.N.; Sert-Dinc, U.Y.; Ali-Algan, C.; Demir, B.; Sahin, D.; Keskin, H.L.; Tayman, C.; Moraloglu-Tekin, Ö. Newborn Hearing Screening Results of Infants Born to Mothers Who Had COVID-19 Disease during Pregnancy: A Retrospective Cohort Study. Ear Hear. 2021, 43, 41–44. [Google Scholar] [CrossRef]
- Mostafa, B.E.; Mostafa, A.; El Fiky, L.M.E.; Omara, A.; Teaima, A. Maternal COVID-19 and neonatal hearing loss: A multicentric survey. Eur. Arch. Otorhinolaryngol. 2021, 279, 3435–3438. [Google Scholar] [CrossRef]
- Ghiselli, S.; Laborai, A.; Biasucci, G.; Carvelli, M.; Salsi, D.; Cuda, D. Auditory evaluation of infants born to COVID19 positive mothers. Am. J. Otolaryngol. 2022, 43, 103379. [Google Scholar] [CrossRef]
- Alan, M.A.; Alan, C. Hearing screening outcomes in neonates of SARS-CoV-2 positive pregnant women. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110754. [Google Scholar] [CrossRef] [PubMed]
- Wastnedge, E.A.N.; Reynolds, R.M.; van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with COVID-19. N. Engl. J. Med. 2021, 384, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Chari, D.A.; Parikh, A.; Kozin, E.D.; Reed, M.; Jung, D.H. Impact of COVID-19 on Presentation of Sudden Sensorineural Hearing Loss at a Single Institution. Otolaryngol. Neck Head Surg. 2021, 165, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Doweck, I.; Yanir, Y.; Najjar-Debbiny, R.; Shibli, R.; Saliba, W. Sudden Sensorineural Hearing Loss during the COVID-19 Pandemic. JAMA Otolaryngol. Neck Head Surg. 2022, 148, 373. [Google Scholar] [CrossRef] [PubMed]
- Formeister, E.J.; Wu, M.J.; Chari, D.A.; Meek, R., 3rd; Rauch, S.D.; Remenschneider, A.K.; Quesnel, A.M.; de Venecia, R.; Lee, D.J.; Chien, W.; et al. Assessment of Sudden Sensorineural Hearing Loss after COVID-19 Vaccination. JAMA Otolaryngol. Neck Head Surg. 2022, 148, 307. [Google Scholar] [CrossRef] [PubMed]
- Yanir, Y.; Doweck, I.; Shibli, R.; Najjar-Debbiny, R.; Saliba, W. Association Between the BNT162b2 Messenger RNA COVID-19 Vaccine and the Risk of Sudden Sensorineural Hearing Loss. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 299. [Google Scholar] [CrossRef]
- Pisani, D.; Leopardi, G.; Viola, P.; Scarpa, A.; Ricciardiello, F.; Cerchiai, N.; Astorina, A.; Chiarella, G. Sudden sensorineural hearing loss after covid-19 vaccine; A possible adverse reaction? Otolaryngol. Case Rep. 2021, 21, 100384. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, H.S. Sudden sensorineural hearing loss after COVID-19 vaccination. Int. J. Infect. Dis. 2021, 113, 341–343. [Google Scholar] [CrossRef]
- Tsetsos, N.; Poutoglidis, A.; Vlachtsis, K.; Kilmpasanis, A.; Gougousis, S. Sudden Sensorineural Hearing Loss following the Second Dose of COVID-19 Vaccine. Cureus 2021, 13, e17435. [Google Scholar] [CrossRef]
- Wichova, H.; Miller, M.E.; Derebery, M.J. Otologic Manifestations After COVID-19 Vaccination: The House Ear Clinic Experience. Otol. Neurotol. 2021, 42, e1213. [Google Scholar] [CrossRef] [PubMed]
- Avcı, H.; Karabulut, B.; Eken, H.D.; Faraşoğlu, A.; Çakil, T.; Çoruk, S.; Özel, H.; Kaya, N.K.; Özbalta, S. Otolaryngology-Specific Symptoms May Be Highly Observed in Patients With a History of Covid-19 Infection after Inactivated Coronavirus Vaccination. Ear Nose Throat J. 2021. [Google Scholar] [CrossRef] [PubMed]



| Authors | Ref. | Year | Country | # | Age (Years) | Sex |
|---|---|---|---|---|---|---|
| Kilic O. et al. | [4] | June 2020 | Turkey | 1 | 29 | M |
| Degen C. et al. | [5] | June 2020 | USA | 1 | 60 | M |
| Abdel Rhman S. et al. | [6] | July 2020 | Egypt | 1 | 52 | M |
| Lang B. et al. | [7] | October 2020 | Ireland | 1 | 30 | F |
| Koumpa FS. et al. | [8] | October 2020 | UK | 1 | 45 | M |
| Lamounier P. et al. | [9] | November 2020 | Brazil | 1 | 67 | F |
| Karimi-Galougahi M. et al. | [10] | December 2020 | Iran | 3 | 22 | M |
| 40 | F | |||||
| 23 | F | |||||
| Chern A. et al. | [11] | January 2021 | USA | 1 | 18 | F |
| Aasfara J. et al. | [12] | January 2021 | Morocco | 1 | 36 | F |
| Beckers E. et al. | [13] | March 2021 | Belgium | 1 | 53 | M |
| Edwards M. et al. | [14] | June 2021 | UK | 1 | 68 | F |
| Ozer F. et al. | [15] | July 2021 | Turkey | 1 | 62 | F |
| Ricciardiello F. et al. | [16] | July 2021 | Italy | 5 | 26 | F |
| 22 | M | |||||
| 61 | M | |||||
| 30 | M | |||||
| 46 | F | |||||
| Gerstacker K. et al. | [17] | August 2021 | Germany | 1 | 38 | M |
| Shah S.M. et al. | [18] | August 2021 | UK | 4 | 46 | F |
| 43 | F | |||||
| 54 | F | |||||
| 51 | M | |||||
| Jeong M. et al. | [19] | October 2021 | USA | 10 | Mean 48.8 | 6 M |
| (range 22–72) | 4 F | |||||
| Pokharel S. et al. | [20] | October 2021 | Nepal | 1 | 27 | M |
| Yaseen N.K. et al. | [21] | October 2021 | Iraq | 26 | Mean 39.23 | 6 M |
| (range 21–66) | 21 F | |||||
| Asfour L. et al. | [22] | November 2021 | USA | 1 | 34 | M |
| Raymaekers V. et al. | [23] | November 2021 | Belgium | 1 | 40 | M |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fancello, V.; Fancello, G.; Hatzopoulos, S.; Bianchini, C.; Stomeo, F.; Pelucchi, S.; Ciorba, A. Sensorineural Hearing Loss Post-COVID-19 Infection: An Update. Audiol. Res. 2022, 12, 307-315. https://doi.org/10.3390/audiolres12030032
Fancello V, Fancello G, Hatzopoulos S, Bianchini C, Stomeo F, Pelucchi S, Ciorba A. Sensorineural Hearing Loss Post-COVID-19 Infection: An Update. Audiology Research. 2022; 12(3):307-315. https://doi.org/10.3390/audiolres12030032
Chicago/Turabian StyleFancello, Virginia, Giuseppe Fancello, Stavros Hatzopoulos, Chiara Bianchini, Francesco Stomeo, Stefano Pelucchi, and Andrea Ciorba. 2022. "Sensorineural Hearing Loss Post-COVID-19 Infection: An Update" Audiology Research 12, no. 3: 307-315. https://doi.org/10.3390/audiolres12030032
APA StyleFancello, V., Fancello, G., Hatzopoulos, S., Bianchini, C., Stomeo, F., Pelucchi, S., & Ciorba, A. (2022). Sensorineural Hearing Loss Post-COVID-19 Infection: An Update. Audiology Research, 12(3), 307-315. https://doi.org/10.3390/audiolres12030032








