HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE−/− Mice
Abstract
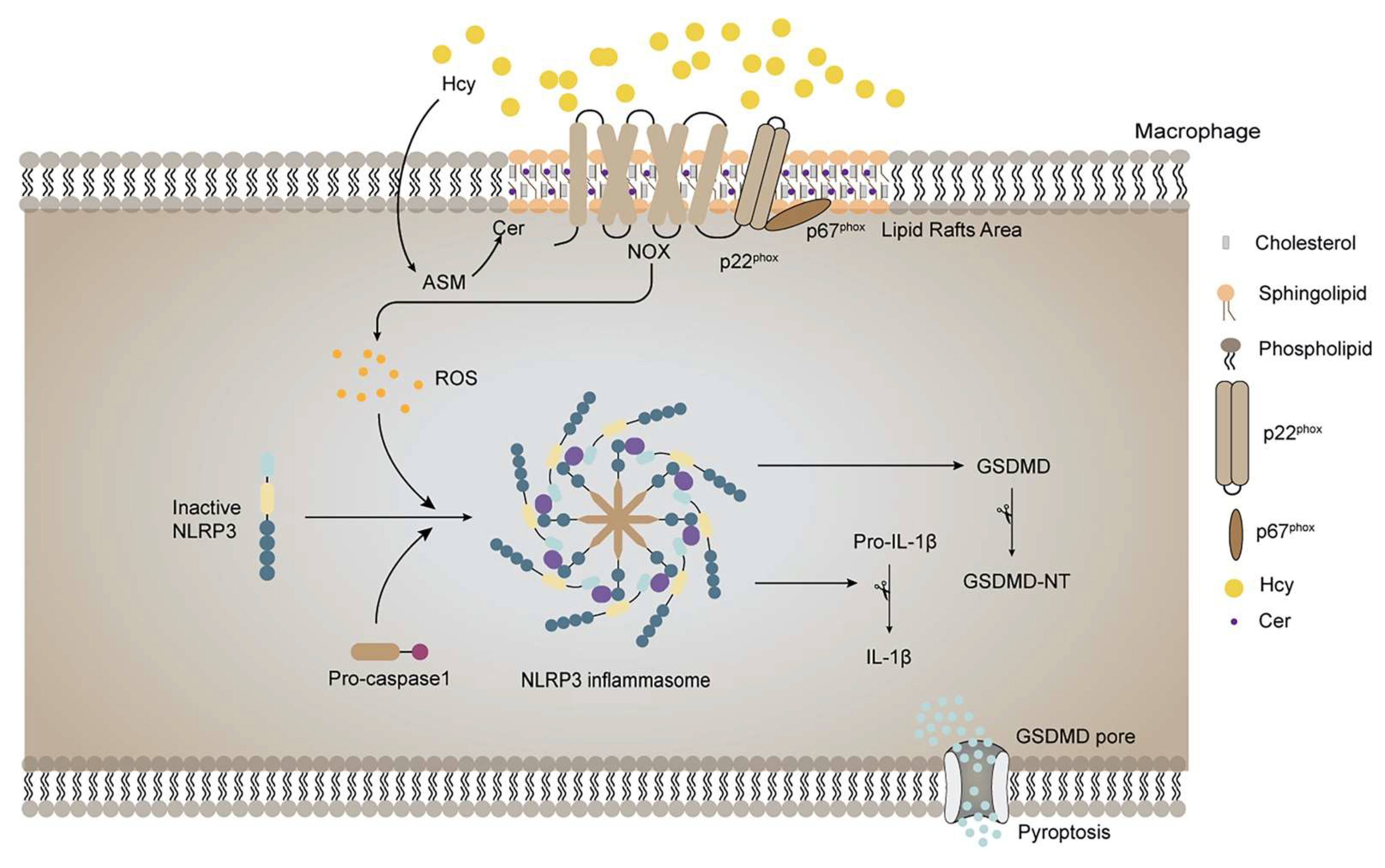
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Small Interfering RNA (siRNA) Transfection
2.3. ROS Assessment
2.4. Lipid Raft Extraction
2.5. NOX Activity Assessment
2.6. Animal Model
2.7. Histological Analysis of the Aortic Root Plaque
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Western Blot Analysis
2.10. Immunofluorescence Analysis
2.11. Statistical Analysis
3. Results
3.1. MβCD Reduced the Intracellular and Mitochondrial ROS Levels in Hcy-Treated Macrophages
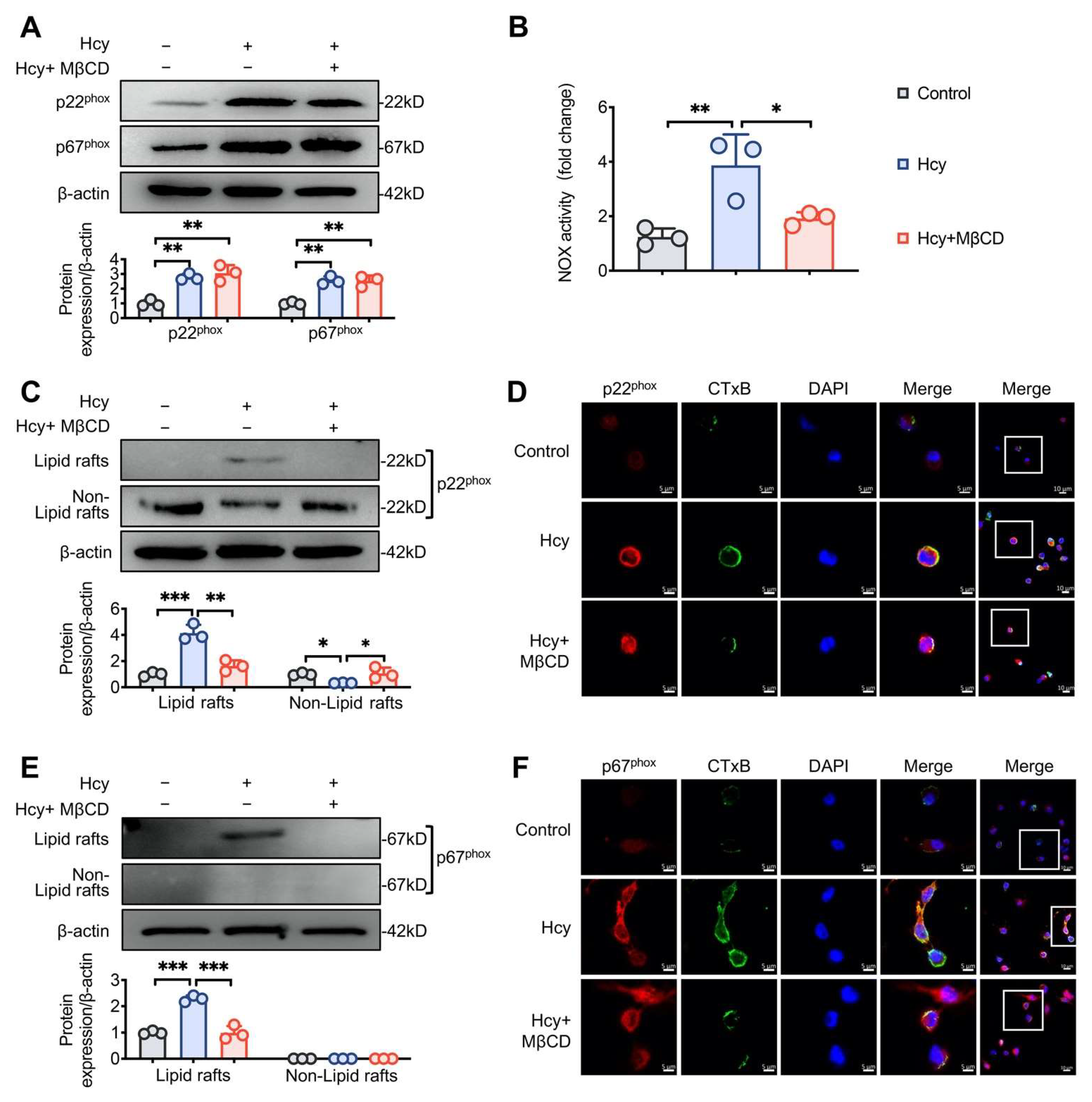
3.2. MβCD Inhibited Hcy-induced p22phox and p67phox Recruitment into Lipid Rafts and Decreased NOX Activity in Macrophages
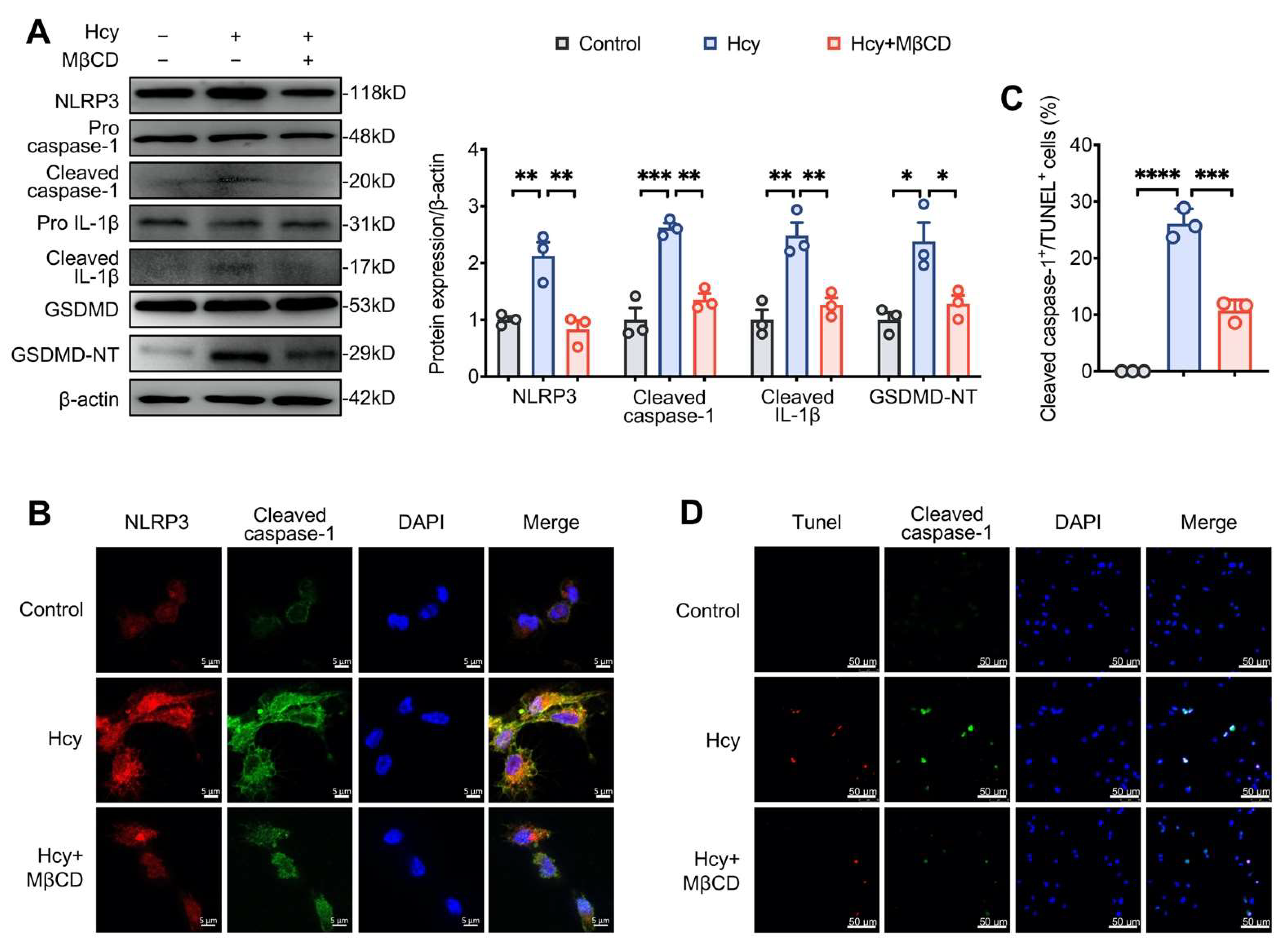
3.3. MβCD Inhibited Hcy-Induced NLRP3 Inflammasome Activation and Pyroptosis in Macrophages
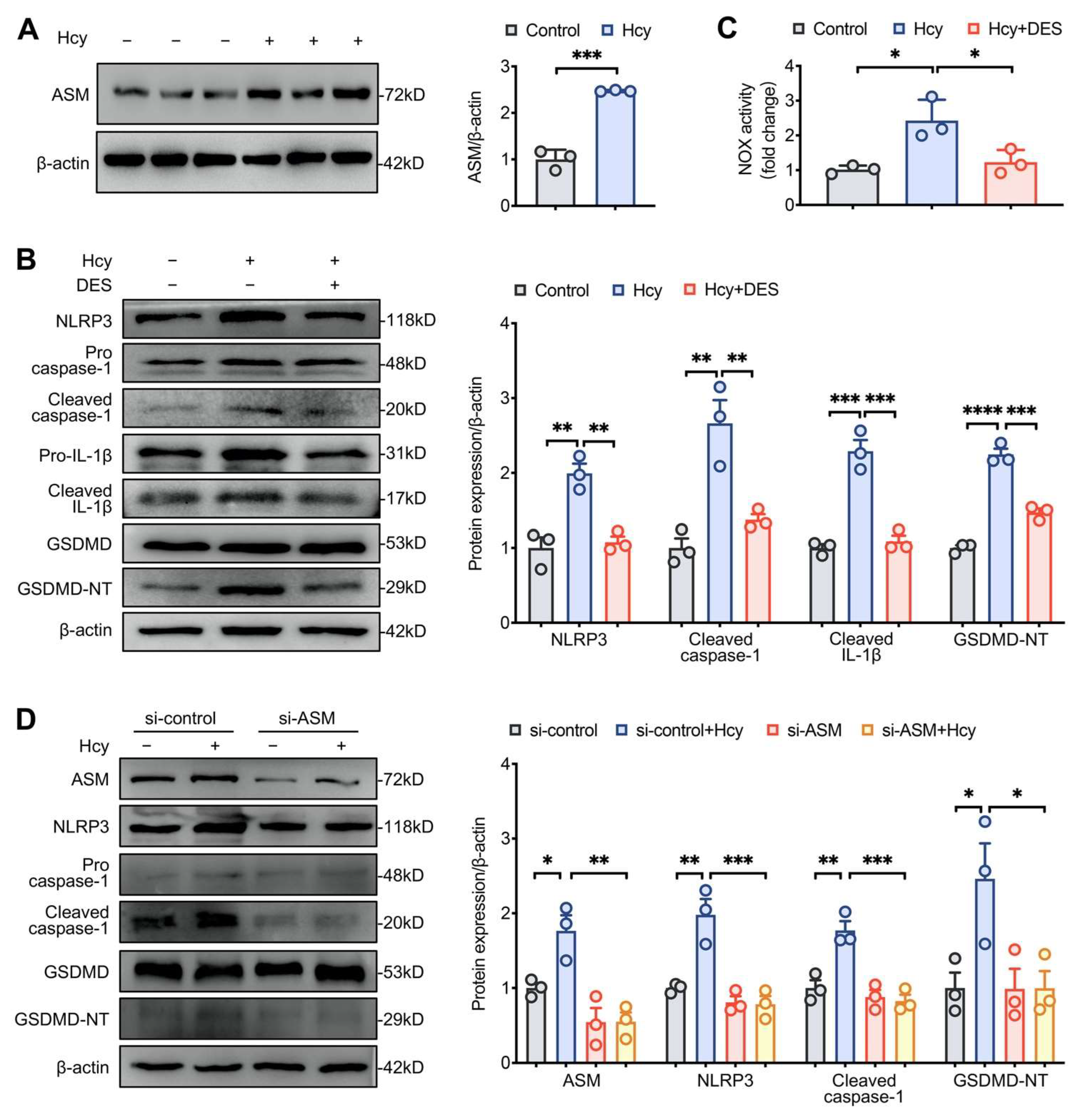
3.4. Disruption of Lipid Raft Clustering Suppressed Hcy-Induced NLRP3 Inflammasome Activation and Pyroptosis in Macrophages
3.5. MβCD Decreased the Levels of Plasma Inflammatory Mediators and Attenuated Atherosclerosis in HHcy Mice
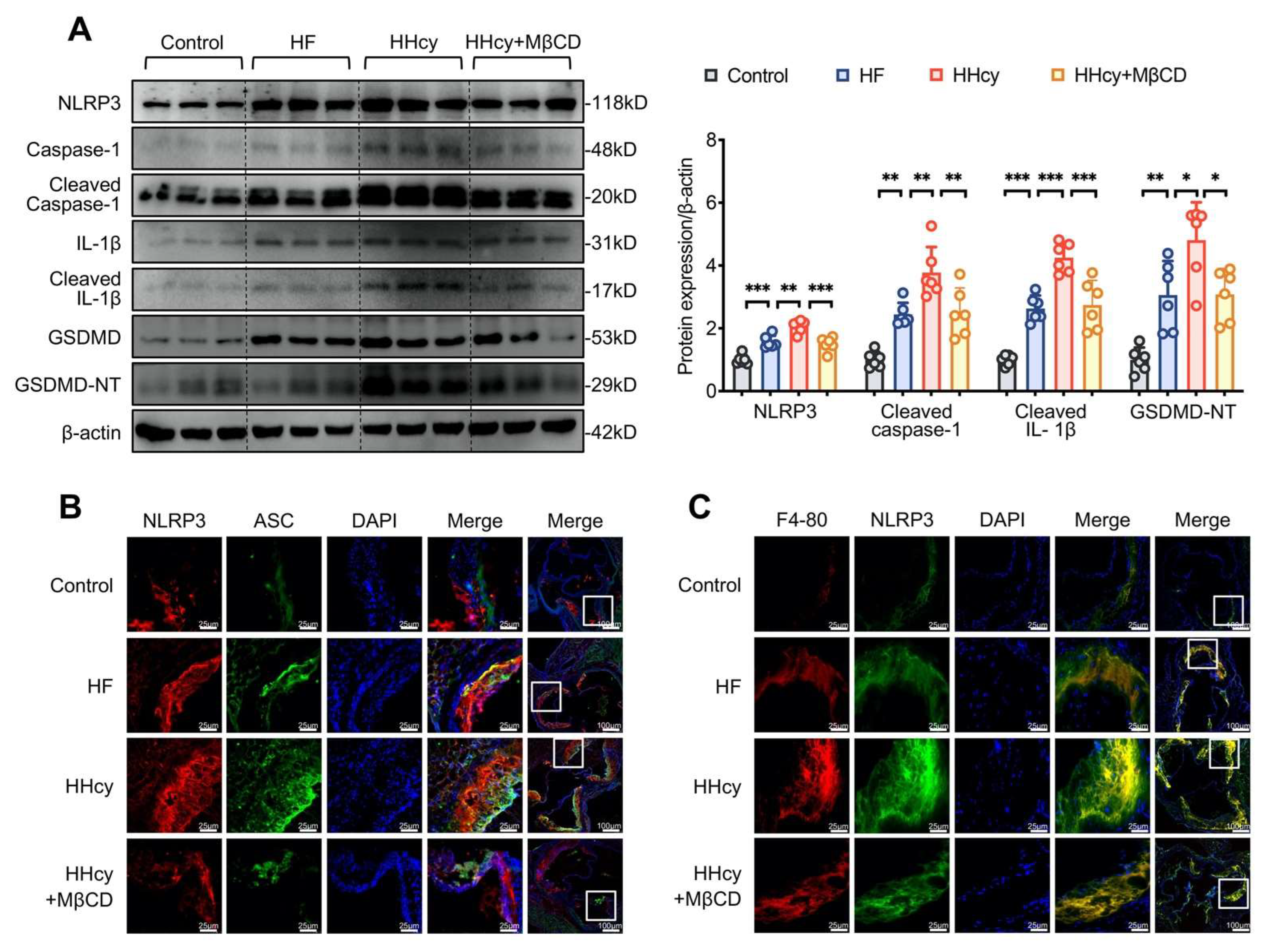
3.6. MβCD Inhibited HHcy-Induced NLRP3 Inflammasome Activation, Pyroptosis, and Macrophage Recruitment into the Aortic Root Plaque in ApoE−/− Mice
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuang, T.; Liu, J.; Chen, X.; Zhang, L.; Pi, J.; Sun, H.; Li, L.; Bauer, R.; Wang, H.; Yu, Z.; et al. Endothelial Foxp1 Suppresses Atherosclerosis via Modulation of Nlrp3 Inflammasome Activation. Circ. Res. 2019, 125, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, H.; Yang, A.; Ma, P.; Sun, L.; Deng, M.; Mao, C.; Xiong, J.; Sun, J.; Wang, N.; et al. Homocysteine accelerates atherosclerosis by inhibiting scavenger receptor class B member1 via DNMT3b/SP1 pathway. J. Mol. Cell. Cardiol. 2020, 138, 34–48. [Google Scholar] [CrossRef]
- Cruciani-Guglielmacci, C.; Meneyrol, K.; Denom, J.; Kassis, N.; Rachdi, L.; Makaci, F.; Migrenne-Li, S.; Daubigney, F.; Georgiadou, E.; Denis, R.; et al. Homocysteine Metabolism Pathway Is Involved in the Control of Glucose Homeostasis: A Cystathionine Beta Synthase Deficiency Study in Mouse. Cells 2022, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Antonopoulos, A.; Tousoulis, D.; Marinou, K.; Stefanadis, C. Homocysteine and coronary atherosclerosis: From folate fortification to the recent clinical trials. Eur. Heart J. 2009, 30, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.; Meijles, D.; Pagano, P. NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 2016, 130, 317–335. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Khotina, V.; Ivanova, E.A.; Orekhov, A.N. NADPH Oxidases and Their Role in Atherosclerosis. Biomedicines 2020, 8, 206. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, Y.; Shen, Y.; Guo, X.; Shang, D.; Zhang, D. Probucol reverses homocysteine induced inflammatory monocytes differentiation and oxidative stress. Eur. J. Pharmacol. 2018, 818, 67–73. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Mu, N.; Lou, X.; Li, W.; Chen, Y.; Fan, D.; Tan, H. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab. Investig. 2017, 97, 922–934. [Google Scholar] [CrossRef]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef]
- Martinet, W.; Coornaert, I.; Puylaert, P.; De Meyer, G. Macrophage Death as a Pharmacological Target in Atherosclerosis. Front. Pharmacol. 2019, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wu, D.; Sun, Y.; Suo, Y.; Yu, Q.; Zeng, M.; Gao, Q.; Yu, B.; Jiang, X.; Wang, Y. The selective NLRP3 inhibitor MCC950 hinders atherosclerosis development by attenuating inflammation and pyroptosis in macrophages. Sci. Rep. 2021, 11, 19305. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Zeng, C.; Liu, S.; Gong, J.; Hu, J.; Li, H.; Tan, H. α1-nAchR-Mediated Signaling Through Lipid Raft Is Required for Nicotine-Induced NLRP3 Inflammasome Activation and Nicotine-Accelerated Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 724699. [Google Scholar]
- Regen, S. The Origin of Lipid Rafts. Biochemistry 2020, 59, 4617–4621. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, F. Lipid raft redox signaling platforms in vascular dysfunction: Features and mechanisms. Curr. Atheroscler. Rep. 2009, 11, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Yi, F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid. Redox Signal. 2007, 9, 1457–1470. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Zhang, X.; Du, Y.; Chen, G.; Xiang, P.; Ling, W.; Wang, D. Terpene Lactucopicrin Limits Macrophage Foam Cell Formation by a Reduction of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 in Lipid Rafts. Mol. Nutr. Food Res. 2022, 66, e2100905. [Google Scholar] [CrossRef]
- Schmitz, G.; Grandl, M. Role of redox regulation and lipid rafts in macrophages during Ox-LDL-mediated foam cell formation. Antioxid. Redox Signal. 2007, 9, 1499–1518. [Google Scholar] [CrossRef]
- Gu, M.; Fu, Y.; Sun, X.; Ding, Y.; Li, C.; Pang, W.; Pan, S.; Zhu, Y. Proteomic analysis of endothelial lipid rafts reveals a novel role of statins in antioxidation. J. Proteome Res. 2012, 11, 2365–2373. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Zhang, L.; Wu, M.; Zhang, F.; Yang, D.; Shen, J.; Chen, J. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic. Biol. Med. 2021, 173, 41–51. [Google Scholar] [CrossRef]
- He, X.; Fan, X.; Bai, B.; Lu, N.; Zhang, S.; Zhang, L. Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol. Res. 2021, 165, 105447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yi, F.; Jin, S.; Xia, M.; Chen, Q.; Gulbins, E.; Li, P. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid. Redox Signal. 2007, 9, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chen, Y.; Leu, H.; Lin, S.; Chen, Y.; Huang, S.; Chen, J. Anti-inflammatory strategies for homocysteine-related cardiovascular disease. Front. Biosci.-Landmark 2009, 14, 3836–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elumalai, S.; Karunakaran, U.; Moon, J.-S.; Won, K.-C. NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction. Cells 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Edirimanne, V.; Woo, C.; Siow, Y.; Pierce, G.; Xie, J.; O, K. Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can. J. Physiol. Pharmacol. 2007, 85, 1236–1247. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Cao, Y.; Si, J.; Li, J.; Cao, K.; Pang, X. Probucol decreases homocysteine-stimulated CRP production in rat aortic smooth muscle cells via regulating HO-1/NADPH oxidase/ROS/p38 pathway. Acta Biochim. Biophys. Sin. 2021, 53, 212–219. [Google Scholar] [CrossRef]
- Ahmed, K.; Sawa, T.; Ihara, H.; Kasamatsu, S.; Yoshitake, J.; Rahaman, M.; Okamoto, T.; Fujii, S.; Akaike, T. Regulation by mitochondrial superoxide and NADPH oxidase of cellular formation of nitrated cyclic GMP: Potential implications for ROS signalling. Biochem. J. 2012, 441, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Qin, Y.; Yu, X.; Xu, X.; Yu, W. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif. 2022, 55, e13167. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, S.; Peng, D.; Zhao, S. High-density lipoprotein affects antigen presentation by interfering with lipid raft: A promising anti-atherogenic strategy. Clin. Exp. Immunol. 2010, 160, 137–142. [Google Scholar] [CrossRef]
- Xiong, J.; Ma, F.; Ding, N.; Xu, L.; Ma, S.; Yang, A.; Hao, Y.; Zhang, H.; Jiang, Y. miR-195-3p alleviates homocysteine-mediated atherosclerosis by targeting IL-31 through its epigenetics modifications. Aging cell 2021, 20, e13485. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, L.; Hu, Y.; Wang, Q. Pyroptosis and its relationship to atherosclerosis. Clin. Chim. Acta 2018, 476, 28–37. [Google Scholar] [CrossRef]
- Frank, P.; Lee, H.; Park, D.; Tandon, N.; Scherer, P.; Lisanti, M. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Han, X.; Zhou, D.; Zhong, J.; Liu, L.; Wang, Y.; Ni, J.; Shen, X.; Liang, C.; Fang, H. BIG1 mediates sepsis-induced lung injury by modulating lipid raft-dependent macrophage inflammatory responses. Acta Biochim. Biophys. Sin. 2021, 53, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, J.; Woo, J.; Jou, I. 25-Hydroxycholesterol suppress IFN-γ-induced inflammation in microglia by disrupting lipid raft formation and caveolin-mediated signaling endosomes. Free Radic. Biol. Med. 2022, 179, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ullah, H.; Zheng, Z.; Gu, X.; Su, C.; Xiao, L.; Wu, X.; Xiong, F.; Li, Q.; Zha, L. Soyasaponins reduce inflammation by downregulating MyD88 expression and suppressing the recruitments of TLR4 and MyD88 into lipid rafts. BMC Complementary Med. Ther. 2020, 20, 167. [Google Scholar] [CrossRef]
- Song, Z.; Gong, Q.; Guo, J. Pyroptosis: Mechanisms and Links with Fibrosis. Cells 2021, 10, 3509. [Google Scholar] [CrossRef]
- Jia, S.; Jin, S.; Zhang, F.; Yi, F.; Dewey, W.; Li, P. Formation and function of ceramide-enriched membrane platforms with CD38 during M1-receptor stimulation in bovine coronary arterial myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1743–H1752. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.; Futerman, A.; Prieto, M. Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations. Biophys. J. 2009, 96, 3210–3222. [Google Scholar] [CrossRef] [Green Version]
- Megha; London, E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): Implications for lipid raft structure and function. J. Biol. Chem. 2004, 279, 9997–10004. [Google Scholar] [CrossRef] [Green Version]
- Serban, K.; Rezania, S.; Petrusca, D.; Poirier, C.; Cao, D.; Justice, M.; Patel, M.; Tsvetkova, I.; Kamocki, K.; Mikosz, A.; et al. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Sci. Rep. 2016, 6, 31596. [Google Scholar] [CrossRef] [PubMed]
- Charla, E.; Mercer, J.; Maffia, P.; Nicklin, S.A. Extracellular vesicle signalling in atherosclerosis. Cell Signal 2020, 75, 109751. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tao, J.; Duan, F.; Li, H.; Tan, H. HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE−/− Mice. Cells 2022, 11, 2438. https://doi.org/10.3390/cells11152438
Liu S, Tao J, Duan F, Li H, Tan H. HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE−/− Mice. Cells. 2022; 11(15):2438. https://doi.org/10.3390/cells11152438
Chicago/Turabian StyleLiu, Sijun, Jun Tao, Fengqi Duan, Huangjing Li, and Hongmei Tan. 2022. "HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE−/− Mice" Cells 11, no. 15: 2438. https://doi.org/10.3390/cells11152438
APA StyleLiu, S., Tao, J., Duan, F., Li, H., & Tan, H. (2022). HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE−/− Mice. Cells, 11(15), 2438. https://doi.org/10.3390/cells11152438




