Advances in Regenerative Dentistry: A Systematic Review of Harnessing Wnt/β-Catenin in Dentin-Pulp Regeneration
Abstract
:1. Introduction
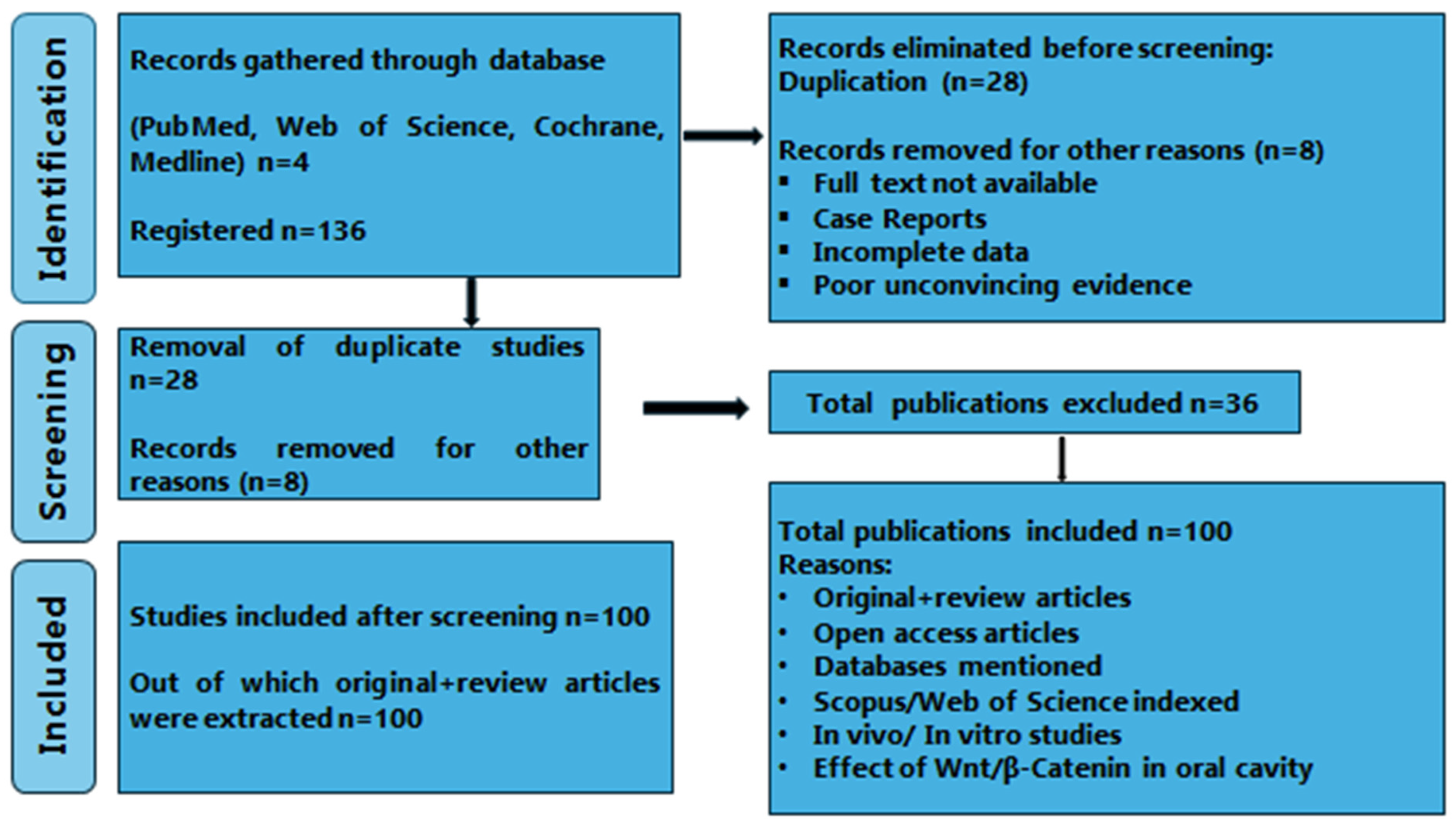
2. Materials and Methods
2.1. Source of Data
2.2. Search Method
- Related studies in Wnt/β-catenin signals
- Human (in vitro) and animal (in vivo) studies.
- Wnt/β-catenin in oral cavity-based publications.
- Bioactive materials on dental pulp cells via Wnt signals
- Unconvincing or poor evidence.
- Case report studies
- Incomplete text publications
- Incomplete data
2.3. PICO Framework
3. Results
4. Discussion
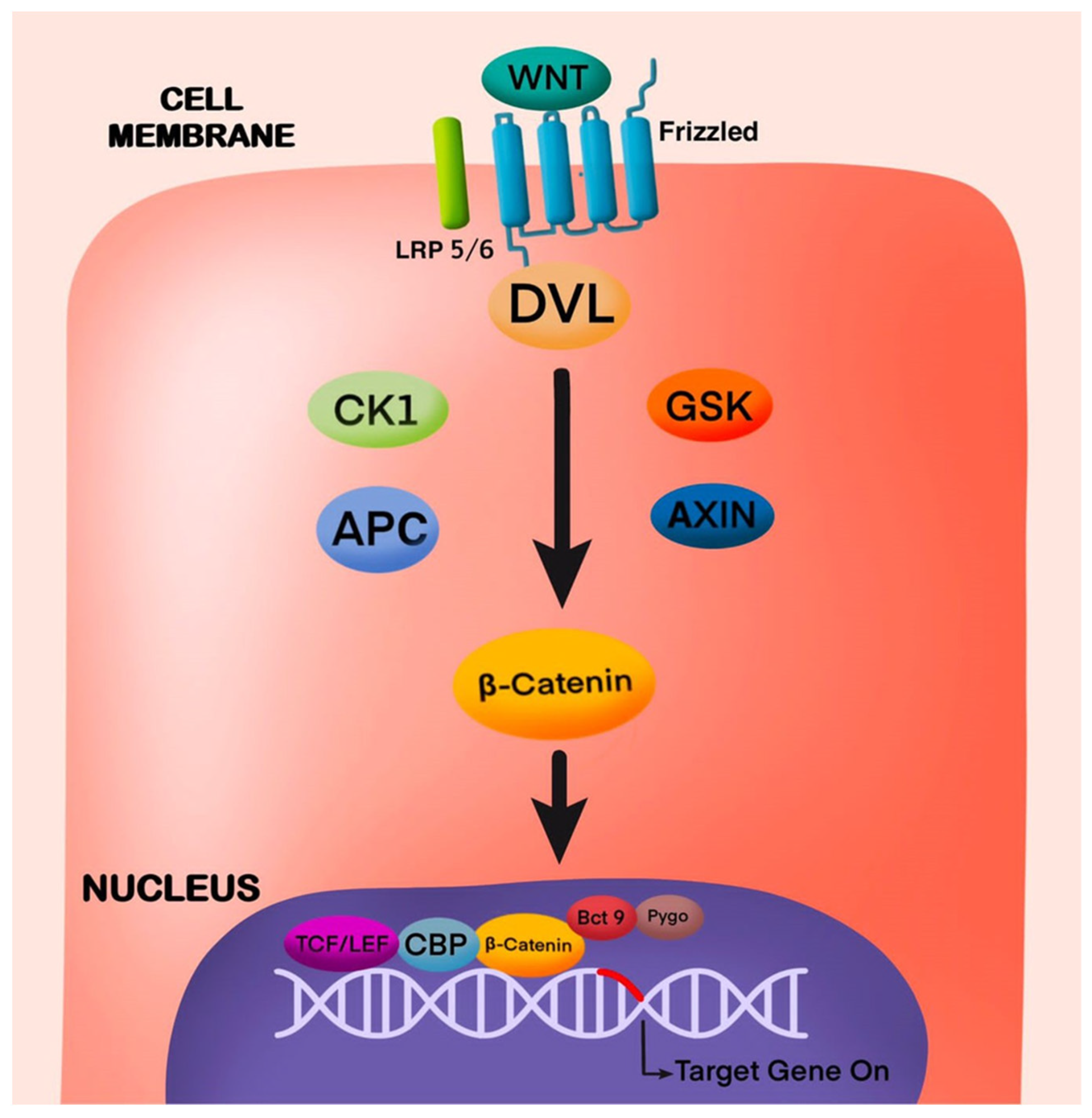
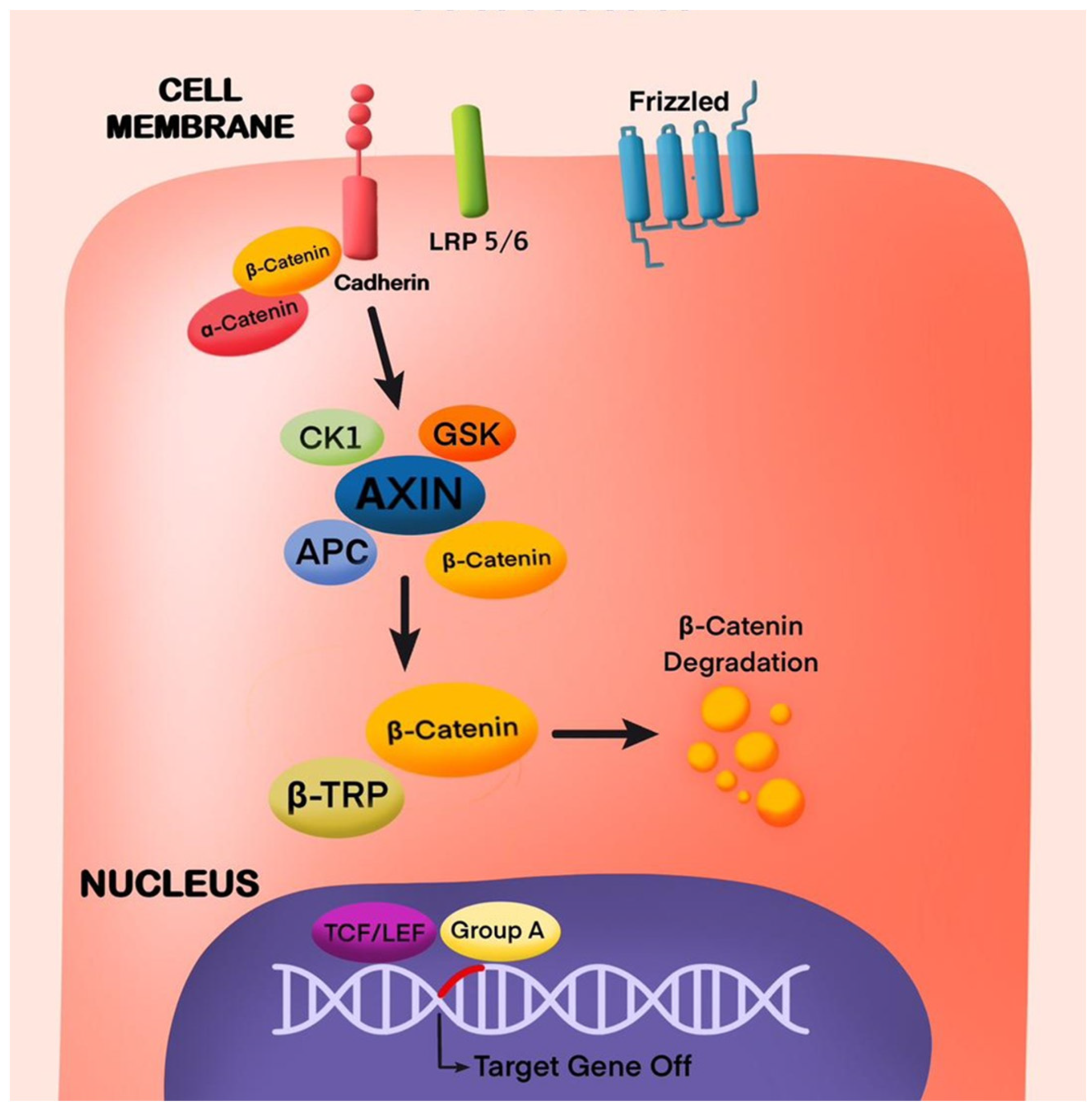
4.1. Wnt/β-Catenin Pathway in Odontogenesis
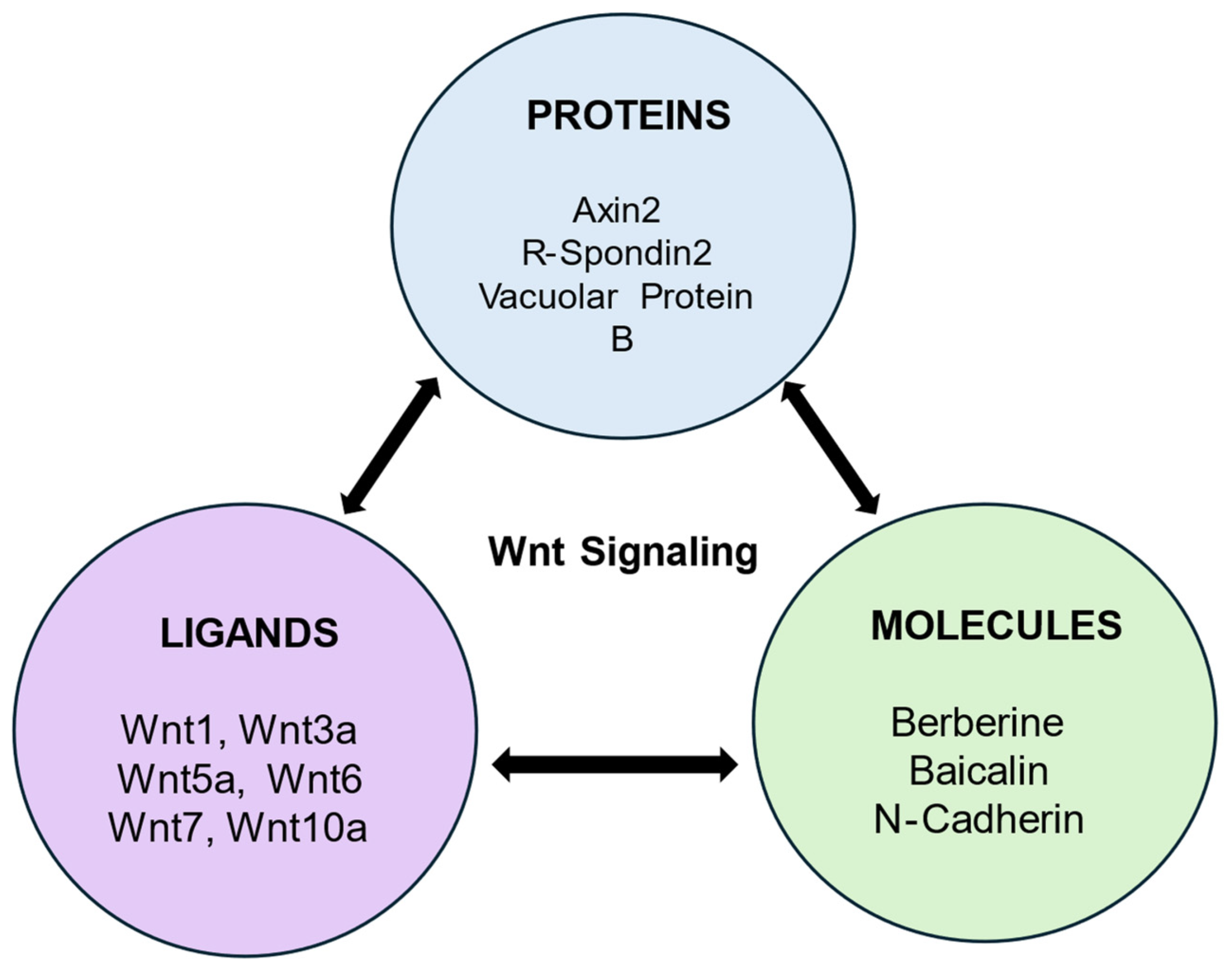
4.2. Wnt Ligands and Their Role in Dentin and Pulp Regeneration
4.2.1. Wnt1
4.2.2. Wnt3a
4.2.3. Wnt3a and Wnt10a in Dentin Bridge Formation
4.2.4. Wnt10a
4.2.5. Wnt10a, a Non-Cellular Agent for Dentin Pulp Regeneration
4.2.6. Wnt7b
4.2.7. Axin2
4.2.8. R-Spondin 2
4.3. Bioactive Molecules and Their Role in Dentin and Pulp Regeneration
4.3.1. Baicalin
4.3.2. Berberine
4.3.3. S-PRG Fillers as Dental Pulp Capping Agents
4.3.4. Vacuolar Protein 4B and Its Effect on the Wnt/β-Catenin Pathway
4.3.5. N-Cadherin
4.4. Lithium Chloride and Wnt/β-Catenin Pathway
4.5. Treated Dentin Matrix and Its Effect on hDPSCs via Wnt/β Catenin Pathway
4.6. Role of Wnt Signalling in Progression of Diabetic-Induced Cellular Aging
4.7. Role of Wnt Pathway in Dentin and Pulp Repair
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Angelova Volponi, A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth Repair and Regeneration. Curr. Oral Health Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, M.; Xing, X.; Li, X.; Shi, C. Effect of Wnt/β-Catenin Signaling Pathway on Repair and Regeneration after Dentin-Pulp Injury. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Prakash, S.; Swaminathan, U. β-Catenin in Health: A Review. J. Oral Maxillofac. Pathol. 2015, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp Capping Materials Modulate the Balance between Inflammation and Regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) In Vitro and In Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Chen, Z.; Chen, J.; Xu, H.; Xu, Y.; Yang, T.; Zhang, Q. RGD-and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration. Int. J. Nanomed. 2020, 15, 6631–6647. [Google Scholar] [CrossRef]
- Inostroza, C.; Vega-Letter, A.M.; Brizuela, C.; Castrillón, L.; Saint Jean, N.; Duran, C.M.; Carrión, F. Mesenchymal Stem Cells Derived from Human Inflamed Dental Pulp Exhibit Impaired Immunomodulatory Capacity In Vitro. J. Endod. 2020, 46, 1091–1098.e2. [Google Scholar] [CrossRef]
- About, I. Dentin–Pulp Regeneration: The Primordial Role of the Microenvironment and Its Modification by Traumatic Injuries and Bioactive Materials. Endod. Top. 2013, 28, 61–89. [Google Scholar] [CrossRef]
- Tsutsui, T. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning Adv. Appl. 2020, 13, 33–42. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. An Integral Program for Tissue Renewal and Regeneration: Wnt Signaling and Stem Cell Control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.L.; Smith, A.A.; Helms, J.A. Wnt Signaling and Injury Repair. Cold Spring Harb. Perspect. Biol. 2012, 4, a008078. [Google Scholar] [CrossRef] [PubMed]
- Babb, R.; Chandrasekaran, D.; Carvalho Moreno Neves, V.; Sharpe, P.T. Axin2-Expressing Cells Differentiate into Reparative Odontoblasts via Autocrine Wnt/β-Catenin Signaling in Response to Tooth Damage. Sci. Rep. 2017, 7, 3102. [Google Scholar] [CrossRef]
- Birjandi, A.A.; Sharpe, P. Wnt Signalling in Regenerative Dentistry. Front. Dent. Med. 2021, 2, 725468. [Google Scholar] [CrossRef]
- Tamura, M.; Nemoto, E. Role of the Wnt Signaling Molecules in the Tooth. Jpn. Dent. Sci. Rev. 2016, 52, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, S.; Langton, P.F.; Zebisch, M.; Howell, S.A.; Chang, T.-H.; Liu, Y.; Feizi, T.; Bineva, G.; O’Reilly, N.; Snijders, A.P.; et al. Notum Deacylates Wnt Proteins to Suppress Signalling Activity. Nature 2015, 519, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nör, F.; Oh, M.; Cucco, C.; Shi, S.; Nör, J.E. Wnt/β-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. Stem Cells 2016, 34, 1576–1587. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The Role of the Wnt/β-Catenin Signaling Pathway in Formation and Maintenance of Bone and Teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef]
- Lu, X.; Yang, J.; Zhao, S.; Liu, S. Advances of Wnt Signalling Pathway in Dental Development and Potential Clinical Application. Organogenesis 2019, 15, 101–110. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Ke, J.; Xu, H.E.; Williams, B.O. Lipid Modification in Wnt Structure and Function. Curr. Opin. Lipidol. 2013, 24, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, C.; Tong, J.; Su, Y.; Lin, Y.; Zhou, X.; Ye, L. WNT6 Promotes the Migration and Differentiation of Human Dental Pulp Cells Partly through C-Jun N-Terminal Kinase Signaling Pathway. J. Endod. 2014, 40, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Kawashima, N.; Yamamoto, M.; Takimoto, K.; Zhou, M.; Suzuki, N.; Saito, M.; Harada, H.; Suda, H. Wnt11 Expression in Rat Dental Pulp and Promotional Effects of W Nt Signaling on Odontoblast Differentiation. Congenit. Anom. 2013, 53, 101–108. [Google Scholar] [CrossRef]
- Peng, L.; Ren, L.B.; Dong, G.; Wang, C.L.; Xu, P.; Ye, L.; Zhou, X.D. Wnt5a Promotes Differentiation of Human Dental Papilla Cells. Int. Endod. J. 2010, 43, 404–412. [Google Scholar] [CrossRef]
- Wang, C.; Ren, L.; Peng, L.; Xu, P.; Dong, G.; Ye, L. Effect of Wnt6 on Human Dental Papilla Cells In Vitro. J. Endod. 2010, 36, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Y.; Su, Y.; Li, R.; Lin, Y.; Zhou, X.; Ye, L. C-Jun N-Terminal Kinase (JNK) Mediates Wnt5a-Induced Cell Motility Dependent or Independent of RhoA Pathway in Human Dental Papilla Cells. PLoS ONE 2013, 8, e69440. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Kim, W.-J.; Chung, S.H.; Woo, K.M. Nanofibrous topography-driven altered responsiveness to Wnt5a mediates the three-dimensional polarization of odontoblasts. Mater. Today Bio. 2022, 17, 100479. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bardet, C.; Mouraret, S.; Liu, B.; Singh, G.; Sadoine, J.; Dhamdhere, G.; Smith, A.; Tran, X.V.; Joy, A.; et al. Wnt Acts as a Prosurvival Signal to Enhance Dentin Regeneration. J. Bone Miner. Res. 2015, 30, 1150–1159. [Google Scholar] [CrossRef]
- Chen, J.; Tu, X.; Esen, E.; Joeng, K.S.; Lin, C.; Arbeit, J.M.; Rüegg, M.A.; Hall, M.N.; Ma, L.; Long, F. WNT7B Promotes Bone Formation in Part through mTORC1. PLoS Genet. 2014, 10, e1004145. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, W.; Lu, M.M.; Morrisey, E.E. Wnt7b Activates Canonical Signaling in Epithelial and Vascular Smooth Muscle Cells through Interactions with Fzd1, Fzd10, and LRP5. Mol. Cell. Biol. 2005, 25, 5022–5030. [Google Scholar] [CrossRef]
- Yamashiro, T.; Zheng, L.; Shitaku, Y.; Saito, M.; Tsubakimoto, T.; Takada, K.; Takano-Yamamoto, T.; Thesleff, I. Wnt10a Regulates Dentin Sialophosphoprotein mRNA Expression and Possibly Links Odontoblast Differentiation and Tooth Morphogenesis. Differentiation 2007, 75, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, D.; Wang, L.; Feng, H. Down-Regulation of Wnt10a Affects Odontogenesis and Proliferation in Mesenchymal Cells. Biochem. Biophys. Res. Commun. 2013, 434, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, Q.; Tian, H.; Lv, P.; Zhou, C.; Gao, X. Effects of WNT10A on Proliferation and Differentiation of Human Dental Pulp Cells. J. Endod. 2014, 40, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Cheng, R.; Wang, F.; Yang, H.; Cheng, L.; Hu, T. β-Catenin and Rho GTPases as Downstream Targets of TGF-Β1 during Pulp Repair. Cell Biol. Int. 2011, 35, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Bae, C.H.; Lee, J.C.; Ko, S.O.; Yang, X.; Jiang, R.; Cho, E.S. β-Catenin Is Required in Odontoblasts for Tooth Root Formation. J. Dent. Res. 2013, 92, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Khalid, S.; Ghafoor, S.; Woo, K.M.; Lee, E.H.; Samie, M.; Konain, K.; Ponnusamy, S.; Arany, P.; Rahman, S.U. Tideglusib-Incorporated Nanofibrous Scaffolds Potently Induce Odontogenic Differentiation. J. Biomater. Appl. 2023, 38, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Birjandi, A.A.; Suzano, F.R.; Sharpe, P.T. Drug Repurposing in Dentistry: Towards Application of Small Molecules in Dentin Repair. Int. J. Mol. Sci. 2020, 21, 6394. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Gu, S.; Liang, J. Effects of Wnt/Β-catenin Signalling on Proliferation and Differentiation of Apical Papilla Stem Cells. Cell Prolif. 2012, 45, 121–131. [Google Scholar] [CrossRef]
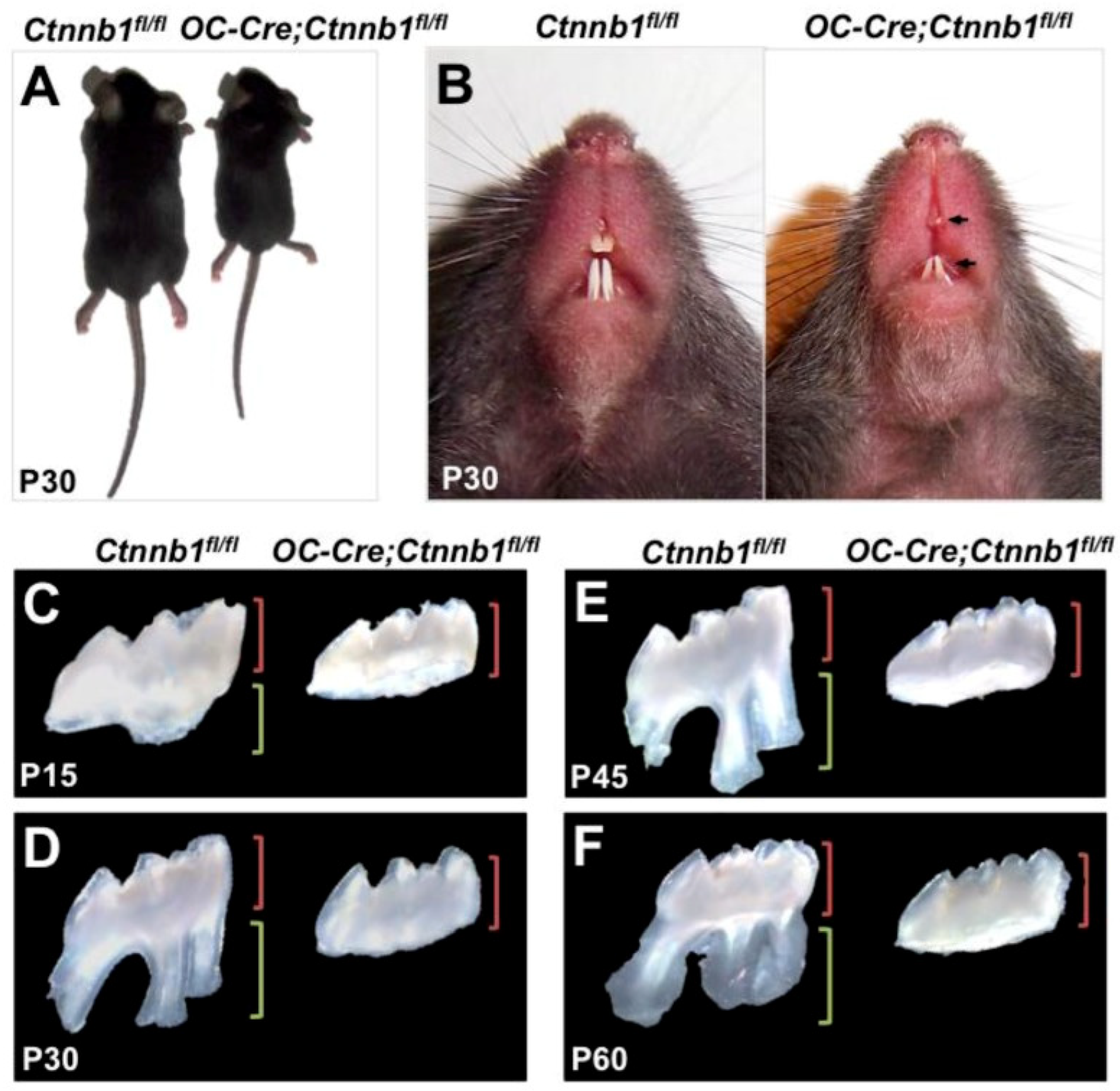
- Zhang, R.; Yang, G.; Wu, X.; Xie, J.; Yang, X.; Li, T. Disruption of Wnt/β-Catenin Signaling in Odontoblasts and Cementoblasts Arrests Tooth Root Development in Postnatal Mouse Teeth. Int. J. Biol. Sci. 2013, 9, 228–236. [Google Scholar] [CrossRef]
- Han, N.; Zheng, Y.; Li, R.; Li, X.; Zhou, M.; Niu, Y.; Zhang, Q. β-Catenin Enhances Odontoblastic Differentiation of Dental Pulp Cells through Activation of Runx2. PLoS ONE 2014, 9, e88890. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Papachristou, E.; Koidis, P.; Geurtsen, W. Wnt/β-Catenin Signaling Regulates Dental Pulp Stem Cells’ Responses to Pulp Injury by Resinous Monomers. Dent. Mater. 2015, 31, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, J.; Wang, C.; Yu, F.; Huang, D.; Ye, L. The WNT7B Protein Promotes the Migration and Differentiation of Human Dental Pulp Cells Partly through WNT/Beta-Catenin and c-Jun N-Terminal Kinase Signalling Pathways. Arch. Oral Biol. 2018, 87, 54–61. [Google Scholar] [CrossRef]
- Ali, M.; Okamoto, M.; Komichi, S.; Watanabe, M.; Huang, H.; Takahashi, Y.; Hayashi, M. Lithium-Containing Surface Pre-Reacted Glass Fillers Enhance hDPSC Functions and Induce Reparative Dentin Formation in a Rat Pulp Capping Model through Activation of Wnt/β-Catenin Signaling. Acta Biomater. 2019, 96, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, X.; Bellido, T.; Helms, J.A. A Correlation between Wnt/Beta-Catenin Signaling and the Rate of Dentin Secretion. J. Endod. 2019, 45, 1357–1364.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Bao, Y.; Yu, H.; Zhou, Y.; Lu, Q. Berberine Accelerates Odontoblast Differentiation by Wnt/β-Catenin Activation. Cell. Reprogramming 2019, 21, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yuan, S.; Sun, J.; Wang, Y.; Liu, S.; Guo, R.; Dong, W.; Li, R. R-Spondin 2 Induces Odontogenic Differentiation of Dental Pulp Stem/Progenitor Cells via Regulation of Wnt/β-Catenin Signaling. Front. Physiol. 2020, 11, 918. [Google Scholar] [CrossRef] [PubMed]
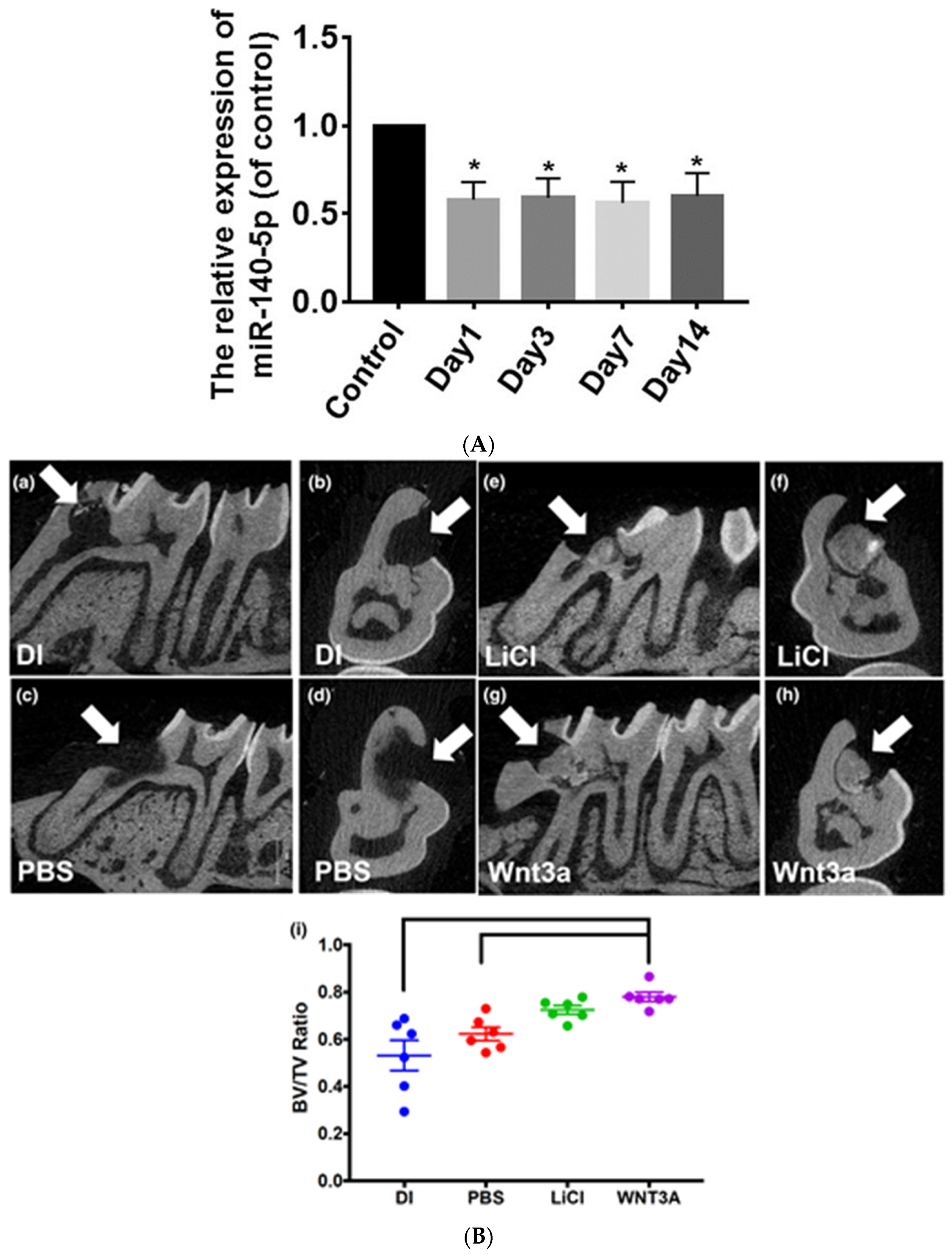
- Lu, X.; Chen, X.; Xing, J.; Lian, M.; Huang, D.; Lu, Y.; Feng, G.; Feng, X. miR-140-5p Regulates the Odontoblastic Differentiation of Dental Pulp Stem Cells via the Wnt1/β-Catenin Signaling Pathway. Stem. Cell. Res. Ther. 2019, 10, 226. [Google Scholar] [CrossRef]
- Yaemkleebbua, K.; Osathanon, T.; Nowwarote, N.; Limjeerajarus, C.N.; Sukarawan, W. Analysis of Hard Tissue Regeneration and Wnt Signalling in Dental Pulp Tissues after Direct Pulp Capping with Different Materials. Int. Endod. J. 2019, 52, 1605–1616. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, T.; Peng, L.; Chen, Z.; Li, M.; Zhang, K.; Xiong, F.; Wu, B. Vacuolar Protein Sorting 4B Regulates the Proliferation and Odontoblastic Differentiation of Human Dental Pulp Stem Cells through the Wnt-β-Catenin Signalling Pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2575–2584. [Google Scholar] [CrossRef]
- Zaugg, L.K.; Banu, A.; Walther, A.R.; Chandrasekaran, D.; Babb, R.C.; Salzlechner, C.; Hedegaard, M.A.B.; Gentleman, E.; Sharpe, P.T. Translation Approach for Dentine Regeneration Using GSK-3 Antagonists. J. Dent. Res. 2020, 99, 544–551. [Google Scholar] [CrossRef]
- Tokavanich, N.; Wein, M.N.; English, J.D.; Ono, N.; Ono, W. The Role of Wnt Signaling in Postnatal Tooth Root Development. Front. Dent. Med. 2021, 2, 769134. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Horibe, K.; Mori, H.; Nakamura, H. The Role of Canonical Wnt Signaling in Dentin Bridge Formation. J. Oral Biosci. 2021, 63, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yan, W.; Dai, X.; Chen, M.; Qu, Q.; Wu, B.; Zhao, W. N-cadherin regulates the odontogenic differentiation of dental pulp stem cells via β-catenin activity. Front. Cell Dev. Biol. 2021, 9, 661116. [Google Scholar] [CrossRef]
- Wang, J.; Ran, S.; Liu, B.; Gu, S. Monitoring of Canonical BMP and Wnt Activities during Postnatal Stages of Mouse First Molar Root Formation. J. Appl. Oral Sci. 2021, 29, e20210281. [Google Scholar] [CrossRef] [PubMed]
- Vijaykumar, A.; Mina, M. Lithium Chloride Exerts Differential Effects on Dentinogenesis and Osteogenesis in Primary Pulp Cultures. Front. Dent. Med. 2021, 2, 649500. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yuan, S.; Yang, Y.; Gong, Y.; Wang, Y.; Guo, R.; Zhang, X.; Liu, Y.; Mi, H.; et al. Treated Dentin Matrix Induces Odontogenic Differentiation of Dental Pulp Stem Cells via Regulation of Wnt/β-Catenin Signaling. Bioact. Mater. 2022, 7, 85–97. [Google Scholar] [CrossRef]
- Asghari, M.; Nasoohi, N.; Hodjat, M. High Glucose Promotes the Aging of Human Dental Pulp Cells through Wnt/Beta-Catenin Signaling. Dent. Med. Probl. 2021, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Kobayashi, Y.; Yamauchi, Y.; Quispe-Salcedo, A.; Chao Feng, Z.; Huang, J.; Partridge, N.C.; Nakatani, T.; D’Armiento, J.; Shimizu, E. The Critical Role of MMP13 in Regulating Tooth Development and Reactionary Dentinogenesis Repair through the Wnt Signaling Pathway. Front. Cell Dev. Biol. 2022, 10, 883266. [Google Scholar] [CrossRef]
- Fu, T.; Liu, Y.; Huang, X.; Guo, Y.; Shen, J.; Shen, H. lncRNA SNHG1 Regulates Odontogenic Differentiation of Human Dental Pulp Stem Cells via miR-328-3p/Wnt/β-Catenin Pathway. Stem. Cell. Res. Ther. 2022, 13, 311. [Google Scholar] [CrossRef]
- Sukarawan, W.; Rattanawarawipa, P.; Yaemkleebbua, K.; Nowwarote, N.; Pavasant, P.; Limjeerajarus, C.N.; Osathanon, T. Wnt3a Promotes Odonto/Osteogenic Differentiation in Vitro and Tertiary Dentin Formation in a Rat Model. Int. Endod. J. 2023, 56, 514–529. [Google Scholar] [CrossRef]
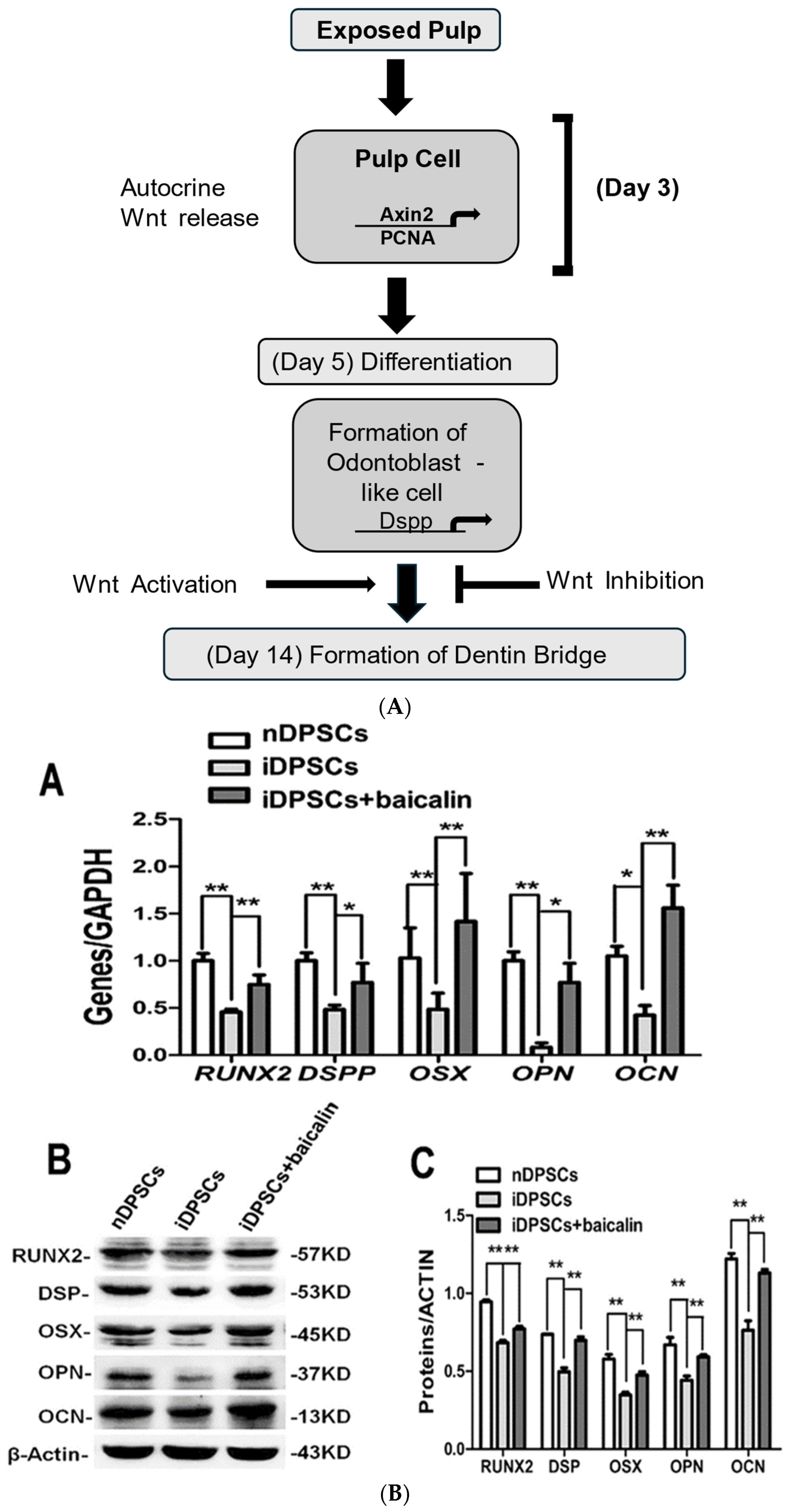
- Li, M.; Wang, Y.; Xue, J.; Xu, Q.; Zhang, Y.; Liu, J.; Xu, H.; Guan, Z.; Bian, C.; Zhang, G.; et al. Baicalin Can Enhance Odonto/Osteogenic Differentiation of Inflammatory Dental Pulp Stem Cells by Inhibiting the NF-κB and β-Catenin/Wnt Signaling Pathways. Mol. Biol. Rep. 2023, 50, 4435–4446. [Google Scholar] [CrossRef] [PubMed]
- Sakatoku, S.; Hayashi, Y.; Futenma, T.; Ishizaka, R.; Gemba, C.; Nawa, H. Wnt10a Is a Candidate as a Non-Cellular Agent for Induction of Dental Pulp Regeneration with Dentine-Inducing Capacity. J. Hard Tissue Biol. 2023, 32, 41–48. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sakatoku, S.; Sugita, Y.; Futenma, T.; Iida, N.; Nakamura, K.; Nawa, H. A Potential Biomarker of Dental Pulp Regeneration: Wnt10a. J. Hard Tissue Biol. 2023, 32, 197–202. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, T.; Wang, H.; Xu, S.; Yu, H.; Luo, X.; Hao, C.; Wu, B.; Ma, D. Stathmin Regulates the Proliferation and Odontoblastic/Osteogenic Differentiation of Human Dental Pulp Stem Cells through Wnt/β-Catenin Signaling Pathway. J. Proteom. 2019, 202, 103364. [Google Scholar] [CrossRef] [PubMed]
- Neves, V.C.M.; Yianni, V.; Sharpe, P.T. Macrophage Modulation of Dental Pulp Stem Cell Activity during Tertiary Dentinogenesis. Sci. Rep. 2020, 10, 20216. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, X.; Feng, X.; Gu, Z.; Gu, Y.; Lian, M.; Xiao, J.; Cao, P.; Zheng, K.; Gu, X.; et al. NRP1 Accelerates Odontoblast Differentiation of Dental Pulp Stem Cells Through Classical Wnt/β-Catenin Signaling. Cell. Reprogramming 2017, 19, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Peng, Y.; Peng, R.; Wei, X. Angelica Sinensis Polysaccharide Promotes the Proliferation and Osteogenic Differentiation of Human Dental Pulp Stem Cells (hDPSCs) by Activating the Wnt/β-Catenin Pathway. Qual. Assur. Saf. Crop. Foods 2021, 13, 31–37. [Google Scholar] [CrossRef]
- Vijaykumar, A.; Root, S.H.; Mina, M. Wnt/β-Catenin Signaling Promotes the Formation of Preodontoblasts In Vitro. J. Dent. Res. 2021, 100, 387–396. [Google Scholar] [CrossRef]
- Cate, A.T. The Role of Epithelium in the Development, Structure and Function of the Tissues of Tooth Support. Oral Dis. 1996, 2, 55–62. [Google Scholar] [CrossRef]
- Bae, C.-H.; Kim, T.-H.; Chu, J.-Y.; Cho, E.-S. New Population of Odontoblasts Responsible for Tooth Root Formation. Gene Expr. Patterns 2013, 13, 197–202. [Google Scholar] [CrossRef]
- Aurrekoetxea, M.; Irastorza, I.; García-Gallastegui, P.; Jiménez-Rojo, L.; Nakamura, T.; Yamada, Y.; Ibarretxe, G.; Unda, F.J. Wnt/β-Catenin Regulates the Activity of Epiprofin/Sp6, SHH, FGF, and BMP to Coordinate the Stages of Odontogenesis. Front. Cell Dev. Biol. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, X.; Liu, B.; Tulu, U.S.; Helms, J.A. Wnt-Responsive Odontoblasts Secrete New Dentin after Superficial Tooth Injury. J. Dent. Res. 2018, 97, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Harichane, Y.; Hirata, A.; Dimitrova-Nakov, S.; Granja, I.; Goldberg, A.; Kellermann, O.; Poliard, A. Pulpal Progenitors and Dentin Repair. Adv. Dent. Res. 2011, 23, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Woo, K.M.; Tahir, M.; Wu, W.; Elango, J.; Mirza, M.R.; Khan, M.; Shamim, S.; Arany, P.R.; Rahman, S.U. Enhancing osteoblast differentiation through small molecule-incorporated engineered nanofibrous scaffold. Clin. Oral Investig. 2022, 26, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Hong, H.; Zhang, X.; Chen, D.; Chen, Z.; Ling, J.; Wu, L. Down-Regulated lncRNA MEG3 Promotes Osteogenic Differentiation of Human Dental Follicle Stem Cells by Epigenetically Regulating Wnt Pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2061–2067. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, Y.; Chen, G.; Yan, Z.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. Hertwig’s Epithelial Root Sheath Cells Regulate Osteogenic Differentiation of Dental Follicle Cells through the Wnt Pathway. Bone 2014, 63, 158–165. [Google Scholar] [CrossRef]
- Trosset, J.; Dalvit, C.; Knapp, S.; Fasolini, M.; Veronesi, M.; Mantegani, S.; Gianellini, L.M.; Catana, C.; Sundström, M.; Stouten, P.F.W.; et al. Inhibition of Protein–Protein Interactions: The Discovery of Druglike Β-catenin Inhibitors by Combining Virtual and Biophysical Screening. Proteins 2006, 64, 60–67. [Google Scholar] [CrossRef]
- Paula, A.B.; Laranjo, M.; Marto, C.-M.; Paulo, S.; Abrantes, A.M.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Direct Pulp Capping: What Is the Most Effective Therapy?—Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2018, 18, 298–314. [Google Scholar] [CrossRef]
- Paula, A.; Laranjo, M.; Marto, C.M.; Abrantes, A.M.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E. BiodentineTM Boosts, WhiteProRoot®MTA Increases and Life® Suppresses Odontoblast Activity. Materials 2019, 12, 1184. [Google Scholar] [CrossRef]
- Paula, A.B.; Laranjo, M.; Marto, C.-M.; Paulo, S.; Abrantes, A.M.; Fernandes, B.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Evaluation of Dentinogenesis Inducer Biomaterials: An in Vivo Study. J. Appl. Oral Sci. 2020, 28, e20190023. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.S.; Melton, D.A. A Molecular Mechanism for the Effect of Lithium on Development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R. Wnt Signaling in Disease and in Development. Cell Res. 2005, 15, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Galler, K.; Schmalz, G.; Cosentino, C.; Rengo, S.; Schweikl, H. Inhibition of Phosphatidylinositol 3-Kinase Amplifies TEGDMA-Induced Apoptosis in Primary Human Pulp Cells. J. Dent. Res. 2004, 83, 703–707. [Google Scholar] [CrossRef] [PubMed]
| S | Year | Author | Type of Study | Sample Size | Objective | Conclusion | Recommendation/Limitation |
|---|---|---|---|---|---|---|---|
| 1 | 2012 | J Wang et al. [38] | In vitro | n = 8 3 molars 18–20 age | Role of Wnt/β-catenin signaling pathway in SCAP proliferation and differentiation. | Proliferation and odonto/osteogenic differentiation of SCAP are promoted by the Wnt/β-catenin signaling pathway. | Further study in SCAP mineralization and proliferation may bring improvement in dental tissue engineering. |
| 2 | 2013 | Ran Zhang et al. [39] | In vivo | Wnt signaling and its importance in cementogenesis and odontogenesis. | Wnt signaling encourages the differentiation of odontoblasts from mesenchyme. | Influence of Wnt signaling on tooth development. | |
| 3 | 2014 | Zichun Zhang et al. [33] | In vivo | 9 | Response of Wnt10a on DPSCs. | Proliferation of DPCS is promoted, and their odontoblastic differentiation is negatively regulated by Wnt10a. | Further research is vital to understand the biological effect of Wnt10a on DPCS. |
| 4 | 2014 | Nana Han et al. [40] | In vivo | β-catenin during the activation of Runx2 in DPCs augments odontoblastic differentiation. | Odontoblastic differentiation in tertiary dentin modulated by β-catenin. | The mechanism of reparative dentin formation remains unclear. | |
| 5 | 2015 | Danial J Hunter et al. [28] | In vivo | 72 mice | Response of injured pulp to amplified Wnt signaling resulting in superior pulp healing. | Amplified Wnt signaling caused pulp cells to differentiate into secretory odontoblasts, thus improving pulp vitality. | The role of amplified Wnt signaling on pulp cells plays a role in promoting pulp vitality, treating dental caries, and alternative approaches for root canal treatment. |
| 6 | 2015 | Athina Bakopoulou et al. [41] | In vitro | 3 molars age 18–24. DPSCs cultured from 3 donors. | The function of the Wnt/β-catenin pathway in the healing of pulpal injury resulted from resinous monomers via DPSC mediation. | DPSCs, under the effect of Wnt/β-catenin, participate in pulp healing after injury. | The analysis of stem cells is important for pulp regenerative therapy and bioactive molecule planning via tissue engineering through Wnt activation. |
| 7 | 2016 | Masato Tamura et al. [15] | Review Article | Determination of function of signaling molecules of Wnt in tooth. | Tooth development, maintenance, and turnover are regulated by both canonical and non-canonical Wnt pathway. | The Wnt signaling pathway and its progress in future therapeutics for tooth regeneration. | |
| 8 | 2017 | Hongyang LV et al. [42] | In vivo/In vitro | HDPCs cultured from 3 molars from 19 to 24 years of age. | Function of Wnt7b protein in HDPCs relocation and differentiation. | Wnt/β-catenin and JNK pathways partly have a role in the differentiation of HDPCs and are promoted by the Wnt7b protein. | Wnt7b can be used in the potential treatment of dental caries and tooth trauma. Wnt7b plays a role in maintaining the vitality of the tooth. |
| 9 | 2017 | Rebecca Babb et al. [13] | In vivo | The role of Axin 2 via Wnt/β-catenin signaling in tertiary dentinogenesis. | As a response to tooth injury, new odontoblast-like cells are formed via the Wnt signaling pathway, and Axin 2 cells provide a signal in reparative dentinogenesis. | Further study is required for stem cell-based tooth repair via the Wnt/β-catenin signaling pathway in secondary and tertiary dentinogenesis | |
| 10 | 2018 | Manahil Ali et al. [43] | In vivo/ In vitro | 21 | To check the enhancement of S-PRG cement through the addition of LiCl and its effect on hDPSCs. | S-PRG cement through the Wnt/β-catenin pathway enhances reparative dentin formation in rat teeth and promotes hDPSC profiles. | Inclination of reparative dentinogenesis in clinical trials needs to be further assessed. The interaction of Li ions with S-PRG fillers is not fully understood. |
| 11 | 2019 | Yuan Zhao et al. [44] | In vivo | 2 strains of Wnt reporter mice | The function of Wnt signaling in age-linked variations in pulp and its ability to respond to subacute traumas. | Amplification of Wnt signaling results in reparative/osteodentin organization, as the secretion of dentin from odontoblasts is regulated by this pathway. | Wnt signaling and its role in dentin secretion can be used to form biological pulp-capping material. |
| 12 | 2019 | Xi Lu et al. [19] | Review Article | Review of Wnt signaling pathway, its modulators, and their function in odontogenesis and therapeutic potential. | Tooth development is closely regulated by the canonical Wnt pathway. | The tooth regeneration method may be provided by a detailed study in Wnt signaling. | |
| 13 | 2019 | Anqian Wu et al. [45] | In vitro | (N) n = 20 pulp Ages 18–25 | Impact of berberine on DPSCs and their odontoblast differentiation. | By the stimulation of the Wnt pathway, berberine encourages odontoblast differentiation. | Berberine might be used as a new drug for the treatment of dental defects. |
| 14 | 2019 | Yuping Gong et al. [46] | In vivo | 20 | Role of Rspo2 in promoting hDPSCs to differentiate odontogenically through the Wnt/β-catenin pathway. | hDPSCs stimulated by Rspo2, both positive and negative, by enhancer and silencer agents, thus enhancing their proliferation and differentiation. | Future work of R-spondins and Wnt ligands could be on therapeutic use clinically. |
| 15 | 2019 | Xiaohui Lu et al. [47] | In vitro | n = 10 3 molars age 14–22 yrs | miR-140-5p impact on the odontoblastic differentiation of DPSC. | The odontoblastic differentiation of DPSCs is regulated by lowering miR-140-5p by targeting the Wnt/β-catenin pathway. | miR-140-5p can be used as a medicinal agent that could control odontoblastic differentiation in dental medicine. |
| 16 | 2019 | K. Yaemkleebua et al. [48] | In vivo | 32 rat molar pulps were used. | Hard tissue formation after application of pulp capping materials on pinpoint exposure and the role of Wnt signaling and cell cycle regulation. | Reparative dentinogenesis occurred with the application of pulp capping materials, and cyclin D1 expression was seen. | The association between Wnt signaling and reparative dentin formation might be used to comprehend the response of normal pulp in injured conditions. |
| 17 | 2019 | Yuhua Pan et al. [49] | In vivo In vitro | DPSCs culture from 3 molars of 18–20 yr Donors | The role of VPS4B in DPSCs via the Wnt/β-catenin signaling pathway. | DPSC differentiation and proliferation are regulated by VPS4B via the Wnt/β-catenin pathway. | Dentin dysplasia type1 could be treated via potential medicinal therapies by the formation of dentin and reparative endodontic therapy. |
| 18 | 2020 | LK Zaugg et al. [50] | In vivo/ In vitro | Adult male Wistar rats (7 wk old) and CD1 mice (6 wk old) | The Wnt/β-catenin signaling and its function in reparative dentinogenesis and how it is affected by the introduction of GSK3β inhibitor drugs. | GSK3β inhibitor results in the tertiary dentin formation at the defect region having the size of a human lesion; however, this is not true regeneration. | Augmentation of reparative dentinogenesis can be used potentially as a treatment in direct pulp capping. |
| 19 | 2021 | Nicha Tokavanich et al. [51] | Review Article | Wnt signaling and its effect on postnatal tooth development. | Wnt signaling may play a vital role as a target for root formation and tooth eruption disorders. | In-depth study of the Wnt signaling pathway may provide a path in the future for regenerative dentistry. | |
| 20 | 2021 | Miroku Hara et al. [52] | In vivo | 321 mice | Dentin bridge formation at pinpoint exposure and how the Wnt signaling and its molecules affect it. | The injured dental pulp is repaired by the stimulation of canonical Wnt signaling. | Wnt pathway activation by the dental pulp capping materials may be a new approach for vital pulp therapy. |
| 21 | 2021 | Zilong Deng et al. [53] | In vitro/In vivo | 3 molars Pulp 18–25 yr n = 5 mice | N-cadherin effect on DPSCs, in-vitro and in-vivo, and its function in odontogenic differentiation. | N-cadherin negatively regulates β-catenin activity in DPSCs. | Further study is required on how neurogenic differentiation of DPSCs is regulated by N-cadherin |
| 22 | 2021 | Jia Wang et al. [54] | In vivo | 2 transgenic mice | Role of Wnt signaling and distribution of BMP during postnatal root development. | Tooth root development is regulated by BMP and Wnt signaling pathway. | Further study is required for Wnt/β-catenin pathway regulation for matrix secretion and differentiation in postnatal tooth development. |
| 23 | 2021 | Anushree Vijaykumar et al. [55] | In vitro | Pulp culture from 1 and 2 molar 5–7 d mice | Dentin pulp complex and role of LiCl in its reparation and regeneration via Wnt/β-catenin pathway by inhibiting GSK3β. | LiCl results in reparative dentin formation, which has an improved structure. | GSK3β controls several transcription factors and various pathways; thus, the influence of LiCl on tertiary dentin formation may not be deemed exclusive to the Wnt/β-catenin pathway. |
| 24 | 2021 | Sirui Liu et al. [56] | In vitro In vivo | DPSCs culture donor’s teeth pulp 18–25 yr Wistar mice 26 | Wnt/β-catenin signaling role in hDPSCs and its odontogenic differentiation via TDM extract. | TDM via GSK3β activates the Wnt/β-catenin pathway in hDPSC and stimulates its odontogenic differentiation. | The mechanism for TDM-induced differentiation requires further study. |
| 25 | 2021 | Mona Asghari et al. [57] | In vitro | 4000 cells | Effect of hyperglycemia and function of Wnt pathway in senescence of pulp cells. | Hyperglycemia causes the aging of pulp cells. Cellular aging is induced via beta-catenin. | Further study is required to determine the role of beta-catenin and pulp regeneration in diabetic patients. |
| 26 | 2022 | Henry F Duncan et al. [58] | In vivo In vitro | MMP13 deficient mice and WT control mice were used for DPCs. | The effect and interaction of MMP13 with the Wnt signaling pathway. Its effect on tooth development, repair, and dentin pulp regeneration. | MMP13 controls the organization and regulation of tooth development in addition to dentin pulp regeneration. | This study found targets for new therapy for traumatized pulp by increasing repair response. |
| 27 | 2022 | Tingting Fu et al. [59] | In vitro | DPSCs Culture Donar teeth ≤16 yr | Role of SNHG1 in hDPSCs in odontogenic differentiation via Wnt/β-catenin signaling. | The Wnt/β-catenin pathway is silenced by IncRNA SNHG1 via miR-328-3p, resulting in the differentiation of hDPSCs. | Useful in regenerative endodontics. |
| 28 | 2023 | Waleerat Sukarawan et al. [60] | In vivo | 6 mice | To check in SHEDs the influence of Wnt3a on the odonto/osteogenic differentiation and reparative dentin production. | Wnt3a causes an increase in osteogenic differentiation, silencing of proliferation in SHEDs, advances tertiary dentin formation, and can be used as a biological molecule in vital pulp therapy. | An in-depth study is required to identify the function and role of Wnt3a in tissue engineering. |
| 29 | 2023 | Mengyuan Li et al. [61] | In vitro | DPSC In 6 well plates | Effect of Baicalin on DPSC differentiation via Wnt/β/NF-kB pathway. | Baicalin impedes both NF-Kb and Wnt/β-catenin pathways, thus promoting the differentiation of DPSCs, causing repair of pulp with early irreversible pulpitis. | Further investigation and research are required to apply it in the long-term |
| 30 | 2023 | Shintaro Sakatoku et al. [62] | In vitro | DPSC in 24 well plates | Role of Wnt10a and odontoblasts in regenerative dental pulp. | Odontoblasts expressing Wnt10a play an increased role in pulp regeneration, with dentin-inducing capacity. | A detailed study after transplantation is required to check the long-term effect of Wnt10a along with DKK1 levels in regenerated dental pulp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amir, M.; Jeevithan, L.; Barkat, M.; Fatima, S.H.; Khan, M.; Israr, S.; Naseer, F.; Fayyaz, S.; Elango, J.; Wu, W.; et al. Advances in Regenerative Dentistry: A Systematic Review of Harnessing Wnt/β-Catenin in Dentin-Pulp Regeneration. Cells 2024, 13, 1153. https://doi.org/10.3390/cells13131153
Amir M, Jeevithan L, Barkat M, Fatima SH, Khan M, Israr S, Naseer F, Fayyaz S, Elango J, Wu W, et al. Advances in Regenerative Dentistry: A Systematic Review of Harnessing Wnt/β-Catenin in Dentin-Pulp Regeneration. Cells. 2024; 13(13):1153. https://doi.org/10.3390/cells13131153
Chicago/Turabian StyleAmir, Mariam, Lakshmi Jeevithan, Maham Barkat, Syeda Habib Fatima, Malalai Khan, Sara Israr, Fatima Naseer, Sarmad Fayyaz, Jeevithan Elango, Wenhui Wu, and et al. 2024. "Advances in Regenerative Dentistry: A Systematic Review of Harnessing Wnt/β-Catenin in Dentin-Pulp Regeneration" Cells 13, no. 13: 1153. https://doi.org/10.3390/cells13131153






