Generation of Therapeutically Potent Spheroids from Human Endometrial Mesenchymal Stem/Stromal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Serum-Free Media
2.3. Spheroid Formation
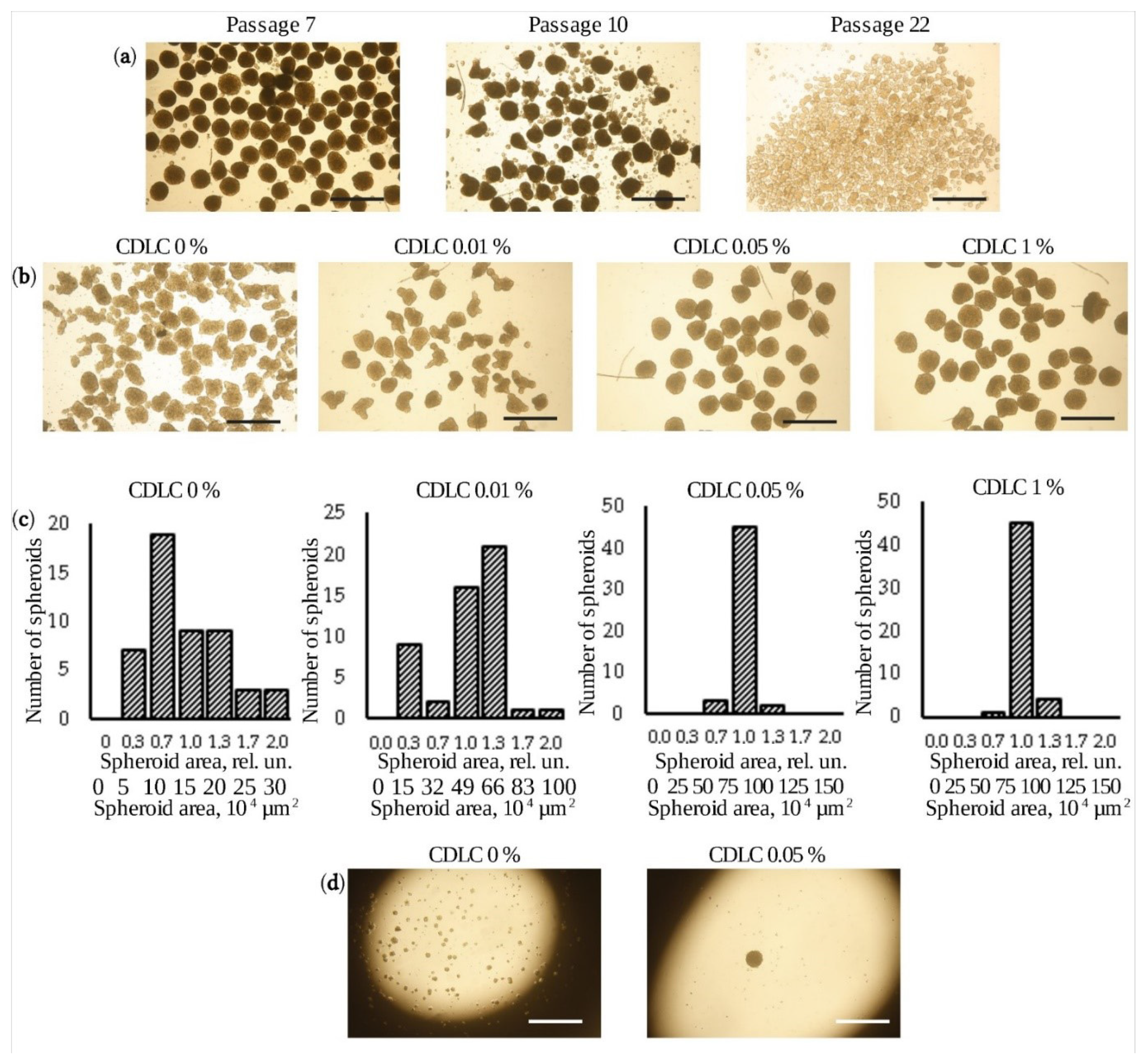
2.4. Selection of Conditions for Spheroid Formation
2.5. Immunophenotyping
2.6. Adipogenic Differentiation
2.7. Osteogenic Differentiation
2.8. qRT-PCR Assays
2.9. Animals
2.10. Animal Wound Model
2.11. Wound Healing Assay
2.12. Histology
2.13. Statistical Analysis
2.14. Ethical Approval
2.15. Statement of Human and Animal Rights
3. Results
3.1. Production of Uniform-Sized Spheroids
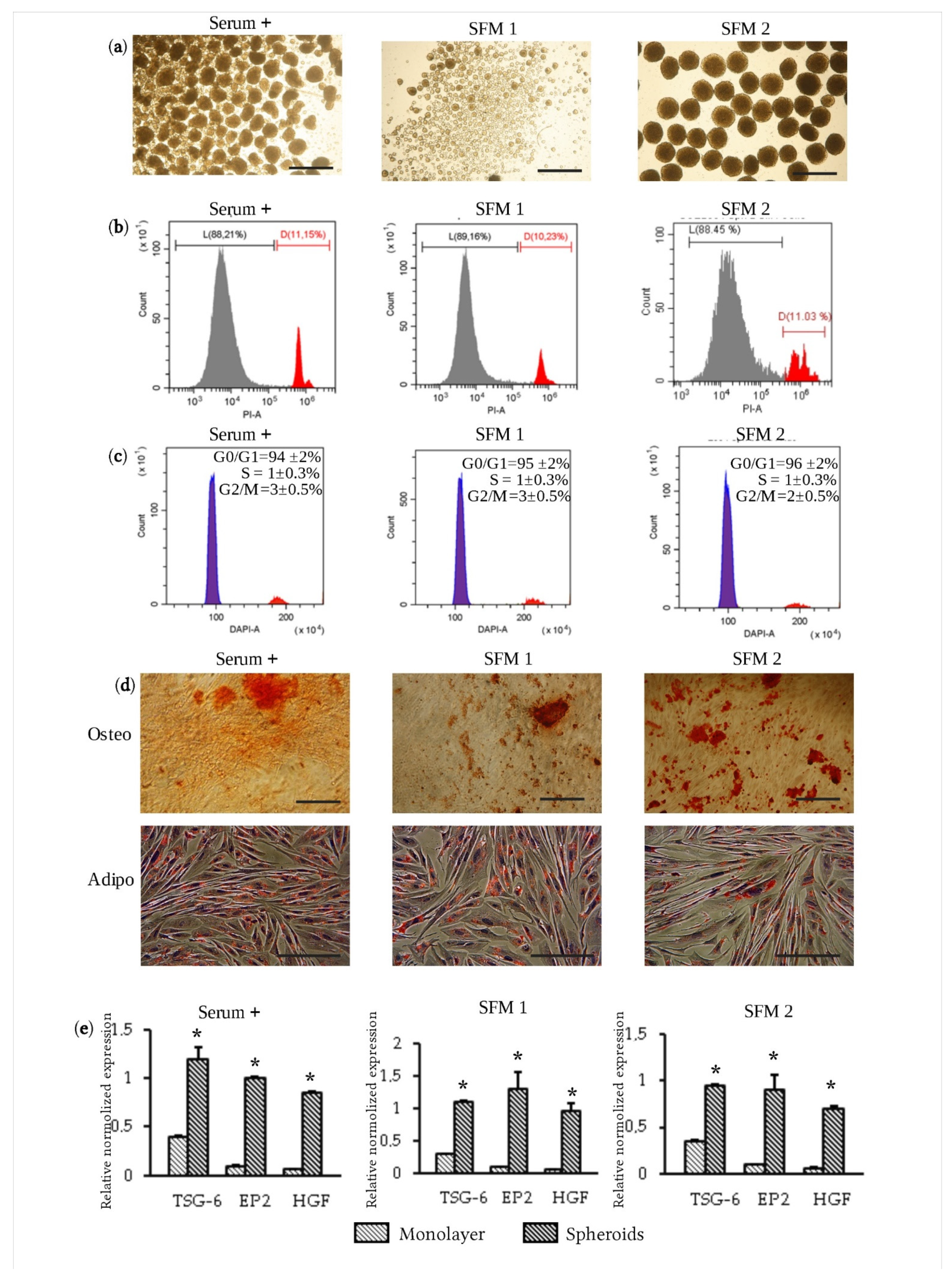
3.2. Formation of Spheroids Using Serum-Free Media
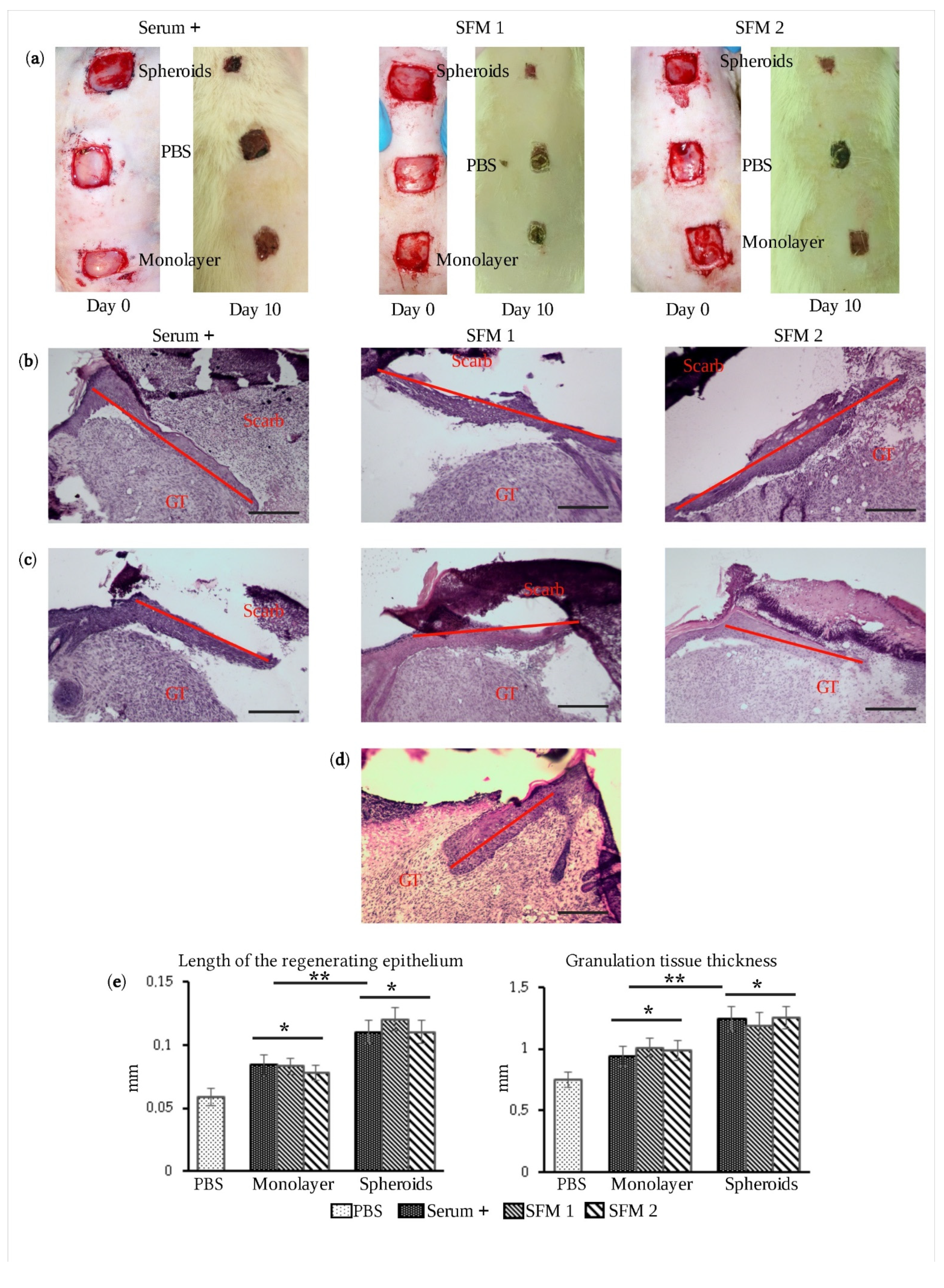
3.3. Therapeutic Efficacy of eMSC Spheroids in Serum-Free Medium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Dominici, M.; Blanc, K.L.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Prockop, D.J. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy 2017, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Kota, D.J.; Bazhanov, N.; Regerl, R.L. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J. Cell. Mol. Med. 2010, 14, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Katz, A.J. Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin. Biol. Ther. 2006, 6, 567–578. [Google Scholar] [CrossRef]
- Harris, D.T.; Badowski, M.; Ahmad, N.; Gaballa, M.A. The potential of cord blood stem cells for use in regenerative medicine. Expert Opin. Biol. Ther. 2007, 7, 1311–1322. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Park, Y.K.; Kim, Y.T.; Yang, H.; Kim, S.K. Lifetime expression of stem cell markers in the uterine endometrium. Fertil. Steril. 2004, 81, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E. Identification and characterisation of human endometrial stem/progenitor cells. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 250–253. [Google Scholar] [CrossRef]
- Zemelko, V.I.; Grinchuk, T.M.; Domnina, A.P.; Artzibasheva, I.V.; Zenin, V.V.; Kirsanov, A.A.; Bichevaia, N.K.; Korsak, V.S.; Nikolsky, N.N. Multipotent mesenchymal stem cells of desquamated endometrium: Isolation, Characterization and use as feeder layer for maintenance of human embryonic stem cell lines. Cell Tissue Biol. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Murphy, M.P.; Wang, H.; Patel, A.N.; Kambhampati, S.; Angle, N.; Chan, K.; Marleau, A.M.; Pyszniak, A.; Carrier, E.; Ichim, T.E.; et al. Allogeneic endometrial regenerative cells: An “Off the shelf solution” for critical limb ischemia? J. Transl. Med. 2008, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Domnina, A.; Novikova, P.; Obidina, J.; Fridlyanskaya, I.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Zenin, V.; Nikolsky, N. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res. Ther. 2018, 9, 50. [Google Scholar] [CrossRef]
- Domnina, A.; Ivanova, J.; Alekseenko, L.; Kozhukharova, I.; Borodkina, A.; Pugovkina, N.; Smirnova, I.; Lyublinskaya, O.; Fridlyanskaya, I.; Nikolsky, N. Three-Dimensional Compaction Switches Stress Response Programs and Enhances Therapeutic Efficacy of Endometrial Mesenchymal Stem/Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Gstraunthaler, G.; Lindl, T.; van der Valk, J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 2013, 65, 791–793. [Google Scholar] [CrossRef]
- European Medicines Agency. Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products (EMA/410/01 rev.3). Off. J. Eur. Union 2011, 73, 1–18. [Google Scholar]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the Optimal Culture Conditions for Clinical Scale Production of Human Mesenchymal Stem Cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef]
- Rowley, J.; Abraham, E.; Campbell, A.; Brandwein, H.; Oh, S. Meeting lot-size challenges of manufacturing adherent cells for therapy. Bioprocess Int. 2012, 10, 16–22. [Google Scholar]
- Spees, J.L.; Gregory, C.A.; Singh, H.; Tucker, H.A.; Peister, A.; Lynch, P.J.; Hsu, S.-C.; Smith, J.; Prockop, D.J. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol. Ther. 2004, 9, 747–756. [Google Scholar] [CrossRef]
- Shahdadfar, A.; Frønsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In Vitro Expansion of Human Mesenchymal Stem Cells: Choice of Serum Is a Determinant of Cell Proliferation, Differentiation, Gene Expression, and Transcriptome Stability. Stem Cells 2005, 23, 1357–1366. [Google Scholar] [CrossRef]
- Tan, K.Y.; Teo, K.L.; Lim, J.F.; Chen, A.K.; Choolani, M.; Reuveny, S.; Chan, J.; Oh, S.K. Serum-free media formulations are cell line-specific and require optimization for microcarrier culture. Cytotherapy 2015, 17, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Kinzebach, S.; Bieback, K. Expansion of mesenchymal stem/stromal cells under xenogenic-free culture conditions. Adv. Biochem. Eng. Biotechnol. 2013, 129, 33–57. [Google Scholar] [PubMed]
- Matthyssen, S.; Dhubhghaill, S.N.; Van Gerwen, V.; Zakaria, N. Xeno-Free Cultivation of Mesenchymal Stem Cells From the Corneal Stroma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2659–2665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Hecker, A.; Kocaömer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klüteret, H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 2009, 27, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Panchalingam, K.M.; Rosenberg, L.; Behieet, L.A. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012, 2012, 123030. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.; Duan, H.; Chai, J.; Yang, J.; Li, X.; Yu, Y.; Zhang, X.; Hu, X.; Xiao, M.; et al. TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci. Rep. 2016, 6, 30121. [Google Scholar] [CrossRef]
- Kunisch, E.; Jansen, A.; Kojima, F.; Löffler, I.; Kapoor, M.; Kawai, S.; Rubio, I.; Crofford, L.J.; Kinne, R.W. Prostaglandin E 2 Differentially Modulates Proinflammatory/Prodestructive Effects of TNF-α on Synovial Fibroblasts via Specific E Prostanoid Receptors/cAMP. J. Immunol. 2009, 183, 1328–1336. [Google Scholar] [CrossRef]
- Jankowski, K.; Kucia, M.; Wysoczynski, M.; Reca, R.; Zhao, D.; Trzyna, E.; Trent, J.; Peiper, S.; Zembala, M.; Ratajczak, J.; et al. Both Hepatocyte Growth Factor (HGF) and Stromal-Derived Factor-1 Regulate the Metastatic Behavior of Human Rhabdomyosarcoma Cells, but only HGF Enhances Their Resistance to Radiochemotherapy. Cancer Res. 2003, 63, 7926–7935. [Google Scholar]
- Liang, W.; Guan, H.; He, X.; Ke, W.; Xu, L.; Liu, L.; Xiao, H.; Li, Y. Down-regulation of SOSTDC1 promotes thyroid cancer cell proliferation via regulating cyclin A2 and cyclin E2. Oncotarget 2015, 6, 31780–31791. [Google Scholar] [CrossRef]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Hwa, L.R.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Domnina, A.P.; Fridlyanskaya, I.I.; Zemelko, V.I.; Pugovkina, N.A.; Kovaleva, Z.V.; Zenin, V.V.; Grinchuk, T.M.; Nikolsky, N.N. Mesenchymal stem cells from human endometrium do not undergo spontaneous transformation during long-term cultivation. Cell Tissue Biol. 2013, 7, 221–226. [Google Scholar] [CrossRef]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cell Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Murphy, K.C.; Whitehead, J.; Zhou, D.; Ho, S.S.; Leach, J.K. Engineering Fibrin Hydrogels to Promote the Wound Healing Potential of Mesenchymal Stem Cell Spheroids. Acta Biomater. 2017, 64, 176–186. [Google Scholar] [CrossRef]
- Kouroupis, D.; Correa, D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 621748. [Google Scholar] [CrossRef]
- Dong, G.; Wang, S.; Ge, Y.; Deng, Q.; Cao, Q.; Wang, Q.; Shang, Z.; Yang, W.O.; Li, J.; Liu, C.; et al. Serum-Free Culture System for Spontaneous Human Mesenchymal Stem Cell Spheroid Formation. Stem Cells Int. 2019, 2019, 6041816. [Google Scholar] [CrossRef]
- Ylostalo, J.H.; Bazhanov, N.; Mohammadipoor, A.; Bartosh, T.J. Production and administration of therapeutic mesenchymal stem/stromal cell (MSC) spheroids primed in 3-D cultures under xeno-free conditions. J. Vis. Exp. 2017, 121, 55126. [Google Scholar] [CrossRef]
- Baptista, L.S.; Kronemberger, G.S.; Côrtes, I.; Charelli, L.E.; Matsui, R.A.M.; Palhares, T.N.; Sohier, J.; Rossi, A.M.; Granjeiro, J.M. Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
| CD Marker | eMSC | Spheroids | eMSC SFM1 | Spheroids SFM1 | eMSC SFM2 | Spheroids SFM2 |
|---|---|---|---|---|---|---|
| CD34 | 0.18 ± 0.3% | 0.32 ± 0.1% | 0.60 ± 0.2% | 0.39 ± 0.1% | 0.45 ± 0.3% | 0.15 ± 0.1% |
| CD 44 | 100.00 ± 2% | 93.14 ± 2% | 99.57 ± 2% | 92.35 ± 2% | 98.48 ± 2% | 96.48 ± 2% |
| CD 45 | 0.83 ± 0.5% | 0.76 ± 0.2% | 0.94 ± 0.2% | 0.66 ± 0.2% | 0.93 ± 0.2% | 0.74 ± 0.1% |
| CD 73 | 99.62 ± 1% | 94.36 ± 2% | 99.86 ± 2% | 96.68 ± 2% | 93.95 ± 3% | 95.95 ± 2% |
| CD 90 | 95.66 ± 2% | 96.34 ± 2% | 96.66 ± 2% | 99.34 ± 2% | 93.07 ± 2% | 99.96 ± 1% |
| CD 105 | 92.90 ± 2% | 94.89 ± 2% | 98.39 ± 1% | 91.89 ± 2% | 93.92 ± 2% | 90.20 ± 2% |
| CD 146 | 97.95 ± 2% | 3.09 ± 1% | 99.95 ± 1% | 0.56 ± 0.3% | 95.00 ± 2% | 0.50 ± 0.1% |
| HLA-1 | 99.59 ± 2% | 99.99 ± 2% | 99.59 ± 2% | 98.44 ± 2% | 98.17 ± 2% | 97.20 ± 2% |
| HLA-DR | 0.31 ± 0.1% | 0.30 ± 0.2% | 0.60 ± 0.2% | 0.38 ± 0.1% | 0.50 ± 0.2% | 0.60 ± 0.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domnina, A.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Shorokhova, M.; Zenin, V.; Fridlyanskaya, I.; Nikolsky, N. Generation of Therapeutically Potent Spheroids from Human Endometrial Mesenchymal Stem/Stromal Cells. J. Pers. Med. 2021, 11, 466. https://doi.org/10.3390/jpm11060466
Domnina A, Alekseenko L, Kozhukharova I, Lyublinskaya O, Shorokhova M, Zenin V, Fridlyanskaya I, Nikolsky N. Generation of Therapeutically Potent Spheroids from Human Endometrial Mesenchymal Stem/Stromal Cells. Journal of Personalized Medicine. 2021; 11(6):466. https://doi.org/10.3390/jpm11060466
Chicago/Turabian StyleDomnina, Alisa, Larisa Alekseenko, Irina Kozhukharova, Olga Lyublinskaya, Mariia Shorokhova, Valeriy Zenin, Irina Fridlyanskaya, and Nikolay Nikolsky. 2021. "Generation of Therapeutically Potent Spheroids from Human Endometrial Mesenchymal Stem/Stromal Cells" Journal of Personalized Medicine 11, no. 6: 466. https://doi.org/10.3390/jpm11060466
APA StyleDomnina, A., Alekseenko, L., Kozhukharova, I., Lyublinskaya, O., Shorokhova, M., Zenin, V., Fridlyanskaya, I., & Nikolsky, N. (2021). Generation of Therapeutically Potent Spheroids from Human Endometrial Mesenchymal Stem/Stromal Cells. Journal of Personalized Medicine, 11(6), 466. https://doi.org/10.3390/jpm11060466






