Impact of Microplastics and Nanoplastics on Human Health
Abstract
:1. Introduction
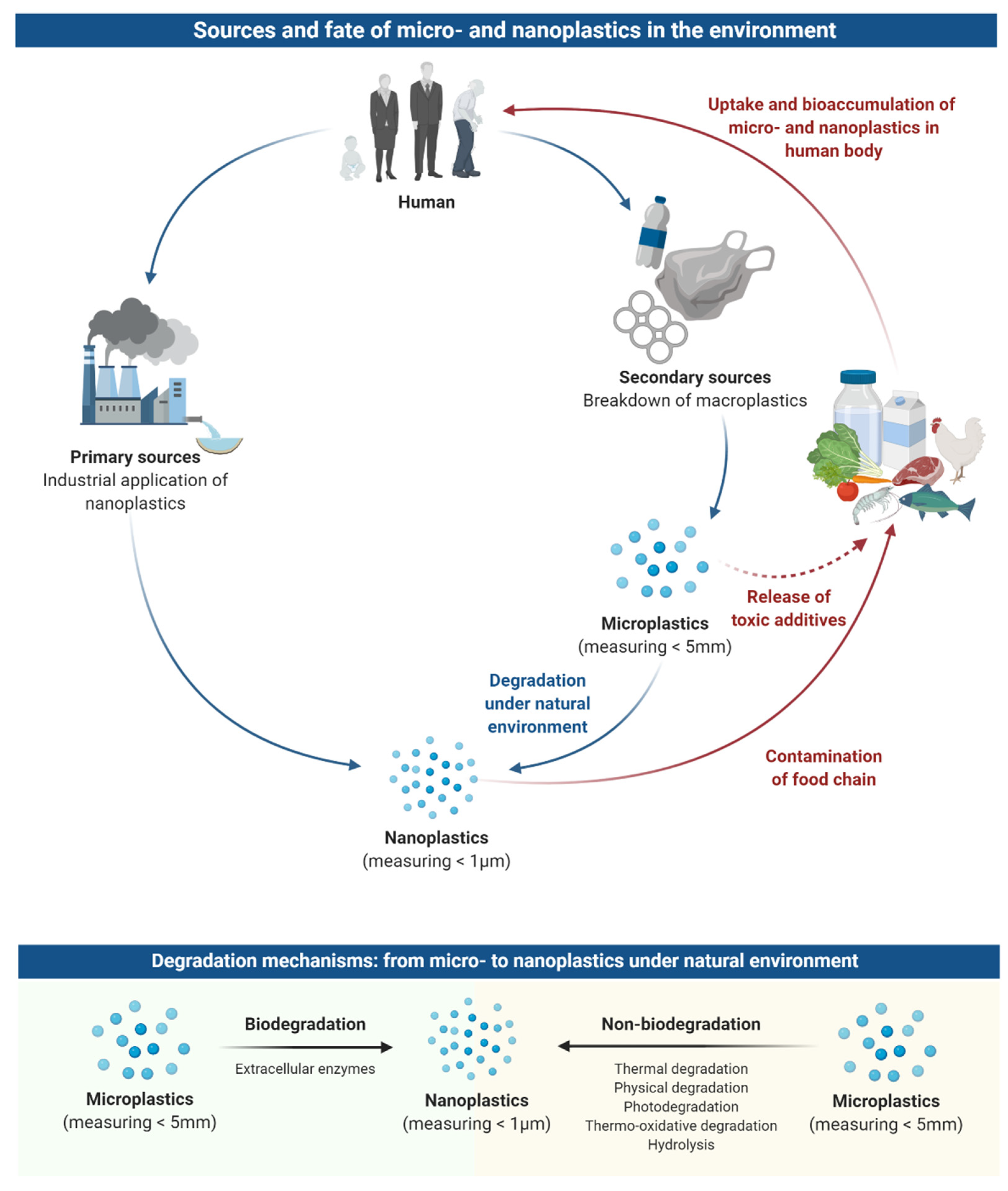
2. Sources and Fate of Microplastics and Nanoplastics in the Environment
3. Occurrence of Microplastics and Nanoplastics in the Food Chain
4. Uptake and Bioaccumulation of Microplastics and Nanoplastics in the Human Body
4.1. Gastric Exposure
4.2. Pulmonary Exposure
4.3. Dermal Exposure
5. Cellular Uptake and Intracellular Fate of Microplastic and Nanoplastic Particles
6. Potential Toxic Effects of Microplastics and Nanoplastics on Human Health
6.1. Inflammation
6.2. Oxidative Stress and Apoptosis
6.3. Metabolic Homeostasis
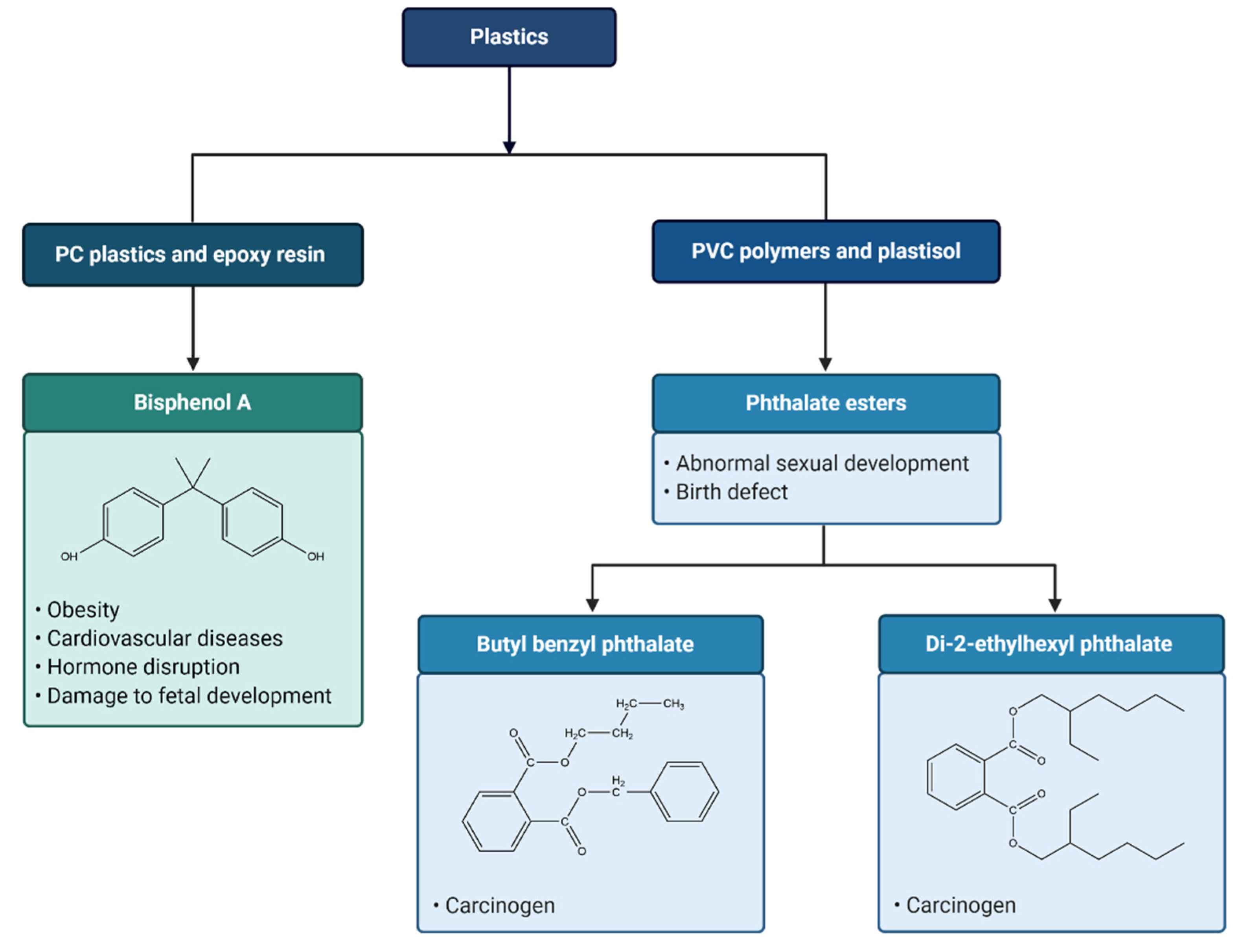
7. Leaching of Toxic Chemicals from Plastics
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garside, M. Global Plastic Production Statistics. Retrieved from Statista. Available online: https://www.statista.com/statistics/282732/global (accessed on 1 August 2020).
- Wagner, S.; Reemtsma, T. Things we know and don’t know about nanoplastic in the environment. Nat. Nanotechnol. 2019, 14, 300–301. [Google Scholar] [CrossRef]
- Shrivastava, A. Polymerization. In Introduction to Plastics Engineering; William Andrew Publishing: New York, NY, USA, 2018; pp. 17–48. [Google Scholar] [CrossRef]
- Liu, T.; Guo, X.; Liu, W.; Hao, C.; Wang, L.; Hiscox, W.C.; Liu, C.; Jin, C.; Xin, J.; Zhang, J. Selective cleavage of ester linkages of anhydride-cured epoxy using a benign method and reuse of the decomposed polymer in new epoxy preparation. Green Chem. 2017, 19, 4364–4372. [Google Scholar] [CrossRef]
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. Additives in Polymers. Modif. Polym. Prop. 2017, 87–108. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.P. Micro- and Nanoplastics in the Environment: Research and Policymaking; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 12–16. [Google Scholar]
- Gigault, J.; Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Contam, E.P.O.C.I.T.F.C. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nat. Cell Biol. 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef]
- Gigault, J.; Pedrono, B.; Maxit, B.; Ter Halle, A. Marine plastic litter: The unanalyzed nano-fraction. Environ. Sci. Nano 2016, 3, 346–350. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Browne, M.A.; Underwood, A.J.; Chapman, M.G.; Williams, R.; Thompson, R.C.; Van Franeker, J.A. Linking effects of anthropogenic debris to ecological impacts. Proc. R. Soc. B Boil. Sci. 2015, 282, 20142929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thushari, G.; Senevirathna, J. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, K.; Jocic, S.; Doverbratt, I.; Hansson, L.-A. Nanoplastics in the Aquatic Environment. In Microplastic Contamination in Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–399. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.; Thompson, R.C.; Fisher, A.S. Metals and marine microplastics: Adsorption from the environment versus addition during manufacture, exemplified with lead. Water Res. 2020, 173, 115577. [Google Scholar] [CrossRef] [PubMed]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar]
- Corcoran, P.L.; Biesinger, M.C.; Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Kolandhasamy, P.; Su, L.; Li, J.; Qu, X.; Jabeen, K.; Shi, H. Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Sci. Total Environ. 2018, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Lee, Y.-H.; Chiu, I.-J.; Lin, Y.-F.; Chiu, H.-W. Potent Impact of Plastic Nanomaterials and Micromaterials on the Food Chain and Human Health. Int. J. Mol. Sci. 2020, 21, 1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. Integr. Environ. Assess. Manag. 2017, 13, 516–521. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yang, D.; Li, L.; Jabeen, K.; Shi, H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Devriese, L.I.; Van Der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-De-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [Green Version]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermato-Endocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Camacho, M.; Herrera, A.; Gómez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic pollutants in marine plastic debris from Canary Islands beaches. Sci. Total Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Viršek, M.K.; Lovšin, M.N.; Koren, Š.; Kržan, A.; Peterlin, M. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar. Pollut. Bull. 2017, 125, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Ge, H.; Yan, Y.; Wu, D.; Huang, Y.; Tian, F. Potential role of LINC00996 in colorectal cancer: A study based on data mining and bioinformatics. OncoTargets Ther. 2018, 11, 4845–4855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Cell Junctions. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Tomazic-Jezic, V.J.; Merritt, K.; Umbreit, T.H. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J. Biomed. Mater. Res. 2001, 55, 523–529. [Google Scholar] [CrossRef]
- Carr, K.E.; Smyth, S.H.; McCullough, M.T.; Morris, J.F.; Moyes, S.M. Morphological aspects of interactions between microparticles and mammalian cells: Intestinal uptake and onward movement. Prog. Histochem. Cytochem. 2012, 46, 185–252. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Tromp, P.; Helsper, J.P.F.G.; Van Der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology 2014, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle Uptake by the Rat Gastrointestinal Mucosa: Quantitation and Particle Size Dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.-J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Interactions of Dissolved Organic Matter with Natural and Engineered Inorganic Colloids: A Review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P. Toxicological considerations of nano-sized plastics. AIMS Environ. Sci. 2019, 6, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Leslie, H.A. Plastic Debris Is a Human Health Issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlwein, S.; Kappeler, R.; Kutlar Joss, M.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.R.; Wu, N.; Wolfarth, M.G.; Sriram, K.; Leonard, S.; Battelli, L.; Schwegler-Berry, D.; Friend, S. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 2010, 269, 136–147. [Google Scholar] [CrossRef]
- Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Varela, J.A.; Bexiga, M.G.; Åberg, C.; Simpson, J.C.; Dawson, K.A. Quantifying size-dependent interactions between fluorescently labeled polystyrene nanoparticles and mammalian cells. J. Nanobiotechnol. 2012, 10, 39. [Google Scholar] [CrossRef]
- Deville, S.; Penjweini, R.; Smisdom, N.; Notelaers, K.; Nelissen, I.; Hooyberghs, J.; Ameloot, M. Intracellular dynamics and fate of polystyrene nanoparticles in A549 Lung epithelial cells monitored by image (cross-) correlation spectroscopy and single particle tracking. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Yacobi, N.R.; DeMaio, L.; Xie, J.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.-J.; Crandall, E.D. Polystyrene nanoparticle trafficking across alveolar epithelium. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Åberg, C.; Dos Santos, T.; Varela, J.; Pinto, P.; Lynch, I.; Dawson, K.A. Experimental and theoretical comparison of intracellular import of polymeric nanoparticles and small molecules: Toward models of uptake kinetics. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 818–826. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Som, C.; Wick, P.; Krug, H.; Nowack, B. Environmental and health effects of nanomaterials in nanotextiles and façade coatings. Environ. Int. 2011, 37, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.; Pilgram, G.; Gooris, G.; Koerten, H.; Ponec, M. New aspects of the skin barrier organization. Ski. Pharmacol. Appl. Ski. Physiol. 2001, 14, 52–62. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.; Guy, R.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Campbell, C.S.J.; Contreras-Rojas, L.R.; Delgado-Charro, M.B.; Guy, R.H. Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. J. Control. Release 2012, 162, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.E.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1500 nm, Nanoparticles Enter Epidermal CD1a+ Cells after Transcutaneous Application on Human Skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef] [Green Version]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, L.J.; Oberdörster, G.; Pentland, A.P.; DeLouise, L.A. In Vivo Skin Penetration of Quantum Dot Nanoparticles in the Murine Model: The Effect of UVR. Nano Lett. 2008, 8, 2779–2787. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Jatana, S.; Callahan, L.M.; Pentland, A.P.; DeLouise, L.A. Impact of Cosmetic Lotions on Nanoparticle Penetration through ex vivo C57BL/6 Hairless Mouse and Human Skin: A Comparison Study. Cosmetics 2016, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.-R.; Wu, C.-L.; Hsu, C.-T.; Lo, W.; Chiang, S.-J.; Lin, S.-J.; Dong, C.-Y.; Chen, C.-C. Chemical enhancer induced changes in the mechanisms of transdermal delivery of zinc oxide nanoparticles. Biomaterials 2009, 30, 3002–3008. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein−Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of Protein Modification on the Protein Corona on Nanoparticles and Nanoparticle–Cell Interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteom. 2016, 137, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, H.F.; Wick, P. Nanotoxicology: An Interdisciplinary Challenge. Angew. Chem. Int. Ed. 2011, 50, 1260–1278. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [Green Version]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Mani, I.; Pandey, K.N. Emerging concepts of receptor endocytosis and concurrent intracellular signaling: Mechanisms of guanylyl cyclase/natriuretic peptide receptor-A activation and trafficking. Cell. Signal. 2019, 60, 17–30. [Google Scholar] [CrossRef]
- Rossi, G.; Barnoud, J.; Monticelli, L. Polystyrene Nanoparticles Perturb Lipid Membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef]
- Fiorentino, I.; Gualtieri, R.; Barbato, V.; Mollo, V.; Braun, S.; Angrisani, A.; Turano, M.; Furia, M.; Netti, P.A.; Guarnieri, D.; et al. Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Exp. Cell Res. 2015, 330, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of Transport Inhibitors on the Cellular Uptake of Carboxylated Polystyrene Nanoparticles in Different Cell Lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef] [Green Version]
- Fazlollahi, F.; Angelow, S.; Yacobi, N.R.; Marchelletta, R.; Yu, A.S.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.-J.; Crandall, E.D. Polystyrene nanoparticle trafficking across MDCK-II. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelmans, A.A.; Besseling, E.; Wegner, A.; Foekema, E.M. Plastic as a Carrier of POPs to Aquatic Organisms: A Model Analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, E.; Meindl, C.; Roblegg, E.; Ebner, B.; Absenger, M.; Pieber, T.R. Action of polystyrene nanoparticles of different sizes on lysosomal function and integrity. Part. Fibre Toxicol. 2012, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Isidoro, C.; Maneerat, E.; Giovia, A.; Carlo, F.; Caputo, G. Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: Effects of size and surface charge groups. Int. J. Nanomed. 2012, 7, 4147–4158. [Google Scholar] [CrossRef] [Green Version]
- Malek, A.; Merkel, O.; Fink, L.; Czubayko, F.; Kissel, T.; Aigner, A. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(–PEG)/siRNA complexes. Toxicol. Appl. Pharmacol. 2009, 236, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, B.; Pahl, S.; Backhaus, T.; Bessa, F.; van Calster, G.; Contzen, N.; Cronin, R.; Galloway, T.; Hart, A.; Henderson, L. A Scientific Perspective on Microplastics in Nature and Society; SAPEA: Berlin, Germany, 2019. [Google Scholar]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Oliveira, M.; Almeida, M.; Miguel, I. A Micro(nano)plastic Boomerang Tale: A Never Ending Story? Elsevier: Amsterdam, The Netherlands, 2019; Volume 112, pp. 196–200. [Google Scholar]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-Dependent Proinflammatory Effects of Ultrafine Polystyrene Particles: A Role for Surface Area and Oxidative Stress in the Enhanced Activity of Ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. In Vitro 2016, 31, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Prietl, B.; Meindl, C.; Roblegg, E.; Pieber, T.R.; Lanzer, G.; Fröhlich, E. Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biol. Toxicol. 2014, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, A.-K.; Syrovets, T.; Haas, K.A.; Loos, C.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Simmet, T. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials 2016, 85, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.H.; Stone, M.; Fisher, J.; Ingham, E. The influence of molecular weight, crosslinking and counterface roughness on TNF-alpha production by macrophages in response to ultra high molecular weight polyethylene particles. Biomaterials 2004, 25, 3511–3522. [Google Scholar] [CrossRef]
- Green, T.R.; Fisher, J.; Stone, M.; Wroblewski, B.M.; Ingham, E. Polyethylene particles of a ’critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998, 19, 2297–2302. [Google Scholar] [CrossRef]
- Veruva, S.Y.; Lanman, T.H.; Isaza, J.E.; Freeman, T.A.; Kurtz, S.M.; Steinbeck, M.J. Periprosthetic UHMWPE Wear Debris Induces Inflammation, Vascularization, and Innervation After Total Disc Replacement in the Lumbar Spine. Clin. Orthop. Relat. Res. 2017, 475, 1369–1381. [Google Scholar] [CrossRef] [Green Version]
- Suñer, S.; Gowland, N.; Craven, R.; Joffe, R.; Emami, N.; Tipper, J.L. Ultrahigh molecular weight polyethylene/graphene oxide nanocomposites: Wear characterization and biological response to wear particles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 106, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Massin, P.; Achour, S. Wear products of total hip arthroplasty: The case of polyethylene. Morphologie 2017, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nich, C.; Goodman, S.B. Role of Macrophages in the Biological Reaction to Wear Debris from Joint Replacements. J. Autom. Inf. Sci. 2014, 24, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Devane, P.A.; Bourne, R.B.; Rorabeck, C.H.; Hardie, R.M.; Horne, J.G. Measurement of polyethylene wear in metal-backed acetabular cups. I. Three-dimensional technique. Clin. Orthop. Relat. Res. 1995, 319, 303–316. [Google Scholar]
- Devane, P.A.; Bourne, R.B.; Rorabeck, C.H.; Macdonald, S.; Robinson, E.J. Measurement of polyethylene wear in metal-backed acetabular cups. II. Clinical application. Clin. Orthop. Relat. Res. 1995, 319, 317–326. [Google Scholar]
- Shanbhag, A.; Jacobs, J.; Glant, T.; Gilbert, J.; Black, J.; Galante, J. Composition and morphology of wear debris in failed uncemented total hip replacement. J. Bone Jt. Surgery. Br. Vol. 1994, 76, 60–67. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [Green Version]
- Inkielewicz-Stepniak, I.; Tajber, L.; Behan, G.; Zhang, H.; Radomski, M.W.; Medina, C.; Santos-Martinez, M.J. The Role of Mucin in the Toxicological Impact of Polystyrene Nanoparticles. Materials 2018, 11, 724. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.-W.; Xia, T.; Lee, Y.-H.; Chen, C.-W.; Tsai, J.-C.; Wang, Y.-J. Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress. Nanoscale 2015, 7, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Zink, J.I.; Nel, A.E. Cationic Polystyrene Nanosphere Toxicity Depends on Cell-Specific Endocytic and Mitochondrial Injury Pathways. ACS Nano 2008, 2, 85–96. [Google Scholar] [CrossRef]
- Liu, X.; Tian, X.; Xu, X.; Lu, J. Design of a phosphinate-based bioluminescent probe for superoxide radical anion imaging in living cells. Luminescence 2018, 33, 1101–1106. [Google Scholar] [CrossRef]
- Paget, V.; Dekali, S.; Kortulewski, T.; Grall, R.; Gamez, C.; Blazy, K.; Aguerre-Chariol, O.; Chevillard, S.; Braun, A.; Rat, P.; et al. Specific Uptake and Genotoxicity Induced by Polystyrene Nanobeads with Distinct Surface Chemistry on Human Lung Epithelial Cells and Macrophages. PLoS ONE 2015, 10, e0123297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruenraroengsak, P.; Tetley, T.D. Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: Robust response of alveolar type 1 epithelial cells. Part. Fibre Toxicol. 2015, 12, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thubagere, A.; Reinhard, B.M. Nanoparticle-Induced Apoptosis Propagates through Hydrogen-Peroxide-Mediated Bystander Killing: Insights from a Human Intestinal EpitheliumIn VitroModel. ACS Nano 2010, 4, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, G.; Valiyaveettil, S. Understanding the interactions of poly(methyl methacrylate) and poly(vinyl chloride) nanoparticles with BHK-21 cell line. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; Gong, X.; Nahirney, D.; Duszyk, M.; Radomski, M. Polystyrene nanoparticles activate ion transport in human airway epithelial cells. Int. J. Nanomed. 2011, 6, 1343–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Gu, W.; Zhang, M.; Chang, Y.-N.; Chen, K.; Bai, X.; Yu, L.; Li, J.; Li, S.; Xing, G. Endocytosed nanoparticles hold endosomes and stimulate binucleated cells formation. Part. Fibre Toxicol. 2016, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- Mahler, G.J.; Esch, M.B.; Tako, E.; Southard, T.L.; Archer, S.D.; Glahn, R.P.; Shuler, M.L. Oral exposure to polystyrene nanoparticles affects iron absorption. Nat. Nanotechnol. 2012, 7, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631, 449–458. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, R.; Chen, X.; Wang, J.; Rui, Q.; Wang, D. Induction of protective response to polystyrene nanoparticles associated with dysregulation of intestinal long non-coding RNAs in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2021, 212, 111976. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.E. The Complex Interaction between Marine Debris and Toxic Chemicals in the Ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro- and Nano-plastics and Human Health. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 343–366. [Google Scholar]
- Koelmans, A.A.; Besseling, E.; Foekema, E.M. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014, 187, 49–54. [Google Scholar] [CrossRef]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; Leblanc, G.A.; Guillette, L.J. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Al-Odaini, N.A.; Song, Y.K.; Hong, S.H. Qualitative Analysis of Additives in Plastic Marine Debris and Its New Products. Arch. Environ. Contam. Toxicol. 2015, 69, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4- tertiary -Octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Guart, A.; Wagner, M.; Mezquida, A.; Lacorte, S.; Oehlmann, J.; Borrell, A. Migration of plasticisers from Tritan™ and polycarbonate bottles and toxicological evaluation. Food Chem. 2013, 141, 373–380. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Godara, S. Use of polycarbonate plastic products and human health. Int. J. Basic Clin. Pharmacol. 2013, 2, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid Hormone Action Is Disrupted by Bisphenol A as an Antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Ropero, A.B.; Alonso-Magdalena, P.; García-García, E.; Ripoll, C.; Fuentes, E.; Nadal, A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int. J. Androl. 2008, 31, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Cipelli, R.; Harries, L.; Okuda, K.; Yoshihara, S.; Melzer, D.; Galloway, T. Bisphenol A modulates the metabolic regulator oestrogen-related receptor-α in T-cells. Reproduction 2014, 147, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.-T.; Wareham, N.J.; et al. Urinary Bisphenol A Concentration and Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, C.; Gallart-Ayala, H. Metabolomics: A tool to characterize the effect of phthalates and bisphenol A. Environ. Rev. 2018, 26, 351–357. [Google Scholar] [CrossRef]
- Cheng, Z.; Nie, X.-P.; Wang, H.-S.; Wong, M.-H. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ. Int. 2013, 57–58, 75–80. [Google Scholar] [CrossRef]
- Brachner, A.; Fragouli, D.; Duarte, I.F.; Farias, P.M.A.; Dembski, S.; Ghosh, M.; Barisic, I.; Zdzieblo, D.; Vanoirbeek, J.; Schwabl, P.; et al. Assessment of Human Health Risks Posed by Nano-and Microplastics Is Currently Not Feasible. Int. J. Environ. Res. Public Health 2020, 17, 8832. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Böhmert, L. Micro- and nanoplastics—Current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv. 2020, 2, 4350–4367. [Google Scholar] [CrossRef]
- Leslie, H.; Depledge, M. Where is the evidence that human exposure to microplastics is safe? Environ. Int. 2020, 142, 105807. [Google Scholar] [CrossRef] [PubMed]


| Toxic Effects | Characteristics of Plastic Particles | Particle Size | Details | References |
|---|---|---|---|---|
| Inflammation | Polystyrene particles | 202 nm and 535 nm |
| [112] |
| Unaltered/Carboxylated polystyrene nanoparticles | 20 nm, 44 nm, 500 nm, and 1000 nm |
| [113,114] | |
| Carboxylated and amino-modified polystyrene particles | 120 nm |
| [115] | |
| Unaltered polyethylene particles | 0.3 μm, 10 μm |
| [117] | |
| Polyethylene particles from plastic prosthetic implants | 0.2 μm and 10 μm |
| [121] | |
| [121,122,123] | |||
| Polystyrene microplastics particles | 5 μm and 20 μm |
| [125] | |
| Oxidative stress and apoptosis | Amine-modified polystyrene nanoparticles | 60 nm |
| [126] |
| Cationic polystyrene nanoparticles | 60 nm |
| [127,128] | |
| Unaltered or functionalized polystyrene | 20 nm, 40 nm, 50 nm, and 100 nm |
| [129,130,131,132] | |
| polyvinyl chloride (PVC) and poly (methyl methacrylate) (PMMA) | 120 nm, 140 nm |
| [133] | |
| Metabolic homeostasis | Pristine and fluorescent polystyrene microplastics | 5 µm |
| [134,135] |
| Anionic carboxylated polystyrene nanoparticles | 20 nm |
| [136] | |
| Polystyrene nanoparticles | 30 nm |
| [137] | |
| Cationic polystyrene nanoparticles | 50 nm and 200 nm |
| [138] | |
| Pristine polystyrene microparticles | 5 µm and 20 µm |
| [125,139,140] | |
| Microplastics | 0.5 µm and 5 µm |
| [135,141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. https://doi.org/10.3390/nano11020496
Yee MS-L, Hii L-W, Looi CK, Lim W-M, Wong S-F, Kok Y-Y, Tan B-K, Wong C-Y, Leong C-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials. 2021; 11(2):496. https://doi.org/10.3390/nano11020496
Chicago/Turabian StyleYee, Maxine Swee-Li, Ling-Wei Hii, Chin King Looi, Wei-Meng Lim, Shew-Fung Wong, Yih-Yih Kok, Boon-Keat Tan, Chiew-Yen Wong, and Chee-Onn Leong. 2021. "Impact of Microplastics and Nanoplastics on Human Health" Nanomaterials 11, no. 2: 496. https://doi.org/10.3390/nano11020496
APA StyleYee, M. S.-L., Hii, L.-W., Looi, C. K., Lim, W.-M., Wong, S.-F., Kok, Y.-Y., Tan, B.-K., Wong, C.-Y., & Leong, C.-O. (2021). Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials, 11(2), 496. https://doi.org/10.3390/nano11020496






