Chemical Vapor Deposition of Uniform and Large-Domain Molybdenum Disulfide Crystals on Glass/Al2O3 Substrates
Abstract
:1. Introduction
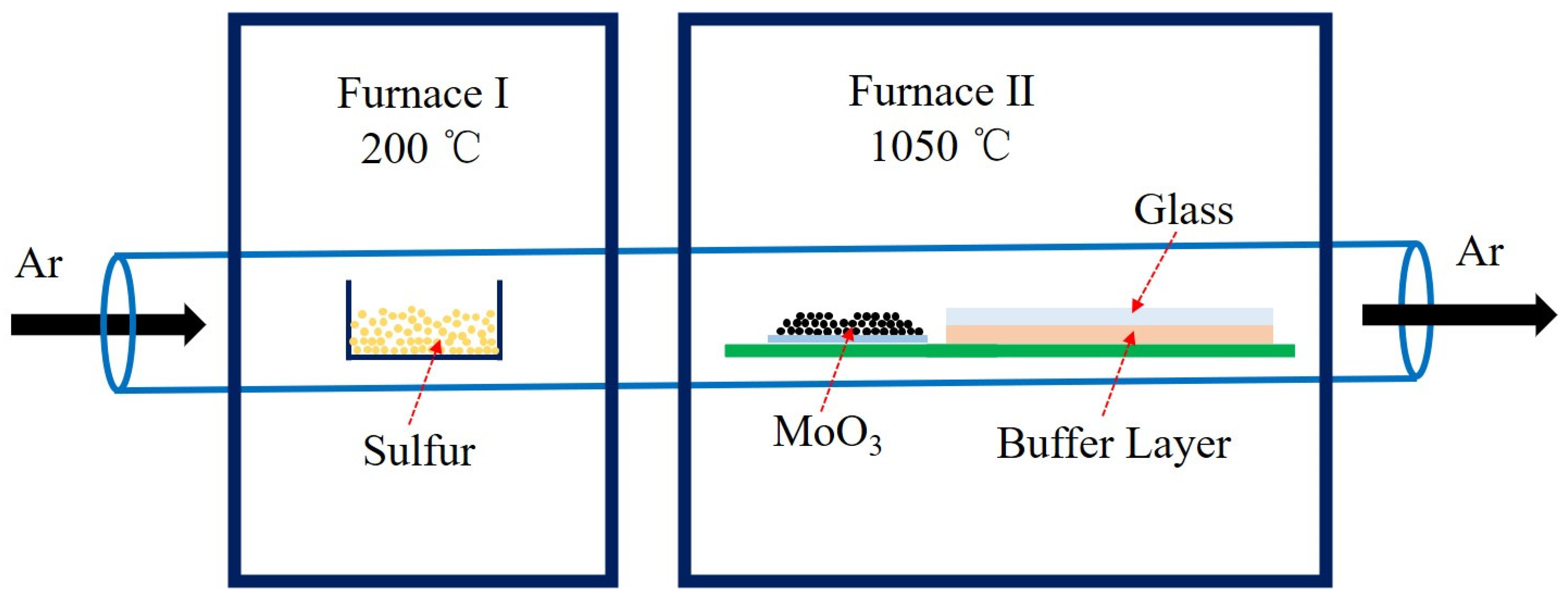
2. Experiments and Methods
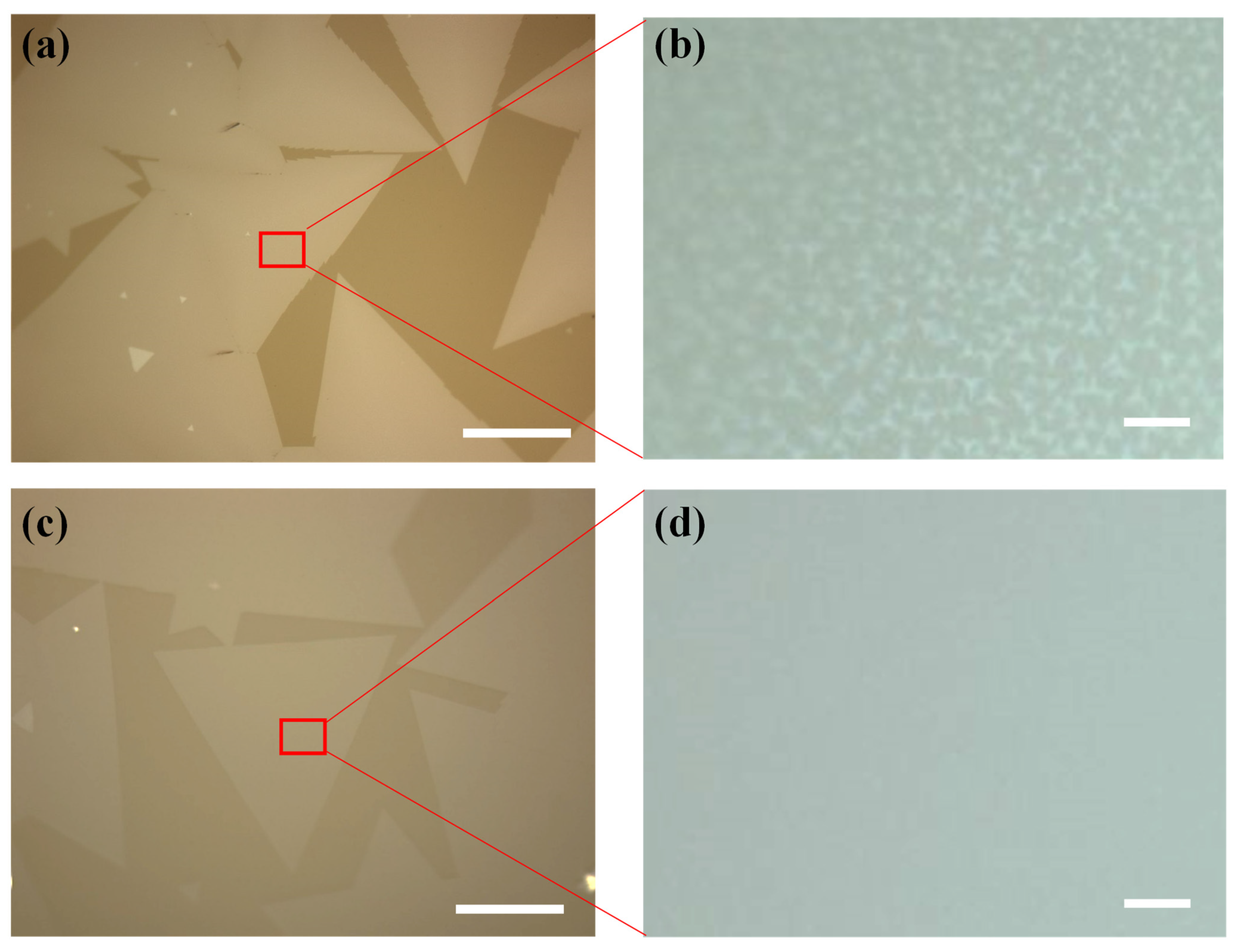
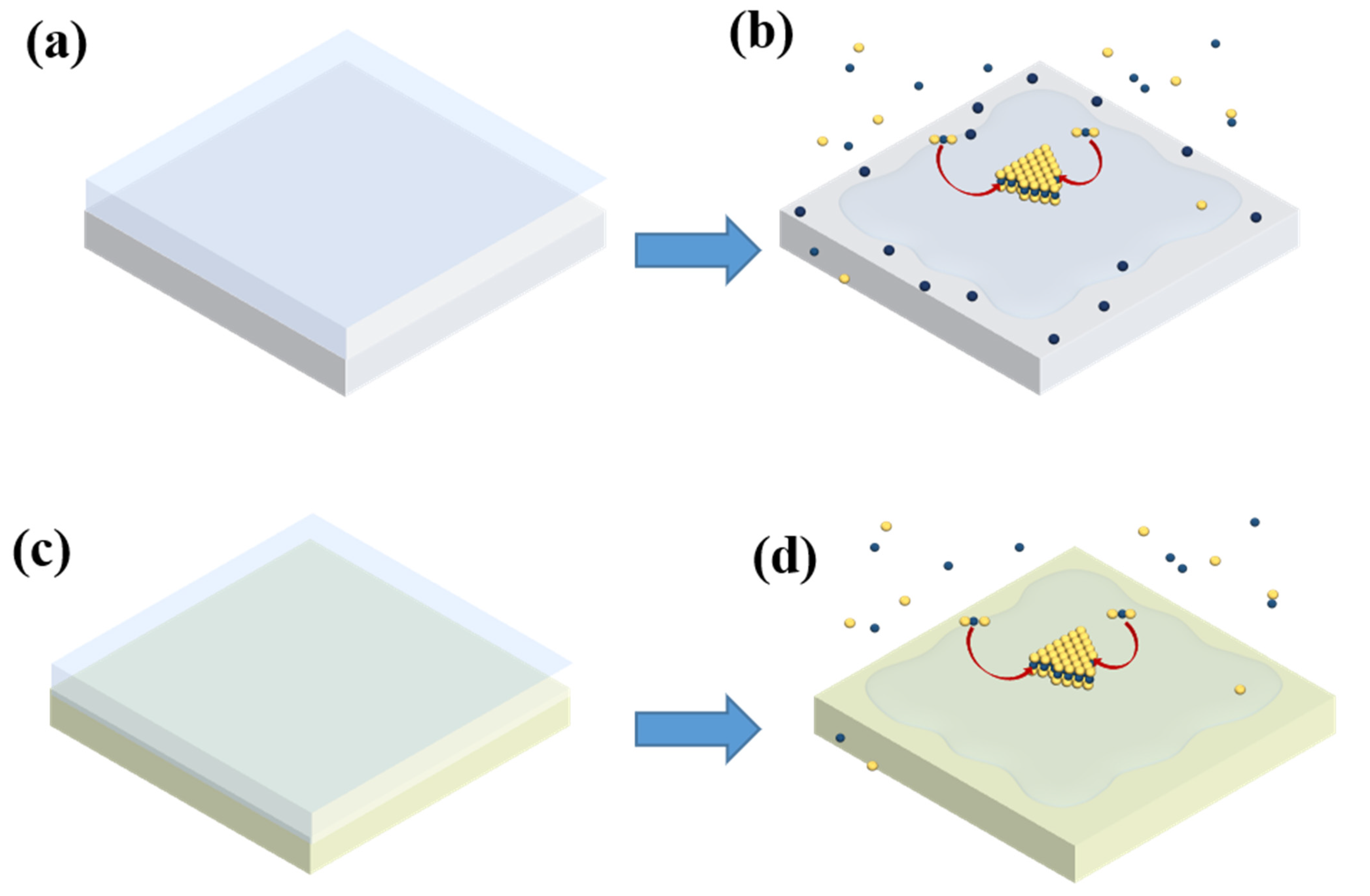
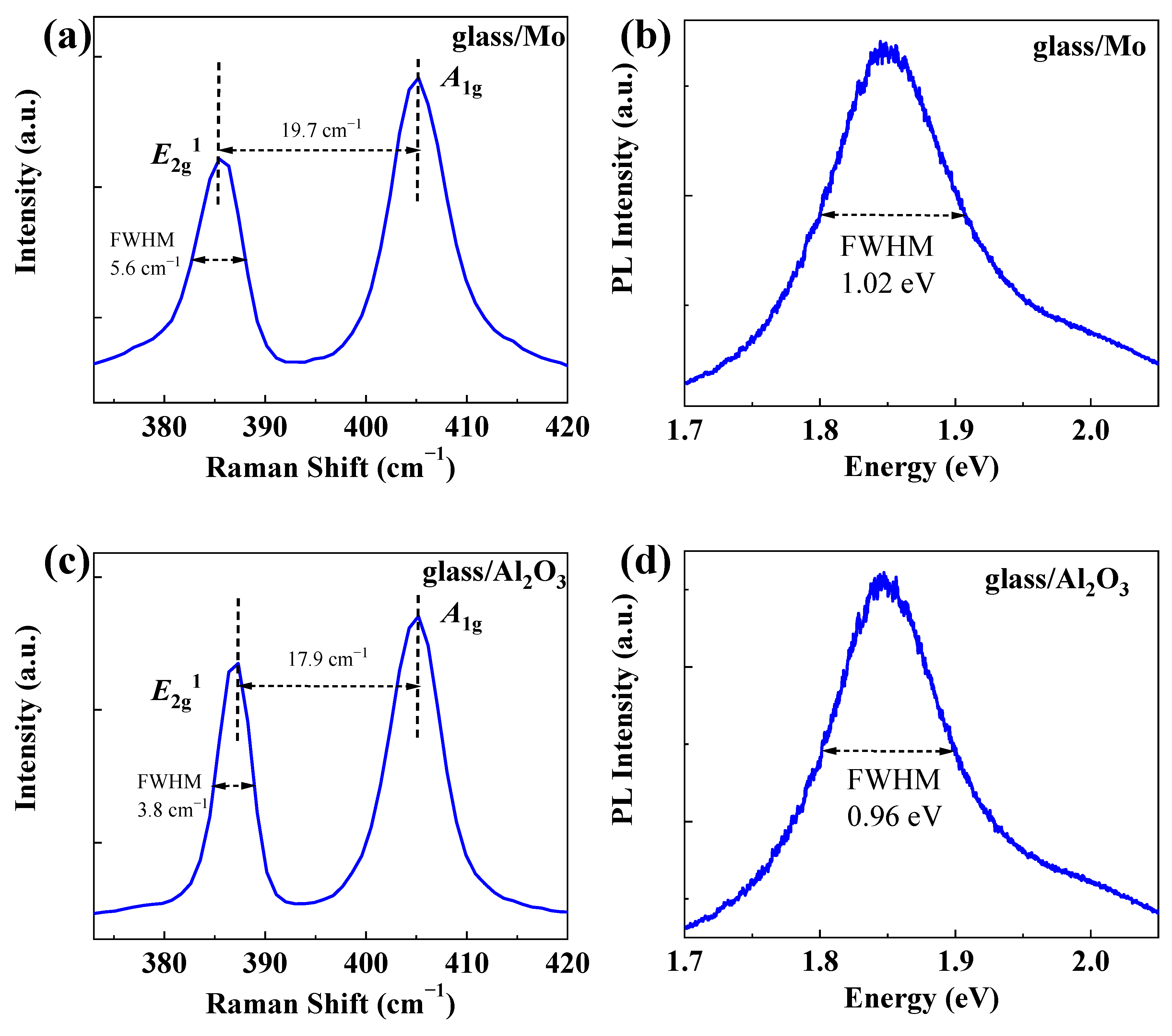
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Tian, H.; Shen, Y.; Hou, Z.; Ren, J.; Gou, G.; Sun, Y.; Yang, Y.; Ren, T.-L. Vertical MoS2 transistors with sub-1-nm gate lengths. Nature 2022, 603, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.-C.; Su, C.; Lin, Y.; Chou, A.-S.; Cheng, C.-C.; Park, J.-H.; Chiu, M.-H.; Lu, A.-Y.; Tang, H.-L.; Tavakoli, M.M.; et al. Ultralow contact resistance between semimetal and monolayer semiconductors. Nature 2021, 593, 211–217. [Google Scholar] [CrossRef]
- Meng, W.; Xu, F.; Yu, Z.; Tao, T.; Shao, L.; Liu, L.; Li, T.; Wen, K.; Wang, J.; He, L.; et al. Three-dimensional monolithic micro-LED display driven by atomically thin transistor matrix. Nat. Nanotechnol. 2021, 16, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Grajal, J.; Vazquez-Roy, J.L.; Radhakrishna, U.; Wang, X.; Chern, W.; Zhou, L.; Lin, Y.; Shen, P.-C.; Ji, X.; et al. Two-dimensional MoS2-enabled flexible rectenna for Wi-Fi-band wireless energy harvesting. Nature 2019, 566, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, Y.; Halim, U.; Ding, M.; Liu, Y.; Wang, Y.; Jia, C.; Chen, P.; Duan, X.; Wang, C.; et al. Solution-processable 2D semiconductors for high-performance large-area electronics. Nature 2018, 562, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.B.; Madhvapathy, S.R.; Sachid, A.B.; Llinas, J.P.; Wang, Q.; Ahn, G.H.; Pitner, G.; Kim, M.J.; Bokor, J.; Hu, C.; et al. MoS 2 transistors with 1-nanometer gate lengths. Science 2016, 354, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef]
- Li, T.; Guo, W.; Ma, L.; Li, W.; Yu, Z.; Han, Z.; Gao, S.; Liu, L.; Fan, D.; Wang, Z.; et al. Epitaxial growth of wafer-scale molybdenum disulfide semiconductor single crystals on sapphire. Nat. Nanotechnol. 2021, 16, 1201–1207. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Ma, L.; Li, W.; Gao, S.; Sun, W.; Dong, R.; Zou, X.; Fan, D.; Shao, L.; et al. Uniform nucleation and epitaxy of bilayer molybdenum disulfide on sapphire. Nature 2022, 605, 69–75. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Pan, S.; Tang, B.; Liang, Y.; Zhao, X.; Zhang, Z.; Shi, J.; Huan, Y.; Shi, Y.; et al. Epitaxial Growth of Centimeter-Scale Single-Crystal MoS2 Monolayer on Au(111). ACS Nano 2020, 14, 5036–5045. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Tang, J.; Zhu, J.; Zhang, Q.; Jia, Q.; Lu, Y.; Wei, Z.; Yu, H.; Zhao, Y.; et al. Wafer-Scale Highly Oriented Monolayer MoS2 with Large Domain Sizes. Nano Lett. 2020, 20, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zou, X.; Zhang, Z.; Zhongfan, L.; Shi, J.; Chen, S.; Shulin, C.; Zhao, L.; Jiang, S.; Zhou, X.; et al. Batch production of 6-inch uniform monolayer molybdenum disulfide catalyzed by sodium in glass. Nat. Commun. 2018, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liao, M.; Zhao, W.; Liu, G.; Zhou, X.J.; Wei, Z.; Xu, X.; Liu, K.; Hu, Z.; Deng, K.; et al. Wafer-Scale Growth and Transfer of Highly-Oriented Monolayer MoS2 Continuous Films. ACS Nano 2017, 11, 12001–12007. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, J.; Huang, X.; Zhou, Y.; Chen, Y.; Xia, J.; Wang, H.; Xie, Y.; Yu, H.; Lei, J.; et al. A library of atomically thin metal chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Su, C.; Mao, N.; Tian, X.; Idrobo, J.-C.; Miao, J.; Tisdale, W.A.; Zettl, A.; Li, J.; Kong, J. Revealing the Brønsted-Evans-Polanyi relation in halide-activated fast MoS2 growth toward millimeter-sized 2D crystals. Sci. Adv. 2021, 7, eabj3274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, J.; Ding, F. Strategies, Status, and Challenges in Wafer Scale Single Crystalline Two-Dimensional Materials Synthesis. Chem. Rev. 2021, 121, 6321–6372. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Salt-assisted chemical vapor deposition of two-dimensional transition metal dichalcogenides. iScience 2021, 24, 103229. [Google Scholar] [CrossRef]
- Zhou, S.; Jiao, L. Growth of Single-crystalline Transition Metal Dichalcogenides Monolayers with Large-size. Chem. Res. Chin. Univ. 2020, 36, 511–517. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Qiu, L.; Zhuang, J.; Zhang, L.; Wang, H.; Liao, C.; Song, H.; Qiao, R.; Gao, P.; et al. Ultrafast growth of single-crystal graphene assisted by a continuous oxygen supply. Nat. Nanotechnol. 2016, 11, 930–935. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Qiu, L.; Wang, S.; Wu, T.; Ding, F.; Peng, H.; Liu, K. The Way towards Ultrafast Growth of Single-Crystal Graphene on Copper. Adv. Sci. 2017, 4, 1700087. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Wang, K.; Li, D.; Luo, X.; Weng, J.; Liu, Z.; Zhang, H. Research progress on the preparations, characterizations and applications of large scale 2D transition metal dichalcogenides films. FlatChem 2020, 21, 100161. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Li, C.; He, J.; Wei, Y.; Zhao, J.; Zhang, R.; Wang, P.; Fu, S.; Chen, F.; et al. Morphological Evolution of Monolayer MoS2 Single-Crystalline Flakes. J. Phys. Chem. C 2022, 126, 3549–3559. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, J.; Zhou, L.; Mendes, R.G.; Dong, Y.; Zhang, M.-Y.; Cui, Z.-H.; Cai, Z.; Zhang, Z.; Zhu, D.; et al. Bandgap tuning of two-dimensional materials by sphere diameter engineering. Nat. Mater. 2020, 19, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Song, J.; Gao, Q.; Li, S.; Hu, Q.; Li, X.; Wu, Y. High-performance transistors based on monolayer CVD MoS2 grown on molten glass. Appl. Phys. Lett. 2018, 113, 202103. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Z.; Xu, X.; Song, J.; Li, X.; Wu, Y. Scalable high performance radio frequency electronics based on large domain bilayer MoS2. Nat. Commun. 2018, 9, 4778. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Grinblat, G.; Chen, Z.; Tan, S.J.R.; Fu, W.; Ding, Z.; Abdelwahab, I.; Li, Y.; Geng, D.; et al. Homoepitaxial Growth of Large-Scale Highly Organized Transition Metal Dichalcogenide Patterns. Adv. Mater. 2017, 30, 1704674. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, X.; Tan, S.J.R.; Xu, H.; Wu, B.; Liu, B.; Fu, D.; Fu, W.; Geng, D.; Liu, Y.; et al. Chemical Vapor Deposition of Large-Size Monolayer MoSe2 Crystals on Molten Glass. J. Am. Chem. Soc. 2017, 139, 1073–1076. [Google Scholar] [CrossRef]
- Cai, Z.; Lai, Y.; Zhao, S.; Zhang, R.; Tan, J.; Feng, S.; Zou, J.; Tang, L.; Lin, J.; Liu, B.; et al. Dissolution-precipitation growth of uniform and clean two dimensional transition metal dichalcogenides. Natl. Sci. Rev. 2020, 8, 115. [Google Scholar] [CrossRef]
- Tang, J.; Wei, Z.; Wang, Q.; Wang, Y.; Han, B.; Li, X.; Huang, B.; Liao, M.; Liu, J.; Li, N.; et al. In Situ Oxygen Doping of Monolayer MoS 2 for Novel Electronics. Small 2020, 16, 2004276. [Google Scholar] [CrossRef]
- Durairaj, S.; Ponnusamy, K.; Shinde, N.B.; Eswaran, S.K.; Asokan, V.; Park, J.B.; Chandramohan, S. Oxygen-Driven Growth Regulation and Defect Passivation in Chemical Vapor Deposited MoS2 Monolayers. Cryst. Growth Des. 2021, 21, 6793–6801. [Google Scholar] [CrossRef]
- Yao, B.; Li, R.; Zhang, C.; Zhou, Z.; Fu, Z.; Huang, X.; Yuan, G.; Xu, J.; Gao, L. Tuning the morphology of 2D transition metal chalcogenides via oxidizing conditions. J. Physics Condens. Matter 2022, 34, 195001. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, J.; Zhang, J.; Gu, L.; Yang, Z.; Li, X.; Yu, H.; Zhu, X.; Yang, R.; Shi, D.; et al. Oxygen-Assisted Chemical Vapor Deposition Growth of Large Single-Crystal and High-Quality Monolayer MoS2. J. Am. Chem. Soc. 2015, 137, 15632–15635. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, T.; Luo, Y.; Feng, S.; Cai, Z.; Zhang, H.; Liu, B.; Cheng, H.-M. Vertical Chemical Vapor Deposition Growth of Highly Uniform 2D Transition Metal Dichalcogenides. ACS Nano 2020, 14, 4646–4653. [Google Scholar] [CrossRef]
- Tang, L.; Tan, J.; Nong, H.; Liu, B.; Cheng, H.-M. Chemical Vapor Deposition Growth of Two-Dimensional Compound Materials: Controllability, Material Quality, and Growth Mechanism. Accounts Mater. Res. 2021, 2, 36–47. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Wang, D.; Tang, B.; Yang, P.; Shi, Y.; Zhou, F.; Fu, J.; Huan, Y.; Cui, F.; et al. Ultrafast Growth of Large-Area Uniform, Millimeter-Size MoSe 2 Single Crystals on Low-Cost Soda-Lime Glass. Adv. Mater. Interfaces 2021, 8, 2100415. [Google Scholar] [CrossRef]
- Han, W.; Liu, K.; Yang, S.; Wang, F.; Su, J.; Jin, B.; Li, H.; Zhai, T. Salt-assisted chemical vapor deposition of two-dimensional materials. Sci. China Ser. B Chem. 2019, 62, 1300–1311. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, C.; Yang, K.; Pan, X.; Zhang, Z.; Yang, J.; Yi, Z.; Chi, F.; Liu, L. High-Performance CVD Bilayer MoS2 Radio Frequency Transistors and Gigahertz Mixers for Flexible Nanoelectronics. Micromachines 2021, 12, 451. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, C.; Liu, P.; Hu, Y.; Yang, K.; Yi, Z.; Liu, L.; Pan, X.; Zhang, Z.; Yang, J.; et al. Effect of Back-Gate Voltage on the High-Frequency Performance of Dual-Gate MoS2 Transistors. Nanomaterials 2021, 11, 1594. [Google Scholar] [CrossRef]
- Wang, Y.; Cong, C.; Qiu, C.; Yu, T. Raman Spectroscopy Study of Lattice Vibration and Crystallographic Orientation of Monolayer MoS2under Uniaxial Strain. Small 2013, 9, 2857–2861. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Chakraborty, B.; Bera, A.; Muthu, D.V.S.; Bhowmick, S.; Waghmare, U.V.; Sood, A.K. Symmetry-dependent phonon renormalization in monolayer MoS22transistor. Phys. Rev. B 2012, 85, 161403. [Google Scholar] [CrossRef] [Green Version]
- Zobel, A.; Boson, A.; Wilson, P.M.; Muratov, D.S.; Kuznetsov, D.V.; Sinitskii, A. Chemical vapour deposition and characterization of uniform bilayer and trilayer MoS2crystals. J. Mater. Chem. C 2016, 4, 11081–11087. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Lu, J.; Chen, S.; Chen, L.; Xu, Z.; Lin, D.; Xu, S.; Liu, P.; Zhang, X.; Cai, W.; et al. Chemical Vapor Deposition of Uniform and Large-Domain Molybdenum Disulfide Crystals on Glass/Al2O3 Substrates. Nanomaterials 2022, 12, 2719. https://doi.org/10.3390/nano12152719
Gao Q, Lu J, Chen S, Chen L, Xu Z, Lin D, Xu S, Liu P, Zhang X, Cai W, et al. Chemical Vapor Deposition of Uniform and Large-Domain Molybdenum Disulfide Crystals on Glass/Al2O3 Substrates. Nanomaterials. 2022; 12(15):2719. https://doi.org/10.3390/nano12152719
Chicago/Turabian StyleGao, Qingguo, Jie Lu, Simin Chen, Lvcheng Chen, Zhequan Xu, Dexi Lin, Songyi Xu, Ping Liu, Xueao Zhang, Weiwei Cai, and et al. 2022. "Chemical Vapor Deposition of Uniform and Large-Domain Molybdenum Disulfide Crystals on Glass/Al2O3 Substrates" Nanomaterials 12, no. 15: 2719. https://doi.org/10.3390/nano12152719





