Does the Femoral Head Size in Hip Arthroplasty Influence Lower Body Movements during Squats, Gait and Stair Walking? A Clinical Pilot Study Based on Wearable Motion Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement Protocol
2.3. Motion Registration and Data Processing
2.4. Statistics
3. Results
3.1. Squats
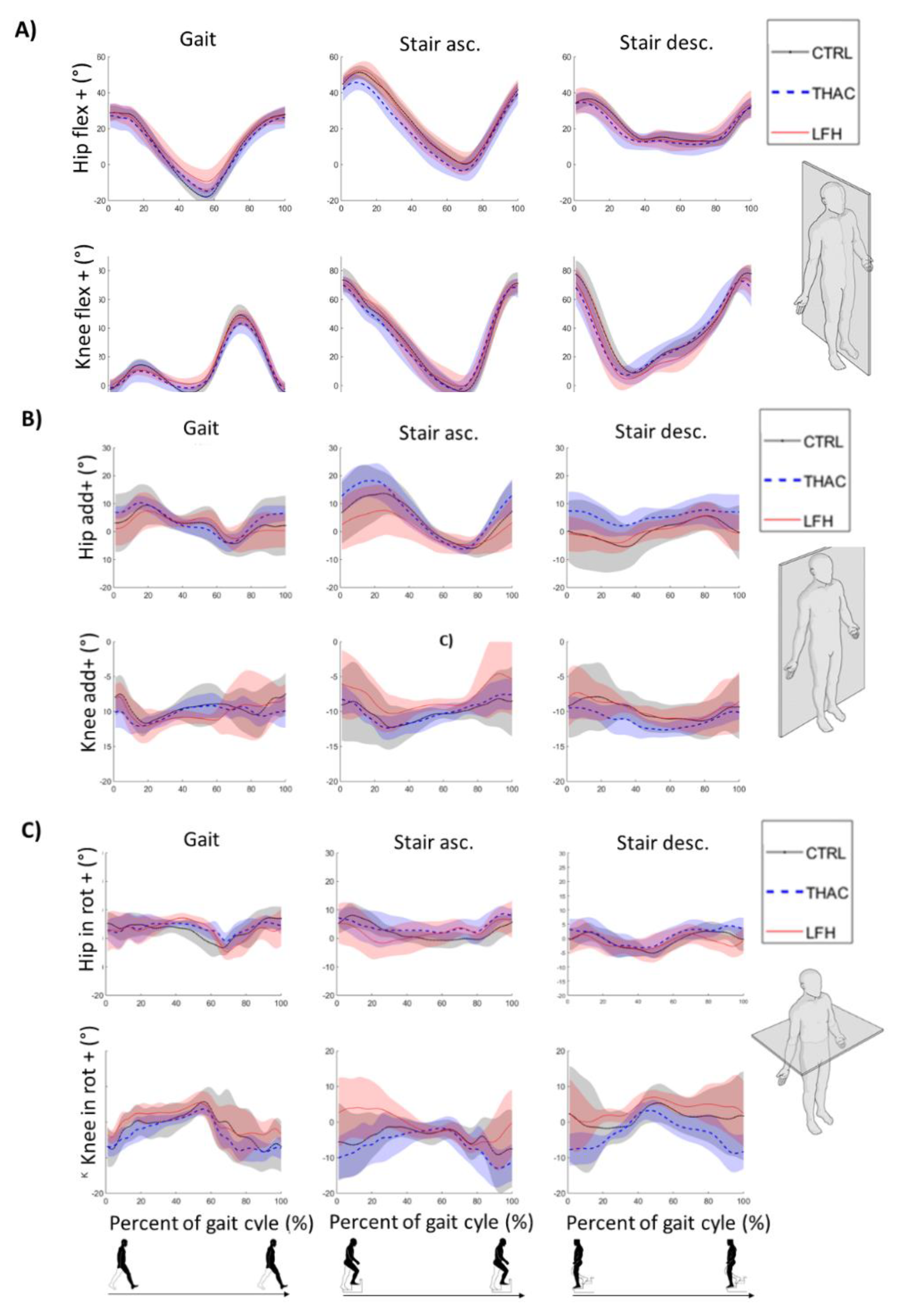
3.2. Gait
3.3. Stair Walking
4. Discussion
4.1. Hip Function in Large Femoral Head (LFH) and Conventional Total Hip Arthroplasty (THAC) Designs: Side Asymmetries and Comparison to Controls
4.2. Potential of Portable Movement Analysis Systems for Clinical Applications
4.3. Strengths and Limitations of the Current Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolk, S.; Minten, M.J.; van Bon, G.E.; Rijnen, W.H.; Geurts, A.C.; Verdonschot, N.; Weerdesteyn, V. Gait and gait-related activities of daily living after total hip arthroplasty: A systematic review. Clin. Biomech. 2014, 29, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.; Lanting, B.; Marsh, J.; Al-Jurayyan, A.; Churchill, L.; Howard, J.; Somerville, L. Postoperative Gait Mechanics After Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. JBJS Rev. 2018, 6, e1. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Cha, Y.H.; Kim, K.J.; Kim, H.Y.; Choy, W.S.; Hwang, S.C. Gait analysis after total hip arthroplasty using direct anterior approach versus anterolateral approach: a systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Ewen, A.M.; Stewart, S.; St Clair Gibson, A.; Kashyap, S.N.; Caplan, N. Post-operative gait analysis in total hip replacement patients—A review of current literature and meta-analysis. Gait Posture 2012, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—Over 100 years of operative history. Orthop. Rev. 2011, 3, e16. [Google Scholar] [CrossRef]
- Del Balso, C.; Teeter, M.G.; Tan, S.C.; Lanting, B.A.; Howard, J.L. Taperosis: Does head length affect fretting and corrosion in total hip arthroplasty? Bone Jt. J. 2015, 97-B, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Lanting, B.A.; Teeter, M.G.; Howard, J.L.; MacDonald, S.J.; Van Citters, D.W. Metal-on-Metal Compared With Metal-on-Polyethylene: The Effect on Trunnion Corrosion in Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 2574–2579. [Google Scholar] [CrossRef]
- MacDonald, S.J. Metal-on-metal total hip arthroplasty: The concerns. Clin. Orthop. Relat. Res. 2004, 429, 86–93. [Google Scholar] [CrossRef]
- Haddad, F.S.; Konan, S.; Tahmassebi, J. A prospective comparative study of cementless total hip arthroplasty and hip resurfacing in patients under the age of 55 years: A ten-year follow-up. Bone Jt. J. 2015, 97-B, 617–622. [Google Scholar] [CrossRef]
- Lu, Y.D.; Yen, S.H.; Kuo, F.C.; Wang, J.W.; Wang, C.J. No benefit on functional outcomes and dislocation rates by increasing head size to 36 mm in ceramic-on-ceramic total hip arthroplasty. Biomed. J. 2015, 38, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Shrader, M.W.; Bhowmik-Stoker, M.; Jacofsky, M.C.; Jacofsky, D.J. Gait and stair function in total and resurfacing hip arthroplasty: A pilot study. Clin. Orthop. Relat. Res. 2009, 467, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Tsikandylakis, G.; Mohaddes, M.; Cnudde, P.; Eskelinen, A.; Kärrholm, J.; Rolfson, O. Head size in primary total hip arthroplasty. EFORT Open Rev. 2018, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Rosenlund, S.; Nielsen, D.B.; Overgaard, S.; Holsgaard-Larsen, A. The use of the Gait Deviation Index for the evaluation of participants following total hip arthroplasty: An explorative randomized trial. Gait Posture 2015, 42, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.K.; Andersen, N.T.; Mogensen, P.; Voight, M.; Soballe, K. Gait analysis after total hip replacement with hip resurfacing implant or Mallory-head Exeter prosthesis: A randomised controlled trial. Int. orthop. 2011, 35, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Nilsdotter, A.K.; Lohmander, L.S.; Klassbo, M.; Roos, E.M. Hip disability and osteoarthritis outcome score (HOOS)—Validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 2003, 4, 10. [Google Scholar] [CrossRef]
- Gerhardt, D.; Mors, T.G.T.; Hannink, G.; Van Susante, J.L.C. Resurfacing hip arthroplasty better preserves a normal gait pattern at increasing walking speeds compared to total hip arthroplasty. Acta Orthop. 2019, 90, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.; Penny, J.O.; Nielsen, D.B.; Overgaard, S.; Holsgaard-Larsen, A. Quantifying Gait Quality in Patients with Large-Head and Conventional Total Hip Arthroplasty—A Prospective Cohort Study. J. Arthroplast. 2015, 30, 2343–2348. [Google Scholar] [CrossRef]
- Camomilla, V.; Cereatti, A.; Cutti, A.G.; Fantozzi, S.; Stagni, R.; Vannozzi, G. Methodological factors affecting joint moments estimation in clinical gait analysis: a systematic review. Biomed. Eng. Online 2017, 16, 106. [Google Scholar] [CrossRef]
- Li, J.D.; Lu, T.W.; Lin, C.C.; Kuo, M.Y.; Hsu, H.C.; Shen, W.C. Soft tissue artefacts of skin markers on the lower limb during cycling: Effects of joint angles and pedal resistance. J. Biomech. 2017, 62, 27–38. [Google Scholar] [CrossRef]
- Jenkyn, T.R.; Nicol, A.C. A multi-segment kinematic model of the foot with a novel definition of forefoot motion for use in clinical gait analysis during walking. J. Biomech. 2007, 40, 3271–3278. [Google Scholar] [CrossRef]
- Wren, T.A.; Otsuka, N.Y.; Bowen, R.E.; Scaduto, A.A.; Chan, L.S.; Sheng, M.; Hara, R.; Kay, R.M. Influence of gait analysis on decision-making for lower extremity orthopaedic surgery: Baseline data from a randomized controlled trial. Gait Posture 2011, 34, 364–369. [Google Scholar] [CrossRef]
- Chang, F.M.; Rhodes, J.T.; Flynn, K.M.; Carollo, J.J. The role of gait analysis in treating gait abnormalities in cerebral palsy. Orthop. Clin. N. Am. 2010, 41, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Filho, M.C.; Yoshida, R.; Carvalho Wda, S.; Stein, H.E.; Novo, N.F. Are the recommendations from three-dimensional gait analysis associated with better postoperative outcomes in patients with cerebral palsy? Gait Posture 2008, 28, 316–322. [Google Scholar] [CrossRef]
- Lofterod, B.; Terjesen, T. Results of treatment when orthopaedic surgeons follow gait-analysis recommendations in children with CP. Dev. Med. Child Neurol. 2008, 50, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Chiari, L.; Holmstrom, L.; Salarian, A.; Horak, F.B. Validity and reliability of an IMU-based method to detect APAs prior to gait initiation. Gait Posture 2016, 43, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Seel, T.; Raisch, J.; Schauer, T. IMU-based joint angle measurement for gait analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, M.; Nicholas, K.; Button, K.; Sparkes, V.; Sheeran, L.; Davies, J.L. Inertial Measurement Units for Clinical Movement Analysis: Reliability and Concurrent Validity. Sensors 2018, 18, 719. [Google Scholar] [CrossRef]
- Kluge, F.; Gassner, H.; Hannink, J.; Pasluosta, C.; Klucken, J.; Eskofier, B.M. Towards Mobile Gait Analysis: Concurrent Validity and Test-Retest Reliability of an Inertial Measurement System for the Assessment of Spatio-Temporal Gait Parameters. Sensors 2017, 17, 1522. [Google Scholar] [CrossRef]
- Ohberg, F.; Backlund, T.; Sundstrom, N.; Grip, H. Portable Sensors Add Reliable Kinematic Measures to the Assessment of Upper Extremity Function. Sensors 2019, 19, 1241. [Google Scholar] [CrossRef]
- Chen, S.; Lach, J.; Lo, B.; Yang, G.Z. Toward Pervasive Gait Analysis with Wearable Sensors: A Systematic Review. IEEE J. Biomed. Health Inform. 2016, 20, 1521–1537. [Google Scholar] [CrossRef]
- Petraglia, F.; Scarcella, L.; Pedrazzi, G.; Brancato, L.; Puers, R.; Costantino, C. Inertial sensors versus standard systems in gait analysis: a systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2019, 55, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Zugner, R.; Tranberg, R.; Timperley, J.; Hodgins, D.; Mohaddes, M.; Karrholm, J. Validation of inertial measurement units with optical tracking system in patients operated with Total hip arthroplasty. BMC Musculoskelet. Disord. 2019, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- McMulkin, M.L.; MacWilliams, B.A. Application of the Gillette Gait Index, Gait Deviation Index and Gait Profile Score to multiple clinical pediatric populations. Gait Posture 2015, 41, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Post, Z.D.; Orozco, F.; Diaz-Ledezma, C.; Hozack, W.J.; Ong, A. Direct anterior approach for total hip arthroplasty: indications, technique, and results. J. Am. Acad. Orthop. Surg. 2014, 22, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.E.; Hunter, J.R. The direct lateral approach to the hip for arthroplasty. Advantages and complications. Orthopedics 1987, 10, 274–280. [Google Scholar] [PubMed]
- Hardinge, K. The direct lateral approach to the hip. J. Bone Jt. Surg. Br. 1982, 64, 17–19. [Google Scholar] [CrossRef]
- Jones, R. Observations on fractures of the neck of the femur. Br. Med. J. 1931, 1, 781–785. [Google Scholar] [CrossRef]
- Gibson, A. Posterior exposure of the hip joint. J. Bone Jt. Surg. Br. 1950, 32-B, 183–186. [Google Scholar] [CrossRef]
- Weaver, J.K. Total hip replacement: A comparison between the transtrochanteric and posterior surgical approaches. Clin. Orthop. Relat. Res. 1975, 147, 201–207. [Google Scholar]
- Öhberg, F.; Lundström, R.; Grip, H. Comparative analysis of different adaptive filters for tracking lower segments of a human body using inertial motion sensors. Meas. Sci. Technol. 2013, 24, 12. [Google Scholar] [CrossRef]
- Favre, J.; Jolles, B.M.; Siegrist, O.; Aminian, K. Quarternion-based fusion of gyroscopes and accelerometers to improve 3D angle measurement. Electron. Lett. 2006, 42, 612–614. [Google Scholar] [CrossRef]
- Rosenlund, S.; Holsgaard-Larsen, A.; Overgaard, S.; Jensen, C. The Gait Deviation Index Is Associated with Hip Muscle Strength and Patient-Reported Outcome in Patients with Severe Hip Osteoarthritis-A Cross-Sectional Study. PLoS ONE 2016, 11, e0153177. [Google Scholar] [CrossRef] [PubMed]
- Shore, W.S.; deLateur, B.J.; Kuhlemeier, K.V.; Imteyaz, H.; Rose, G.; Williams, M.A. A comparison of gait assessment methods: Tinetti and GAITRite electronic walkway. J. Am. Geriatr. Soc. 2005, 53, 2044–2045. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Esquenazi, A.; Benedetti, M.G.; Desloovere, K. Gait analysis: clinical facts. Eur. J. Phys. Rehabil. Med. 2016, 52, 560–574. [Google Scholar] [PubMed]
- Winiarski, S.; Aleksandrowicz, K.; Jarzab, S.; Pozowski, A.; Rutkowska-Kucharska, A. Assessment of gait after bilateral hip replacement. Case study. Ortop. Traumatol. Rehabil. 2014, 16, 197–208. [Google Scholar] [PubMed]
- Brognara, L.; Palumbo, P.; Grimm, B.; Palmerini, L. Assessing Gait in Parkinson's Disease Using Wearable Motion Sensors: A Systematic Review. Diseases 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, C.P.; Williams, S.A.; Grisbrook, T.; Elliott, C.; Imms, C.; Campbell, A. Measurement of Upper Limb Range of Motion Using Wearable Sensors: A Systematic Review. Sports Med. Open 2018, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.M.; Atkins, P.R.; Kutschke, M.J.; Goebel, J.M.; Foreman, K.B.; Anderson, A.E. Soft tissue artifact causes significant errors in the calculation of joint angles and range of motion at the hip. Gait Posture 2017, 55, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zemp, R.; List, R.; Gulay, T.; Elsig, J.P.; Naxera, J.; Taylor, W.R.; Lorenzetti, S. Soft tissue artefacts of the human back: comparison of the sagittal curvature of the spine measured using skin markers and an open upright MRI. PLoS ONE 2014, 9, e95426. [Google Scholar] [CrossRef]
- Cockcroft, J.; Louw, Q.; Baker, R. Proximal placement of lateral thigh skin markers reduces soft tissue artefact during normal gait using the Conventional Gait Model. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 1497–1504. [Google Scholar] [CrossRef]
- Laroche, D.P.; Marques, N.R.; Shumila, H.N.; Logan, C.R.; Laurent, R.S.; Goncalves, M. Excess body weight and gait influence energy cost of walking in older adults. Med. Sci. Sports Exerc. 2015, 47, 1017–1025. [Google Scholar] [CrossRef] [PubMed]


| THAC (N = 6) | LFH (N = 9) | Control (N = 8) | ANOVA (p Value) | Post hoc (p Value) | |
|---|---|---|---|---|---|
| Age (year) | 56 ± 9 | 49 ± 9 | 45 ± 12 | 0.370 | |
| Weight (kg) | 93 ± 12 | 90 ± 11 | 76 ± 7 | 0.009 | LFH vs. THAC: 1.00 LFH > Control: 0.036 THAC > Control: 0.017 |
| Height (m) | 1.80 ± 0.07 | 1.82 ± 0.06 | 1.81 ± 0.05 | 0.803 | |
| Body Mass Index | 28 ± 2 | 27 ± 4 | 23 ± 2 | 0.010 | LFH vs. THAC: 0.922 LFH vs. Control: 0.055 THAC > Control: 0.013 |
| Years since operation | 3.2 ± 1.2 | 4.7 ± 1.1 | n/a | 0.055 | |
| Leg dominance; Right/Left | 6/0 | 8/1 | 6/2 | 0.462 | |
| Prosthesis head; diameter (mm) Range within brackets | 32.7 (1.6) [32–36] | 53.5 (3.2) [49–57] | - | 0.000 |
| Task | Group | Strides per Minute Mean (SE) | GPS Mean (SE) | GDI* Mean (SE) |
|---|---|---|---|---|
| Gait (num = 460) | CTRL THAC LFH | 51.1 (2.8) 46.0 (3.3) 47.9 (2.7) | 6.7 (0.4) 5.9 (0.5) 7.0 (0.4) | 87.7 (1.1) 89.7 (1.2) 86.9 (1.0) |
| CTRL-THAC | −5.1 (4.3) | −0.9 (0.6) | 2.0 (1.6) | |
| CTRL-LFH | −3.2 (3.9) | 0.2 (0.6) | −0.8 (1.5) | |
| Fixed effect | NS | NS | NS | |
| Stair ascending (num = 460) | CTRL THAC LFH | 53.0 (3.4) 52.5 (4.0) 53.0 (3.4) | 7.4 (0.5) 7.7 (0.6) 7.4 (0.5) | 87.9 (1.0) 86.8 (1.2) 85.9 (1.0) |
| CTRL-THAC | −0.4 (5.1) | 0.4 (0.8) | −1.8 (1.5) | |
| CTRL-LFH | −7.8 (4.7) | 0.9 (0.7) | −2.0 (1.4) | |
| Fixed effect | NS | NS | NS | |
| Stair descending (num = 460) | CTRL THAC LFH | 61.9 (3.4) 55.4 (4.0) 52.9 (3.3) | 7.1(0.4) 7.5 (0.5) 7.3 (0.4) | 87.8(1.0) 86.5 (1.1) 87.2 (0.9) |
| CTRL-THAC | −6.5 (5.2) | 0.4 (0.7) | −1.3 (1.5) | |
| CTRL-LFH | −9.0 (4.8) | 0.2 (0.6) | −0.6 (1.3) | |
| Fixed effect | NS | NS | NS |
| Task | Group | RoM Pelvis (°) | RoM Hip (°) | RoM Knee (°) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FE | Abd-Add | Rot | FE | Abd-Add | Rot | FE | AbAdd | Rot | ||
| Squat (num = 92) | CTRL THAC LFH | 24.5 (3.9) 16.8 (4.5) 28.1 (3.7) | 4.6 (1.8) 4.7 (2.0) 9.6 (1.7) | 4.4 (1.2) 3.7 (1.4) 8.9 (1.2) | 79.4 (6.8) 63.6 (7.8) 91.0 (6.4) | 13.3 (2.6) 11.9 (2.9) 17.0 (2.4) | 9.9 (2.9) 13.2 (3.4) 18.0 (2.8) | 90.5 (5.3) 80.9 (6.2) 95.7 (5.0) | 13.2 (1.9) 9.2 (2.2) 16.8 (1.8) | 15.2 (1.8) 12.2 (2.1) 19.4 (1.7) |
| CTRL-THAC | −7.7 (6.0) | 0.1 (2.7) | −0.7 (1.9) | −15.8 (10.3) | −1.4 (3.9) | 3.3 (4.5) | −9.6 (8.1) | −4.0 (2.9) | −3.1 (2.8) | |
| CTRL-LFH | 3.6 (5.4) | 5.0 (2.4) | 4.5 (1.7) | 11.6 (9.3) | 3.8 (3.5) | 8.1 (4.0) | 5.2 (7.3) | 3.6 (2.7) | 4.1 (2.5) | |
| Fixed effects | NS | NS | p = 0.014 | p = 0.043 | NS | NS | NS | p = 0.050 | p = 0.046 | |
| Post hoc | LFH>THAC LFH>CTRL | LFH>THAC | LFH>THAC | LFH>THAC | ||||||
| Gait (num = 460) | CTRL THAC LFH | 5.7 (0.4) 6.6 (0.5) 6.4 (0.4) | 7.1 (0.7) 6.9 (0.8) 7.3 (0.7) | 11.9 (1.0) 9.8 (1.2) 9.8 (1.0) | 49.9 (2.5) 43.9 (2.9) 40.5 (2.4) | 19.7 (1.6) 16.0 (1.9) 15.2 (1.5) | 16.9 (1.4) 12.9 (1.6) 16.2 (1.3) | 57.3 (1.8) 50.1 (2.1) 51.7 (1.7) | 16.0 (1.2) 12.0 (1.4) 15.2 (1.1) | 19.1 (1.2) 16.0 (1.4) 15.7 (1.1) |
| CTRL-THAC | 0.9 (0.7) | −0.2 (1.1) | −2.2 (1.6) | −6.0 (3.8) | −3.7 (2.4) | −4.0 (2.1) | 7.1 (2.7) | 4.0 (1.8) | −3.1 (1.8) | |
| CTRL-LFH | 0.7 (0.6) | 0.2 (0.9) | −2.1 (1.4) | −9.4 (3.5) | −4.5 (2.2) | −0.7 (2.0) | −5.6 (2.5) | −0.8 (1.7) | −3.3 (1.6) | |
| Fixed effects | NS | NS | NS | p = 0.044 | NS | NS | p = 0.037 | NS | NS | |
| Post hoc | CTRL>LFH | No sig. post hoc effects | ||||||||
| Stair Ascending (num = 460) | CTRL THAC LFH | 7.0 (0.7) 7.8 (0.8) 7.0 (0.7) | 12.8 (1.2) 15.8 (1.4) 14.1 (1.1) | 9.4 (1.1) 6.1 (1.2) 7.4 (1.0) | 53.4 (1.8) 51.7 (2.1) 54.1 (1.7) | 26.1 (1.9) 28.0 (2.2) 17.4 (1.81) | 12.1 (1.1) 14.5 (1.2) 14.9 (1.0 | 79.3 (1.8) 77.3 (2.1) 75.9 (1.7) | 13.9 (1.6) 13.8 (1.8) 17.5 (1.5) | 16.3 (1.8) 18.5 (2.1) 20.3 (1.7) |
| CTRL-THAC | 0.8 (1.1) | 3.0 (1.8) | −3.2 (1.6) | −1.7 (2.8) | 1.9 (2.9) | 2.4 (1.6) | −2.0 (2.7) | −0.1 (2.4) | 2.2 (2.7) | |
| CTRL-LFH | 0.1 (1.0) | 1.2 (1.7) | −2.0 (1.5) | 0.7 (2.5) | −8.8 (2.7) | 2.8 (1.4) | −3.4 (2.5) | 3.6 (2.1) | 4.0 (2.5) | |
| Fixed effects | NS | NS | NS | NS | p = 0.002 | NS | NS | NS | NS | |
| Post-hoc | CTRL> LFH THAC> LFH | |||||||||
| Stair Descending (num = 460) | CTRL THAC LFH | 6.4 (0.4) 6.4 (0.5) 6.2 (0.4) | 8.3 (0.9) 8.4 (1.1) 8.7 (0.9) | 10.2 (0.8) 6.3 (1.0) 7.3 (0.8) | 29.1 (1.3) 27.1 (1.6) 27.4 (1.3) | 12.8 (1.0) 10.6 (1.1) 12.6 (0.9) | 14.7 (0.7) 13.9 (0.8) 14.3 (0.7) | 74.4 (1.4) 73.3 (1.7) 75.1 (1.4) | 11.4 (1.2) 11.4 (1.3) 14.1 (1.1) | 16.4 (1.2) 18.2 (1.4) 17.0 (1.2) |
| CTRL-THAC | −0.0 (0.6) | −0.1 (1.4) | −3.9 (1.3) | −2.1 (2.1) | −2.2 (1.5) | −0.8 (1.1) | −1.1 (2.2) | −0.0 (1.9) | 1.9 (1.8) | |
| CTRL-LFH | −0.2 (0.6) | 0.4 (1.3) | −2.9 (1.1) | −1.7 (1.9) | −0.2 (1.3) | −0.4 (1.0) | 0.7 (2.0) | 2.7 (1.6) | 0.6 (1.7) | |
| Fixed effects | NS | NS | p = 0.013 | NS | NS | NS | NS | NS | NS | |
| Post hoc | CTRL>THAC | |||||||||
| Task | RoM | Direction | THAC Mean (SE) | LFH Mean (SE) | Fixed Effects (p Value) | Significant group and side difference specified. Post hoc tests given instead if Grp × Side is significant. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-op | Op | Non-op | Op | Group | Side | Grp× Side | ||||
| Squat (num = 120) | Pelvis (°) | FE | 16.8 (3.8) | 16.8 (3.8) | 28.1 (3.2) | 28.1 (3.2) | 0.005 | NS | NS | Group: THAC<LFH |
| Abd-Add | 4.7 (2.5) | 4.7 (2.5) | 9.6 (2.0) | 9.6 (2.0) | NS | NS | NS | |||
| Rot | 3.7 (1.7) | 3.7 (1.7) | 8.9 (1.4) | 8.9 (1.4) | 0.036 | NS | NS | Group: THAC<LFH | ||
| Hip (°) | FE | 63.2 (7.0) | 63.6 (7.0) | 91.0 (5.8) | 91.0 (5.8) | 0.010 | NS | NS | Group: THAC<LFH | |
| Abd-Add | 17.4 (3.6) | 11.9 (3.6) | 22.8 (3.1) | 17.0 (3.1) | NS | 0.000 | NS | Side: Non-op > Op | ||
| Rot | 9.9 (3.3) | 13.2 (3.3) | 20.0 (2.7) | 18.0 (2.7) | NS | NS | 0.002 | Post hoc tests not significant | ||
| Knee (°) | FE | 80.4 (6.2) | 80.9 (6.2) | 97.8 (4.9) | 95.7 (4.9) | NS | NS | NS | ||
| Abd-Add | 13.8 (1.9) | 9.2 (1.9) | 15.3 (1.5) | 16.8 (1.5) | NS | NS | 0.001 | THAC:Non-op > Op, Op:LFH>THAC | ||
| Rot | 14.6 (2.7) | 12.2 (2.7) | 21.8 (2.2) | 19.4 (2.2) | NS | 0.008 | NS | Side: Non-op > Op | ||
| Gait (num = 600) | Pelvis (°) | FE | 6.9 (0.4) | 6.6 (0.4) | 6.4 (0.4) | 6.4 (0.4) | NS | NS | NS | |
| Abd-Add | 7.0 (0.7) | 6.9 (0.7) | 7.5 (0.6) | 7.3 (0.6) | NS | NS | NS | |||
| Rot | 10.2 (1.3) | 10.2 (1.3) | 9.9 (1.2) | 9.8 (1.2) | NS | NS | NS | |||
| Hip (°) | FE | 45.2 (2.6) | 43.9 (2.6) | 44.8 (2.4) | 40.5 (2.4) | NS | 0.000$ | 0.000 | LFH:Non-op > Op | |
| Abd-Add | 17.4 (1.1) | 16.0 (1.1) | 17.0 (1.0) | 15.2 (1.0) | NS | 0.000 | NS | Side: Non-op > Op | ||
| Rot | 15.9 (1.2) | 13.3 (1.2) | 15.3 (1.0) | 16.2 (1.0) | NS | NS | 0.000 | THAC:Non-op > Op LFH:Non-op < Op | ||
| Knee (°) | FE | 49.3 (1.8) | 48.4 (1.8) | 54.6 (1.4) | 51.7 (1.4) | NS | 0.000$ | 0.002 | LFH:Non-op>Op | |
| Abd-Add | 13.7 (1.0) | 12.2 (1.0) | 13.5 (0.9) | 15.2 (0.9) | NS | NS | 0.000 | THAC:Non-op > Op LFH:Non-op < Op | ||
| Rot | 19.5 (1.2) | 16.0 (1.2) | 18.1 (1.1) | 15.7 (1.1) | NS | 0.000$ | NS | Side: Non-op > Op | ||
| Task | Range of Motion | Direction | THAC Non-op | THAC Op | LFH Non-op | LFH Op | Group | Side | Grp × Side | |
| Stair Ascending (num = 600) | RoM Pelvis (°) | FE | 8.0 (0.8) | 7.8 (0.8) | 6.5 (0.6) | 7.0 (0.6) | NS | NS | 0.027* | LFH:Non-op < Op |
| Abd-Add | 16.2 (1.5) | 15.8 (1.5) | 13.9 (1.2) | 14.1 (1.2) | NS | NS | NS | |||
| Rot | 6.3 (1.0) | 6.1 (1.0) | 7.2 (0.8) | 7.4 (0.8) | NS | NS | NS | |||
| RoM Hip (°) | FE | 50.9 (1.3) | 51.7 (1.3) | 58.1 (1.0) | 54.1 (1.0) | 0.009$ | 0.000$ | 0.000 | LFH:Non-op > Op LFH Non-Op > THAC Op LFH Non-Op > THAC Non-Op | |
| Abd-Add | 31.3 (2.0) | 28.0 (2.1) | 26.3 (1.7) | 17.4 (1.7) | 0.010$ | 0.000$ | 0.000 | LFH, THAC:Non-Op>Op THAC Non-Op > LFH Op THAC Op > LFH Op | ||
| Rot | 12.2 (0.9) | 14.5 (0.9) | 14.7 (0.8) | 14.9 (0.8) | NS | 0.000$ | 0.000 | THAC:Non-op < Op | ||
| RoM Knee (°) | FE | 76.3 (1.9) | 77.3 (1.9) | 78.4 (1.6) | 75.9 (1.6) | NS | 0.005$ | 0.000 | LFH:Non-op > Op | |
| Abd-Add | 15.3 (1.6) | 13.8 (1.6) | 19.6 (1.3) | 17.5 (1.3) | NS | 0.000 | NS | Side: Non-Op > Op | ||
| Rot | 17.2 (1.5) | 18.5 (1.5) | 17.8 (1.2) | 20.3 (1.2) | NS | 0.000 | NS | Side: Non-Op < Op | ||
| Stair Descending (num = 600) | RoM Pelvis (°) | FE | 6.1 (0.6) | 6.4 (0.6) | 6.3 (0.5) | 6.2 (0.5) | NS | NS | NS | |
| Abd-Add | 8.8 (1.0) | 8.4 (1.0) | 8.5 (0.9) | 8.7 (0.9) | NS | NS | NS | |||
| Rot | 6.3 (0.8) | 6.3 (0.8) | 7.2 (0.6) | 7.3 (0.6) | NS | NS | NS | |||
| RoM Hip (°) | FE | 27.9 (1.5) | 27.1 (1.5) | 28.6 (1.3) | 27.4 (1.3) | NS | 0.000 | NS | Side: Non-op > Op | |
| Abd-Add | 12.6 (1.0) | 10.6 (1.0) | 11.8 (0.8) | 12.6 (0.8) | NS | NS | 0.000 | THAC:Non-op > Op LFH:Non-op < Op | ||
| Rot | 14.0 (0.9) | 13.9 (0.9) | 14.0 (0.7) | 14.3 (0.7) | NS | NS | NS | |||
| RoM Knee (°) | FE | 70.5 (1.5) | 73.3 (1.5) | 74.5 (1.2) | 75.1(1.2) | NS | 0.000$ | 0.002 | THAC:Non-Op < Op | |
| Abd-Add | 14.3 (1.4) | 11.4 (1.3) | 14.2 (1.0) | 14.1 (1.0) | NS | 0.000$ | 0.000 | THAC:Non-op > Op | ||
| Rot | 21.5 (1.4) | 18.2 (1.5) | 17.9 (1.2) | 17.0 (1.2) | NS | 0.000$ | 0.001 | THAC:Non-Op > Op | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grip, H.; Nilsson, K.G.; Häger, C.K.; Lundström, R.; Öhberg, F. Does the Femoral Head Size in Hip Arthroplasty Influence Lower Body Movements during Squats, Gait and Stair Walking? A Clinical Pilot Study Based on Wearable Motion Sensors. Sensors 2019, 19, 3240. https://doi.org/10.3390/s19143240
Grip H, Nilsson KG, Häger CK, Lundström R, Öhberg F. Does the Femoral Head Size in Hip Arthroplasty Influence Lower Body Movements during Squats, Gait and Stair Walking? A Clinical Pilot Study Based on Wearable Motion Sensors. Sensors. 2019; 19(14):3240. https://doi.org/10.3390/s19143240
Chicago/Turabian StyleGrip, Helena, Kjell G Nilsson, Charlotte K Häger, Ronnie Lundström, and Fredrik Öhberg. 2019. "Does the Femoral Head Size in Hip Arthroplasty Influence Lower Body Movements during Squats, Gait and Stair Walking? A Clinical Pilot Study Based on Wearable Motion Sensors" Sensors 19, no. 14: 3240. https://doi.org/10.3390/s19143240
APA StyleGrip, H., Nilsson, K. G., Häger, C. K., Lundström, R., & Öhberg, F. (2019). Does the Femoral Head Size in Hip Arthroplasty Influence Lower Body Movements during Squats, Gait and Stair Walking? A Clinical Pilot Study Based on Wearable Motion Sensors. Sensors, 19(14), 3240. https://doi.org/10.3390/s19143240







