Comparison between the Airgo™ Device and a Metabolic Cart during Rest and Exercise †
Abstract
:1. Introduction
2. Materials and Methods
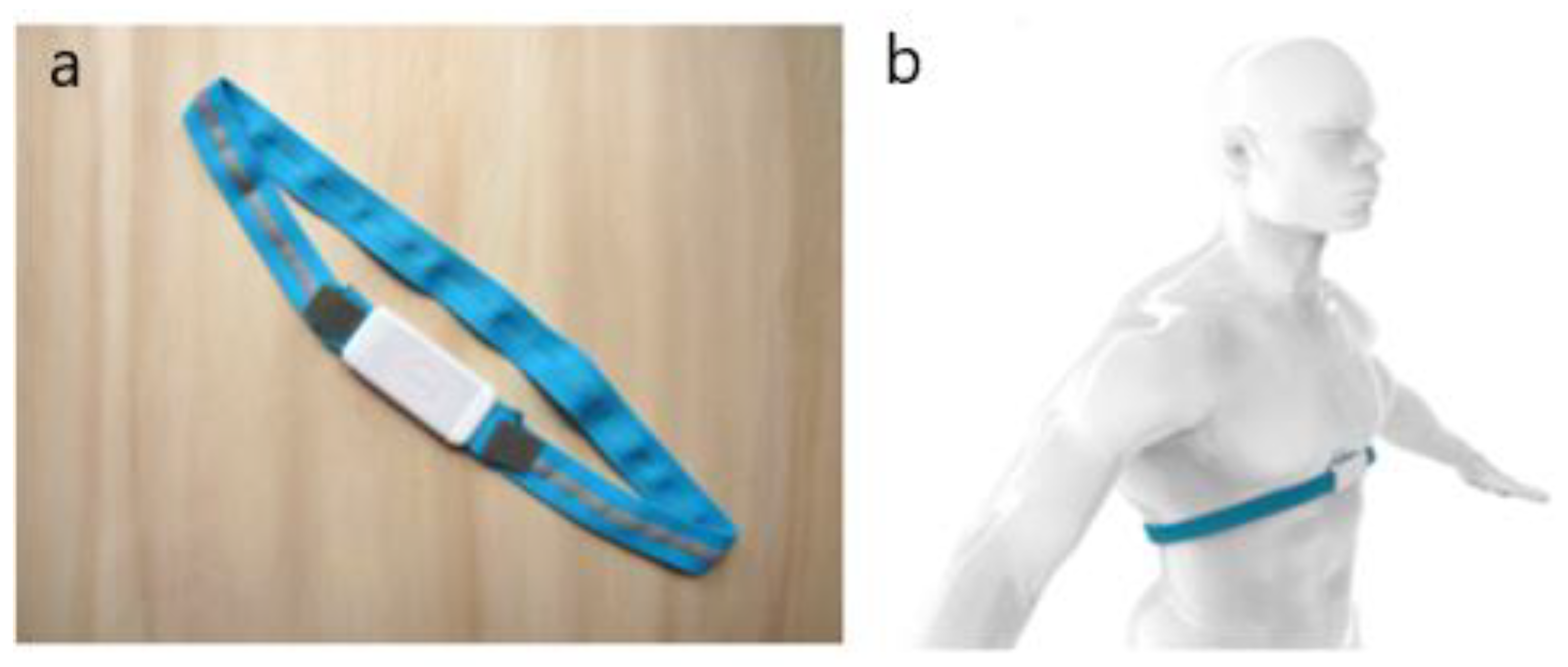
2.1. Description of the Hardware
2.2. Study Design
- Non-smoking healthy volunteers;
- Volunteers able to perform a routine respiratory function test (spirometry and cardiopulmonary exercise testing);
- Age between 18 and 75;
- Volunteers able to understand an informed consensus.
- Presence of cardiac comorbidities (e.g., coronary artery diseases, heart failure, arrhythmias);
- History of coronary artery disease of heart failure;
- Presence of respiratory comorbidities (e.g., asthma, COPD, sleep apnea, lung interstitial disease);
- Pregnancy;
- Former smoker of >5 pack-year;
- Severe trauma or major surgery in the last year;
- Chest pain;
- Obesity (BMI > 30).
2.3. Acquisition Protocol
- (1)
- Preparation: measurement of the subject’s thoracic oblique circumference length (C) at the end of a forced expiration maneuver. Knowing this measurement, the initial length of the elastic band (L0) was decided to be 7% shorter than C in order to obtain a pre-tensioned girth band to ensure an effective fit around the torso. After being cut, the band was coupled with the Airgo™ electronic device and positioned against the subject’s skin;
- (2)
- Test at rest: recording of respiratory parameters while letting the subject breathe quietly in five different standardized positions (standing, seated, supine, right lateral decubitus, left lateral decubitus) for 4 min. The supine position required an elevation of the subject’s head not greater than 10° with a pillow under the subject’s head. Tidal volume, respiratory rate, minute ventilation, inspiratory time, expiratory time and duty cycle (explained in Table 1) were simultaneously recorded by the Airgo™ system and the SensorMedics 2900 metabolic cart. In order to facilitate the off-line synchronization between the two systems, subjects were asked to perform a big, deep breath at the beginning of the test for each position;
- (3)
- Test under physical exercise: execution of a cardiopulmonary exercise test on the Ergoline cycle ergometer 800S, followed by a recovery period. A symptom-limited incremental exercise test was performed and designed to achieve a maximum load in 10 ± 2 min in each subject wearing the Airgo™ device. The physical exercise test followed a linear incremental protocol (with a slope between 15 and 20 Watts per minute) was identical for men and women and chosen based on the level of training of each subject so that each test lasted approximately between 6 and 12 min, as recommended in the guidelines. Subjects were asked to cycle at a velocity of 60 revolutions/minute for 2 min with no load, then to pedal at incremental workload maintaining the same speed as before until the maximal effort was reached. Once the subject reached his/her maximal effort and he/she was not able to ride anymore, the exercise test was interrupted, and the recovery phase started. This phase consisted of cycling for 2 min without any resistance followed by 2 min at rest. The same respiratory parameters as test at rest have been acquired with the big-breath synchronization maneuver.
2.4. Airgo™ Signal Processing Algorithm
- Identification of maximum and minimum of each breathing cycle;
- Representation of each breath by means of a vector that connects the maximum and the minimum;
- Automatic reconstruction of segmented breath, e.g., due to obstructions or movement;
- Automatic removal of fake breaths;
- Computation of tidal volume, respiratory rate and minute ventilation.
2.5. Features Extraction
2.6. Alignment and Data Organization
- Airgo™’s normalized amplitude (AmpA/AmpA0);
- Airgo™’s normalized minute ventilation (MVA/MVA0);
- SensorMedics’ normalized tidal volume (VtSM/VtSM0);
- SensorMedics’ normalized minute ventilation (VtSM/VtSM0).
2.7. Statistical Analysis
3. Results
3.1. Relative Error Medians, Interquartile Ranges and Limits of Agreement of the Parameters
3.2. Kruskal–Wallis Tests between Positions and Intensities
3.3. Box and Whiskers Plots
3.4. Bland–Altman Plots
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Fieselmann, J.F.; Hendryx, M.S.; Helms, C.M.; Wakefield, D.S. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J. Gen. Intern. Med. 1993, 8, 354–360. [Google Scholar] [CrossRef]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory rate: The neglected vital sign. Med. J. Aust. 2008, 188, 657–659. [Google Scholar] [CrossRef]
- Yañez, A.M.; Guerrero, D.; Calle-Rubio, M.; Torrent, M.; Ussetti, P.; Alvarez-Sala, J.L.; de Molina, R.M.; Falcones, M.V.; Sauleda, J.; Gay, M.F.; et al. Monitoring breathing rate at home allows early identification of COPD exacerbations. Chest 2012, 142, 1524–1529. [Google Scholar]
- Shah, S.A.; Velardo, C.; Farmer, A.; Tarassenko, L. Exacerbations in Chronic Obstructive Pulmonary Disease: Identification and Prediction Using a Digital Health System. J. Med. Internet Res. 2017, 19, e69. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, F.; Karatzanis, I.; Zacharioudakis, G.; Meriggi, P.; Rizzo, F.; Stratakis, M.; Louloudakis, S.; Biniaris, C.; Valentini, M.; Di Rienzo, M.; et al. Measurement of heart rate and respiratory rate using a textile-based wearable device in heart failure patients. In Proceedings of the 2008 Computers in Cardiology, Bologna, Italy, 14–17 September 2008; pp. 901–904. [Google Scholar]
- Sun, Q.; Qiu, H.; Huang, M.; Yang, Y. Lower mortality of COVID-19 by early recognition and intervention: Experience from Jiangsu Province. Ann. Intensive Care 2020, 10, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Rajeh, A.; Hurst, J. Monitoring of Physiological Parameters to Predict Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review. J. Clin. Med. 2016, 5, 108. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolò, A.; Schena, E.; Sacchetti, M. Remote Respiratory Monitoring in the Time of COVID-19. Front. Physiol. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Villar, R.; Beltrame, T.; Hughson, R.L. Validation of the Hexoskin wearable vest during lying, sitting, standing, and walking activities. Appl. Physiol. Nutr. Metab. 2015, 40, 1019–1024. [Google Scholar] [CrossRef]
- Sarmento, A.; Vignati, C.; Paolillo, S.; Lombardi, C.; Scoccia, A.; Nicoli, F.; Mapelli, M.; Leonardi, A.; Ossola, D.; Rigoni, R.; et al. Qualitative and quantitative evaluation of a new wearable device for ECG and respiratory Holter monitoring. Int. J. Cardiol. 2018, 272, 231–237. [Google Scholar] [CrossRef]
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Jain, R.; Radom-Aizik, S.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Talaminos-Barroso, A.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rostan, G.; Cejudo-Ramos, P.; Marquez-Martin, E.; Ortega-Ruiz, F. Smart vest for respiratory rate monitoring of copd patients based on non-contact capacitive sensing. Sensors 2018, 18, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of breathing parameters using an inertial measurement unit (IMU)-based system. Sensors 2019, 19, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.Z.; Guo, Y.W.; Zhu, Q.S.; Huang, B.Y.; Wang, L. Estimation of respiration rate from three-dimensional acceleration data based on body sensor network. Telemed. J. E-Health 2011, 17, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, C.; Venanzi, C.; Silvatti, A.P.; Presti, D.L.; Saccomandi, P.; Formica, D.; Giurazza, F.; Caponero, M.A.; Schena, E. Smart textile for respiratory monitoring and thoraco-abdominal motion pattern evaluation. J. Biophotonics 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downey, C.; Ng, S.; Jayne, D.; Wong, D. Reliability of a wearable wireless patch for continuous remote monitoring of vital signs in patients recovering from major surgery: A clinical validation study from the TRaCINg trial. BMJ Open 2019, 9, e031150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitlock, J.; Sill, J.; Jain, S. A-spiro: Towards continuous respiration monitoring. Smart Health 2020, 15, 100105. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, L.; Bao, N.; Qi, L.; Shi, J.; Yang, Y.; Yao, Y. Research on non-contact monitoring system for human physiological signal and body movement. Biosensors 2019, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Aliverti, A. Wearable technology: Role in respiratory health and disease. Breathe 2017, 13, e27–e36. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, A.; Aliverti, A. Telemonitoring systems for respiratory patients: Technological aspects. Pulmonology 2020, 26, 221–232. [Google Scholar] [CrossRef]
- Nicolò, A.; Massaroni, C.; Passfield, L. Respiratory Frequency during Exercise: The Neglected Physiological Measure. Front. Physiol. 2017, 8, 922. [Google Scholar] [CrossRef]
- Antonelli, A.; Stanzi, A.; Mazza, F.; Venturino, M.; Noceti, P.; Guilizzoni, D.; Aliverti, A.; Melloni, G. Validation study of the Airgo™ device for continuous monitoring of respiratory function. Eur. Respir. J. 2018, 52, PA2418. [Google Scholar]
- Qi, W.; Aliverti, A. A Multimodal Wearable System for Continuous and Real-time Breathing Pattern Monitoring During Daily Activity. IEEE J. Biomed. Health Inform. 2019, 1. [Google Scholar] [CrossRef]
- Romei, M.; Mauro, A.L.; D’Angelo, M.G.; Turconi, A.C.; Bresolin, N.; Pedotti, A.; Aliverti, A. Effects of gender and posture on thoraco-abdominal kinematics during quiet breathing in healthy adults. Respir. Physiol. Neurobiol. 2010, 172, 184–191. [Google Scholar] [CrossRef] [PubMed]















| Parameter | Definition | Measurement Unit |
|---|---|---|
| Tidal volume | Amount of air moving into and out of the lungs during spontaneous breathing | Liters [L] |
| Respiratory rate | Number of breaths performed in a minute | Breaths/minute [bpm] |
| Minute ventilation | Tidal volume multiplied by respiratory rate | Liters/minute [L/min] |
| Inspiratory time | Duration of the inspiration phase | Seconds [s] |
| Expiratory time | Duration of the expiration phase | Seconds [s] |
| Duty cycle | Inspiratory time divided by total time | Percentage [%] |
| Parameter | Standing | Seated | Supine | Side R | Side L |
|---|---|---|---|---|---|
| Normalized amplitude | 6.8% (21.5/±12.2) | 9.0% (26.0/±18.0) | 0.8% (33.2/±33.7) | 4.4% (28.7/±21.1) | 1.1% (27.0/±20.4) |
| Respiratory rate (bpm) | 0% (3.5/±5.9) | 0% (8.9/±7.9) | 0% (15.0/±10.6) | 0% (6.8/±8.6) | 0% (4.7/±6.3) |
| Normalized minute ventilation | 5.9% (23.4/±12.5) | 7.0% (27.6/±16.2) | 3.4% (34.9/±38.0) | 3.6% (29.9/±22.6) | 1.2% (28.1/±20.7) |
| Duty cycle (%) | −0.29 (0.18/±0.07) | −0.31 (0.20/±0.08) | −0.32 (0.21/±0.12) | −0.30 (0.18/±0.05) | −0.28 (0.18/±0.06) |
| Parameter | Low Intensity | Medium Intensity | High Intensity | Maximum Intensity | Recovery Phase 1 | Recovery Phase 2 |
|---|---|---|---|---|---|---|
| Normalized amplitude | −11.1% (24.9/±56.5) | −29.6% (18.5/±131.5) | −36.4% (14.8/±194.0) | −39.7% (13.8/±188.1) | −34.3% (14.5/±189.1) | −24.8% (20.6/±128.0) |
| Respiratory rate (bpm) | 0% (22.4/±25.8) | 0% (18.9/±19.3) | 0% (17.1/±19.6) | 0% (15.9/±31.5) | 0% (16.2/±30.8) | 0% (20.0/±50.8) |
| Normalized minute ventilation | −9.3% (28.4/±57.1) | −33.3% (18.1/±160.6) | −36.0% (13.6/±296.0) | −40.6% (14.1/±373.7) | −33.7% (14.7/±307.0) | −22.8% (22.1/±168.1) |
| Duty cycle (%) | −0.21 (0.26/±0.10) | −0.27 (0.24/±0.11) | −0.24 (0.25/±0.13) | −0.23 (0.33/±0.13) | −0.14 (0.18/±0.10) | −0.17 (0.22/±0.09) |
| Parameter | p-Value (Test at Rest) | (p-Value Exercise Test) |
|---|---|---|
| Normalized amplitude | 0.107 | <0.01 |
| Normalized minute ventilation | 0.174 | 0.001 |
| Respiratory rate (bpm) | 0.932 | 0.631 |
| Duty cycle | <0.05 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonelli, A.; Guilizzoni, D.; Angelucci, A.; Melloni, G.; Mazza, F.; Stanzi, A.; Venturino, M.; Kuller, D.; Aliverti, A. Comparison between the Airgo™ Device and a Metabolic Cart during Rest and Exercise. Sensors 2020, 20, 3943. https://doi.org/10.3390/s20143943
Antonelli A, Guilizzoni D, Angelucci A, Melloni G, Mazza F, Stanzi A, Venturino M, Kuller D, Aliverti A. Comparison between the Airgo™ Device and a Metabolic Cart during Rest and Exercise. Sensors. 2020; 20(14):3943. https://doi.org/10.3390/s20143943
Chicago/Turabian StyleAntonelli, Andrea, Dario Guilizzoni, Alessandra Angelucci, Giulio Melloni, Federico Mazza, Alessia Stanzi, Massimiliano Venturino, David Kuller, and Andrea Aliverti. 2020. "Comparison between the Airgo™ Device and a Metabolic Cart during Rest and Exercise" Sensors 20, no. 14: 3943. https://doi.org/10.3390/s20143943
APA StyleAntonelli, A., Guilizzoni, D., Angelucci, A., Melloni, G., Mazza, F., Stanzi, A., Venturino, M., Kuller, D., & Aliverti, A. (2020). Comparison between the Airgo™ Device and a Metabolic Cart during Rest and Exercise. Sensors, 20(14), 3943. https://doi.org/10.3390/s20143943







