Interpretability of Input Representations for Gait Classification in Patients after Total Hip Arthroplasty
Abstract
:1. Introduction
- (i)
- Many studies have used simple descriptive statistics of the gait waveforms such as peak values, range of motion, or respective side differences [13,14]. They are straight forward to interpret and are often mentioned in the literature for describing gait characteristics. However, it is unclear if important information would be a priori discarded, and model performance is consequently negatively affected. A further limitation is the dependence on expert or prior knowledge.
- (ii)
- An alternative approach, which is independent of prior knowledge, is the use of entire concatenated waveforms as input features [7,15,16]. This allows for an interpretation of group differences through the determination of important areas of the waveforms. However, it is unclear if this shows better discriminative power compared with the abovementioned extracted statistical features and, therefore, enhances classification performance. Correlations and redundancy of the inputs may further be problematic.
- (iii)
- Lastly, automated feature extraction using a vast amount of possibly meaningful statistics can be applied [17]. Feature extraction algorithms such as tsfresh [18] or featuretools [19] can be used for this. However, the extracted features are often nested, complex, and hard to interpret, therefore showing limited comparability with the literature, which results in questionable clinical relevance.
2. Materials and Methods
2.1. Subjects, Data Acquisition, and Data Preprocessing
- without data scaling,
- removal of the mean and scaling to unit variance (StandardScaler),
- scaling to a feature range between 0 and 1 (MinMaxScaler).
2.2. Model Training and Classification
2.3. Model Interpretation
3. Results
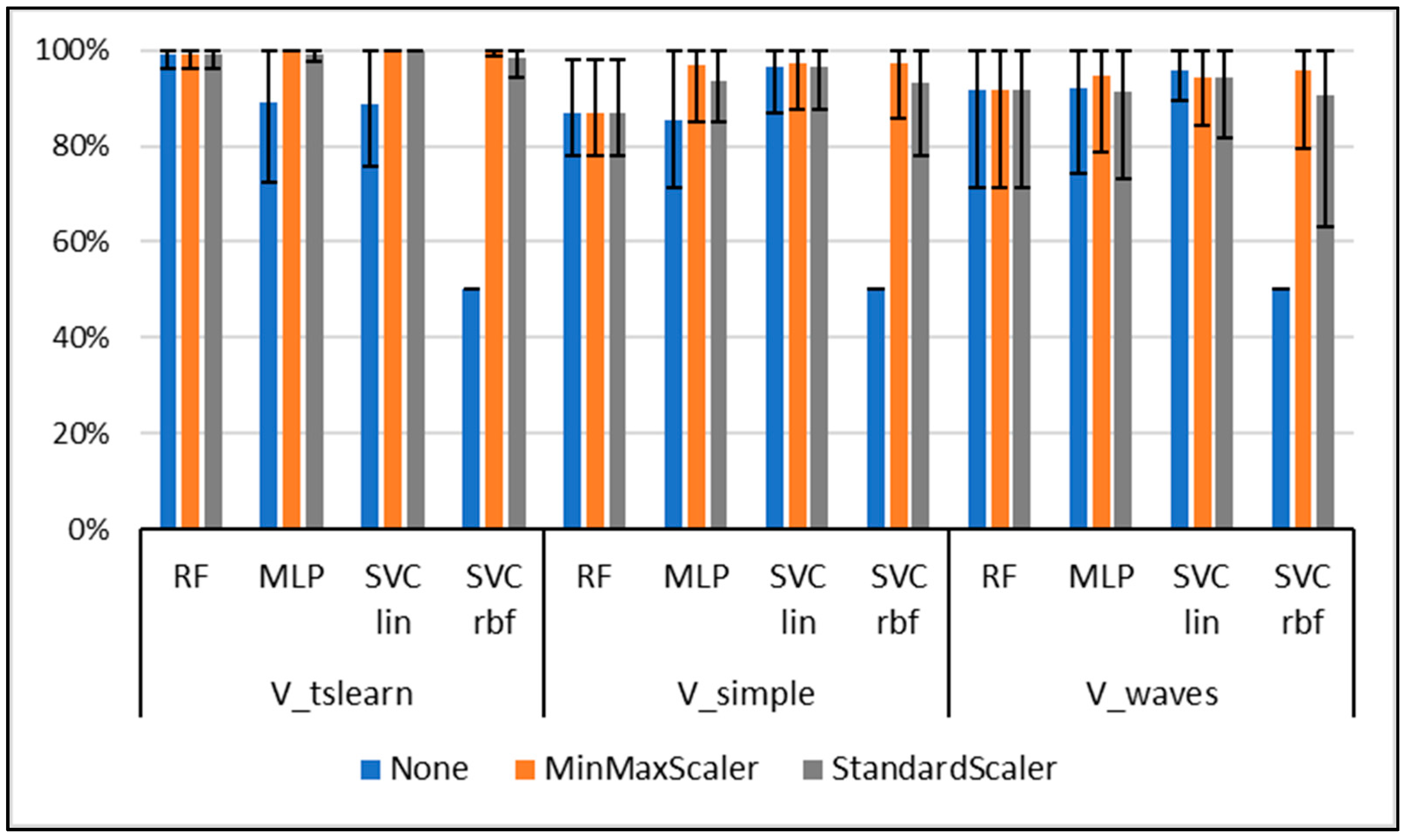
3.1. Classification Results
3.2. Model Interpretation Based on Waveforms
3.3. Model Interpretation: Discrete Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eskofier, B.M.; Kraus, M.; Worobets, J.T.; Stefanyshyn, D.J.; Nigg, B.M. Pattern classification of kinematic and kinetic running data to distinguish gender, shod/barefoot and injury groups with feature ranking. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ferber, R.; McClay Davis, I.; Williams, D.S., III. Gender differences in lower extremity mechanics during running. Clin. Biomech. 2003, 18, 350–357. [Google Scholar] [CrossRef]
- Phinyomark, A.; Petri, G.; Ibáñez-Marcelo, E.; Osis, S.T.; Ferber, R. Analysis of Big Data in Gait Biomechanics: Current Trends and Future Directions. J. Med. Biol. Eng. 2018, 38, 244–260. [Google Scholar] [CrossRef] [Green Version]
- Halilaj, E.; Rajagopal, A.; Fiterau, M.; Hicks, J.L.; Hastie, T.J.; Delp, S.L. Machine learning in human movement biomechanics: Best practices, common pitfalls, and new opportunities. J. Biomech. 2018, 81, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kobsar, D.; Charlton, J.M.; Tse, C.T.F.; Esculier, J.-F.; Graffos, A.; Krowchuk, N.M.; Thatcher, D.; Hunt, M.A. Validity and reliability of wearable inertial sensors in healthy adult walking: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2020, 17, 62. [Google Scholar] [CrossRef]
- Bzdok, D.; Altman, N.; Krzywinski, M. Statistics versus machine learning. Nat. Methods 2018, 15, 233–234. [Google Scholar] [CrossRef]
- Laroche, D.; Tolambiya, A.; Morisset, C.; Maillefert, J.F.; French, R.M.; Ornetti, P.; Thomas, E. A classification study of kinematic gait trajectories in hip osteoarthritis. Comput. Biol. Med. 2014, 55, 42–48. [Google Scholar] [CrossRef]
- Lau, H.-Y.; Tong, K.-Y.; Zhu, H. Support vector machine for classification of walking conditions of persons after stroke with dropped foot. Hum. Mov. Sci. 2009, 28, 504–514. [Google Scholar] [CrossRef]
- Wahid, F.; Begg, R.K.; Hass, C.J.; Halgamuge, S.; Ackland, D.C. Classification of Parkinson’s Disease Gait Using Spatial-Temporal Gait Features. IEEE J. Biomed. Health Inform. 2015, 19, 1794–1802. [Google Scholar] [CrossRef]
- Adadi, A.; Berrada, M. Peeking Inside the Black-Box: A Survey on Explainable Artificial Intelligence (XAI). IEEE Access 2018, 6, 52138–52160. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2016/679 of the european parliament and of the council of 27 april 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing directive 95/46/ec (General Data Protection Regulation). Off. J. Eur. Union 2016, L119, 1–88. [Google Scholar]
- Slijepcevic, D.; Zeppelzauer, M.; Schwab, C.; Raberger, A.-M.; Breiteneder, C.; Horsak, B. Input representations and classification strategies for automated human gait analysis. Gait Posture 2020, 76, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Teufl, W.; Taetz, B.; Miezal, M.; Lorenz, M.; Pietschmann, J.; Jöllenbeck, T.; Fröhlich, M.; Bleser, G. Towards an Inertial Sensor-Based Wearable Feedback System for Patients after Total Hip Arthroplasty: Validity and Applicability for Gait Classification with Gait Kinematics-Based Features. Sensors 2019, 19, 5006. [Google Scholar] [CrossRef] [Green Version]
- Begg, R.; Kamruzzaman, J. A machine learning approach for automated recognition of movement patterns using basic, kinetic and kinematic gait data. J. Biomech. 2005, 38, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Dindorf, C.; Teufl, W.; Taetz, B.; Becker, S.; Bleser, G.; Fröhlich, M. Feature extraction and gait classification in hip replacement patients on basis of kinematic waveform data. (under review).
- Horst, F.; Slijepcevic, D.; Lapuschkin, S.; Raberger, A.-M.; Zeppelzauer, M.; Samek, W.; Breiteneder, C.; Schöllhorn, W.I.; Horsak, B. On the Understanding and Interpretation of Machine Learning Predictions in Clinical Gait Analysis Using Explainable Artificial Intelligence. Available online: http://arxiv.org/pdf/1912a.07737v1 (accessed on 10 March 2020).
- Dindorf, C.; Konradi, J.; Wolf, C.; Taetz, B.; Bleser, G.; Huthwelker, J.; Drees, P.; Fröhlich, M.; Betz, U. General method for automated feature extraction and selection and its application for gender classification and biomechanical knowledge discovery of sex differences in spinal posture during stance and gait. (under review).
- Christ, M.; Kempa-Liehr, A.W.; Feindt, M. Distributed and Parallel Time Series Feature Extraction for Industrial Big Data Applications. Available online: http://arxiv.org/pdf/1610.07717v3 (accessed on 10 January 2020).
- Feature Labs, I. Featuretools: Automated Feature Engineering. Available online: https://www.featuretools.com/ (accessed on 30 May 2020).
- Eskofier, B.M.; Federolf, P.; Kugler, P.F.; Nigg, B.M. Marker-based classification of young-elderly gait pattern differences via direct PCA feature extraction and SVMs. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yu, L. Toward Integrating Feature Selection Algorithms for Classification and Clustering. IEEE Trans. Knowl. Data Eng. 2005, 17, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Gunning, D.; Stefik, M.; Choi, J.; Miller, T.; Stumpf, S.; Yang, G.-Z. XAI—Explainable artificial intelligence. Sci. Robot. 2019, 4, eaay7120. [Google Scholar] [CrossRef] [Green Version]
- Bologna, G.; Hayashi, Y. Characterization of Symbolic Rules Embedded in Deep DIMLP Networks: A Challenge to Transparency of Deep Learning. J. Artif. Intell. Soft Comput. Res. 2017, 7, 265–286. [Google Scholar] [CrossRef] [Green Version]
- Samek, W.; Müller, K.-R. Towards explainable artificial intelligence. In Explainable AI: Interpreting, Explaining and Visualizing Deep Learning, 1st ed.; Samek, W., Montavon, G., Vedaldi, A., Hansen, L.K., Muller, K.-R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 5–22. [Google Scholar]
- Holzinger, A.; Biemann, C.; Pattichis, C.S.; Kell, D.B. What do We Need to Build Explainable AI Systems for the Medical Domain? Available online: http://arxiv.org/pdf/1712.09923v1 (accessed on 20 February 2020).
- Ribeiro, M.T.; Singh, S.; Guestrin, C. "Why Should I Trust You?": Explaining the Predictions of Any Classifier. In Proceedings of the KDD '16: The 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Shrikumar, A.; Greenside, P.; Kundaje, A. Learning important features through propagating activation differences. In Proceedings of the 34th International Conference on Machine Learning, Sydney, Australia, 6–11 August 2017; pp. 3145–3153. [Google Scholar]
- OECD. Health at a Glance: Europe 2016. State of Health in the EU Cycle; OECD Publishing: Paris, France, 2016; ISBN 978-92-64-26559-2.
- Teufl, W.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Validity, Test-Retest Reliability and Long-Term Stability of Magnetometer Free Inertial Sensor Based 3D Joint Kinematics. Sensors 2018, 18, 1980. [Google Scholar] [CrossRef] [Green Version]
- Miezal, M.; Taetz, B.; Bleser, G. On Inertial Body Tracking in the Presence of Model Calibration Errors. Sensors 2016, 16, 1132. [Google Scholar] [CrossRef]
- Miezal, M.; Taetz, B.; Bleser, G. Real-time inertial lower body kinematics and ground contact estimation at anatomical foot points for agile human locomotion. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017; pp. 3256–3263. [Google Scholar]
- Teufl, W.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Validity of inertial sensor based 3D joint kinematics of static and dynamic sport and physiotherapy specific movements. PLoS ONE 2019, 14, e0213064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teufl, W.; Lorenz, M.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Towards Inertial Sensor Based Mobile Gait Analysis: Event-Detection and Spatio-Temporal Parameters. Sensors 2018, 19, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewen, A.M.; Stewart, S.; St Clair Gibson, A.; Kashyap, S.N.; Caplan, N. Post-operative gait analysis in total hip replacement patients-a review of current literature and meta-analysis. Gait Posture 2012, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M.L.; Lamontagne, M.; Beaulé, P.E. Lower limb biomechanics during gait do not return to normal following total hip arthroplasty. Gait Posture 2010, 32, 269–273. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. JAIR 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Schöllhorn, W.I.; Nigg, B.M.; Stefanyshyn, D.J.; Liu, W. Identification of individual walking patterns using time discrete and time continuous data sets. Gait Posture 2002, 15, 180–186. [Google Scholar] [CrossRef]
- Horst, F.; Kramer, F.; Schäfer, B.; Eekhoff, A.; Hegen, P.; Nigg, B.M.; Schöllhorn, W.I. Daily changes of individual gait patterns identified by means of support vector machines. Gait Posture 2016, 49, 309–314. [Google Scholar] [CrossRef]
- Hering, J.; Metzenthin, E.; Zenner, A. LIME For Time. Available online: https://github.com/emanuel-metzenthin/Lime-For-Time (accessed on 20 February 2020).
- Molnar, C. Interpretable Machine Learning. A Guide for Making Black Box Models Explainable. 2018. Available online: https://christophm.github.io/interpretable-ml-book/index.html (accessed on 20 February 2020).
- Ataky, T.C. One-dimensional statistical parametric mapping in Python. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 295–301. [Google Scholar] [CrossRef]
- Horstmann, T.; Listringhaus, R.; Haase, G.-B.; Grau, S.; Mündermann, A. Changes in gait patterns and muscle activity following total hip arthroplasty: A six-month follow-up. Clin. Biomech. 2013, 28, 762–769. [Google Scholar] [CrossRef]
- Begg, R.K.; Palaniswami, M.; Owen, B. Support vector machines for automated gait classification. IEEE Trans. Biomed. Eng. 2005, 52, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Kaufman, K.R. Effects of total hip arthroplasty on gait. In Handbook of Human Motion; Müller, B., Wolf, S., Eds.; Springer: Cham, Germany, 2018; pp. 1–15. ISBN 978-3-319-30808-1. [Google Scholar]
- Perron, M.; Malouin, F.; Moffet, H.; McFadyen, B.J. Three-dimensional gait analysis in women with a total hiparthroplasty. Clin. Biomech. 2000, 15, 504–515. [Google Scholar] [CrossRef]
- Madsen, M.S.; Ritter, M.A.; Morris, H.H.; Meding, J.B.; Berend, M.E.; Faris, P.M.; Vardaxis, V.G. The effect of total hip arthroplasty surgical approach on gait. J. Orthop. Res. 2004, 22, 44–50. [Google Scholar] [CrossRef]
- Miller, T. Explanation in artificial intelligence: Insights from the social sciences. Artif. Intell. 2019, 267, 1–38. [Google Scholar] [CrossRef]
- Levinger, P.; Lai, D.T.H.; Begg, R.K.; Webster, K.E.; Feller, J.A. The application of support vector machines for detecting recovery from knee replacement surgery using spatio-temporal gait parameters. Gait Posture 2009, 29, 91–96. [Google Scholar] [CrossRef]
- Nüesch, C.; Valderrabano, V.; Huber, C.; von Tscharner, V.; Pagenstert, G. Gait patterns of asymmetric ankle osteoarthritis patients. Clin. Biomech. 2012, 27, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.P.; de Castro, M.P.; Mendes, E.A.; Machado, L. Principal component analysis in ground reaction forces and center of pressure gait waveforms of people with transfemoral amputation. Prosthet. Orthot. Int. 2016, 40, 729–738. [Google Scholar] [CrossRef]
- Barlow, H.; Mao, S.; Khushi, M. Predicting High-Risk Prostate Cancer Using Machine Learning Methods. Data 2019, 4, 129. [Google Scholar] [CrossRef] [Green Version]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.-S. Validity and Reliability of Wearable Sensors for Joint Angle Estimation: A Systematic Review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Long, F.; Ding, C. Feature Selection Based on Mutual Information: Criteria of Max-Dependency, Max-Relevance, and Min-Redundancy. IEEE Trans. Pattern Anal. Mach. Intell. 2005, 27, 1226–1238. [Google Scholar] [CrossRef]




| Abbreviation | Description | Size (GC × Feature) |
|---|---|---|
| V_waves | Concatenated time-normalized GC for the measured variables. | 940 × 2100 |
| V_simple | Calculated features based on simple descriptive statistics which are commonly mentioned in the literature [35,36]. Maxima, minima, and ROM for every variable as well as the difference between affected and unaffected sides for the respective variables were calculated. | 940 × 74 |
| V_tsfresh | Automated feature extraction with the tsfresh algorithm [18]. | 940 × 8349 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dindorf, C.; Teufl, W.; Taetz, B.; Bleser, G.; Fröhlich, M. Interpretability of Input Representations for Gait Classification in Patients after Total Hip Arthroplasty. Sensors 2020, 20, 4385. https://doi.org/10.3390/s20164385
Dindorf C, Teufl W, Taetz B, Bleser G, Fröhlich M. Interpretability of Input Representations for Gait Classification in Patients after Total Hip Arthroplasty. Sensors. 2020; 20(16):4385. https://doi.org/10.3390/s20164385
Chicago/Turabian StyleDindorf, Carlo, Wolfgang Teufl, Bertram Taetz, Gabriele Bleser, and Michael Fröhlich. 2020. "Interpretability of Input Representations for Gait Classification in Patients after Total Hip Arthroplasty" Sensors 20, no. 16: 4385. https://doi.org/10.3390/s20164385
APA StyleDindorf, C., Teufl, W., Taetz, B., Bleser, G., & Fröhlich, M. (2020). Interpretability of Input Representations for Gait Classification in Patients after Total Hip Arthroplasty. Sensors, 20(16), 4385. https://doi.org/10.3390/s20164385







