A Wearable Device for Breathing Frequency Monitoring: A Pilot Study on Patients with Muscular Dystrophy
Abstract
:1. Introduction
2. Materials and Methods
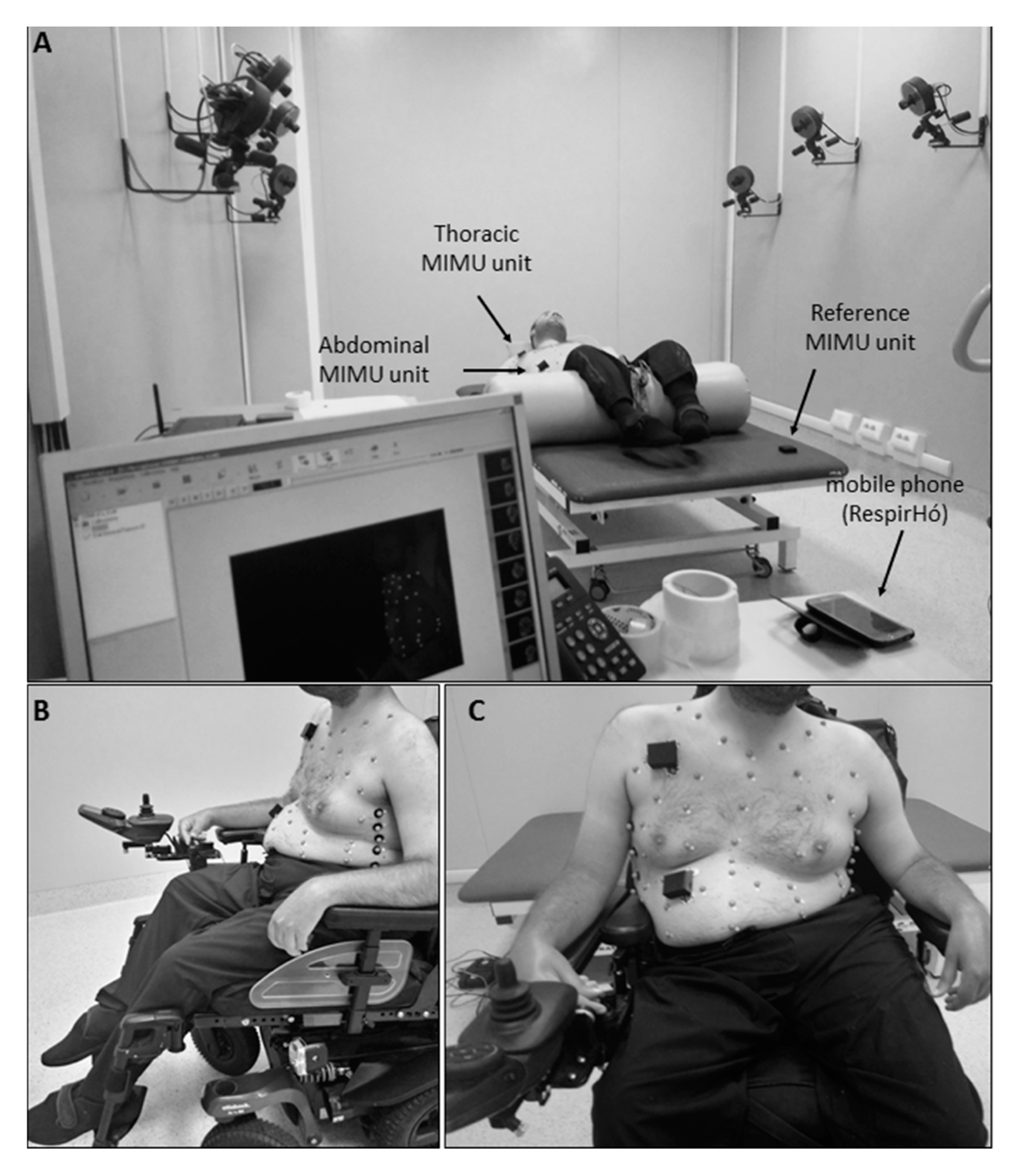
2.1. Device Description
2.2. Analysis Algorithm
2.3. Clinical Protocol
2.3.1. Participants
2.3.2. Respiratory Function Assessment
2.3.3. Experimental Procedures
Phase A: Laboratory Validation
Phase B: Daily Use Assessment
2.4. Measurements and Statistical Analysis
2.4.1. Validation in Static Conditions
2.4.2. Long-Term Breathing Pattern Monitoring (Daily Use)
- Autonomous use time: duration of time during which the patient autonomously used the device.
- Intrinsic data waste: equal to the difference between the expected duration of the acquisition and the actual duration of the recorded data. The latter was obtained as the number of recorded files multiplied by the expected duration of each file (155 s). The intrinsic waste of data is due to limitations of the transmission protocol that requires: (1) resynchronization of data sent by the abdominal, thoracic, and reference units with a consequent waste of initial and final data for each block; and (2) sending of each 3-min block data from the reference unit to the smartphone, which is an operation requiring about 45 s during which the system cannot acquire data.
- Efficiency in terms of data analysis: expressed as the number of files that can be actually analyzed (at least 30 s of consecutive data must be available to compute the PSD and correctly execute the analysis algorithm), with respect to the number of total files recorded.
- Number of unexpected interruptions (N. of unexpected interruption).
2.4.3. Usability and Acceptability
2.4.4. Breathing Frequency: A Potential Marker of Respiratory Dysfunction
3. Results
3.1. Participants
3.2. Respiratory Function
3.3. Validation in Static Conditions
3.4. Long-Term Breathing Pattern Monitoring (Daily Use)
3.5. Usability and Acceptability
3.6. Breathing Frequency: A Potential Marker of Respiratory Dysfunction
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- It is possible to use the device without the need to modify my habits.
- It was difficult to learn using the device.
- I think the device is easy to wear and place.
- I think I would need someone to help me managing the device.
- The fixation method of the device units facilitates the placement and improve the wearability.
- Sometimes I preferred to remove the device for a period.
- I think I would be able to use the device autonomously (placement, activation, app management, etc.…).
- I found the fixation method uncomfortable.
- I think I could wear the device for a long period.
- I think that the use of the device would negatively affect my daily activities.
References
- Gozal, D. Pulmonary Manifestations of Neuromuscular Disease with Special Reference to Duchenne Muscular Dystrophy and Spinal Muscular Atrophy. Pediatr. Pulmonol. 2000, 29, 141–150. [Google Scholar] [CrossRef]
- Fardeau, M.; Hillaire, D.; Mignard, C.; Feingold, N.; Feingold, J.; Mignard, D.; De Ubeda, B.; Collin, H.; Tomé, F.; Richard, I. Juvenile Limb-Girdle Muscular Dystrophy: Clinical, Histopathological and Genetic Data from a Small Community Living in the Reunion Island. Brain 1996, 119, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groen, E.J.; Charlton, R.; Barresi, R.; Anderson, L.V.; Eagle, M.; Hudson, J.; Koref, M.S.; Straub, V.; Bushby, K.M. Analysis of the UK Diagnostic Strategy for Limb Girdle Muscular Dystrophy 2A. Brain 2007, 130, 3237–3249. [Google Scholar] [CrossRef] [PubMed]
- Urtasun, M.; Saenz, A.; Roudaut, C.; Poza, J.J.; Urtizberea, J.A.; Cobo, A.M.; Richard, I.; Garcia Bragado, F.; Leturcq, F.; Kaplan, J.C.; et al. Limb-Girdle Muscular Dystrophy in Guipuzcoa (Basque Country, Spain). Brain 1998, 121, 1735–1747. [Google Scholar] [CrossRef] [Green Version]
- Wagner, K.R.; Lechtzin, N.; Judge, D.P. Current Treatment of Adult Duchenne Muscular Dystrophy. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Narayanaswami, P.; Weiss, M.; Selcen, D.; David, W.; Raynor, E.; Carter, G.; Wicklund, M.; Barohn, R.J.; Ensrud, E.; Griggs, R.C.; et al. Evidence-Based Guideline Summary: Diagnosis and Treatment of Limb-Girdle and Distal Dystrophies: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2014, 83, 1453–1463. [Google Scholar]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory Rate: The Neglected Vital Sign. Med. J. Aust. 2008, 188, 657. [Google Scholar] [CrossRef]
- Subbe, C.; Davies, R.; Williams, E.; Rutherford, P.; Gemmell, L. Effect of Introducing the Modified Early Warning Score on Clinical Outcomes, cardio-pulmonary Arrests and Intensive Care Utilisation in Acute Medical Admissions. Anaesthesia 2003, 58, 797–802. [Google Scholar] [CrossRef]
- Castagna, J.; Weil, M.H.; Shubin, H. Factors Determining Survival in Patients with Cardiac Arrest. Chest 1974, 65, 527–529. [Google Scholar] [CrossRef]
- Fieselmann, J.F.; Hendryx, M.S.; Helms, C.M.; Wakefield, D.S. Respiratory Rate Predicts Cardiopulmonary Arrest for Internal Medicine Inpatients. J. Gen. Intern. Med. 1993, 8, 354–360. [Google Scholar] [CrossRef]
- Browning, I.B.; D’Alonzo, G.E.; Tobin, M.J. Importance of Respiratory Rate as an Indicator of Respiratory Dysfunction in Patients with Cystic Fibrosis. Chest 1990, 97, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Gravelyn, T.R.; Weg, J.G. Respiratory Rate as an Indicator of Acute Respiratory Dysfunction. JAMA 1980, 244, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.P.; Price, R.C.; Eastwood, H.D.; Briggs, R.S. Raised Respiratory Rate in Elderly Patients: A Valuable Physical Sign. Br. Med. J. 1982, 284, 626–627. [Google Scholar] [CrossRef] [Green Version]
- Villar, R.; Beltrame, T.; Hughson, R.L. Validation of the Hexoskin wearable vest during lying, sitting, standing, and walking activities. Appl. Physiol. Nutr. Metab. 2015, 40, 1019–1124. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, A.; Vignati, C.; Paolillo, S.; Lombardi, C.; Scoccia, A.; Nicoli, F.; Mapelli, M.; Leonardi, A.; Ossola, D.; Rigoni, R.; et al. Qualitative and quantitative evaluation of a new wearable device for ECG and respiratory Holter monitoring. Int. J. Cardiol. 2018, 272, 231–237. [Google Scholar] [CrossRef]
- Antonelli, A.; Guilizzoni, D.; Angelucci, A.; Melloni, G.; Mazza, F.; Stanzi, A.; Venturino, M.; Kuller, D.; Aliverti, A. Comparison between the Airgo™ Device and a Metabolic Cart during Rest and Exercise. Sensors 2020, 20, 3943. [Google Scholar] [CrossRef]
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Radom-Aizik, S.; Jain, R.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Talaminos-Barroso, A.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rosta, G.; Cejudo-Ramos, P.; Márquez-Martín, E.; Ortega-Ruiz, F. Smart Vest for Respiratory Rate Monitoring of COPD Patients Based on Non-Contact Capacitive Sensing. Sensors 2018, 18, 2144. [Google Scholar] [CrossRef] [Green Version]
- Massaroni, C.; Venanzi, C.; Silvatti, A.P.; Lo Presti, D.; Saccomandi, P.; Formica, D.; Giurazza, F.; Caponero, M.A.; Schena, E. Smart textile for respiratory monitoring andthoraco-abdominal motion pattern evaluation. J. Biophotonics 2018, 11, e201700263. [Google Scholar] [CrossRef] [Green Version]
- Hung, P.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Yen, P.T.N. Estimation of Respiratory Waveform using an Accelerometer. In Proceedings of the 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 14–17 May 2008; pp. 1493–1496. [Google Scholar]
- Jin, A.; Yin, B.; Morren, G.; Duric, H.; Aarts, R.M. Performance Evaluation of a Tri-Axial Accelerometry-Based Respiration Monitoring for Ambient Assisted Living. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 5677–5680. [Google Scholar]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D. Respiratory Rate and Flow Waveform Estimation from Tri-Axial Accelerometer Data. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Liu, G.; Guo, Y.; Zhu, Q.; Huang, B.; Wang, L. Estimation of Respiration Rate from Three-Dimensional Acceleration Data Based on Body Sensor Network. Telemed. E-Health 2011, 17, 705–711. [Google Scholar] [CrossRef]
- Mann, J.; Rabinovich, R.; Bates, A.; Giavedoni, S.; MacNee, W.; Arvind, D. Simultaneous Activity and Respiratory Monitoring using an Accelerometer. In Proceedings of the 2011 International Conference on Body Sensor Networks, Dallas, TX, USA, 23–25 May 2011; pp. 139–143. [Google Scholar]
- Fekr, A.R.; Janidarmian, M.; Radecka, K.; Zilic, Z. A Medical Cloud-Based Platform for Respiration Rate Measurement and Hierarchical Classification of Breath Disorders. Sensors 2014, 14, 11204–11224. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, A.; Gandolfi, S.; Pini, I.; Biffi, E.; Reni, G.; Aliverti, A. A Novel, Low Cost, Wearable Contact-Based Device for Breathing Frequency Monitoring. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 2402–2405. [Google Scholar]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of Breathing Parameters using an Inertial Measurement Unit (IMU)-Based System. Sensors 2019, 19, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesareo, A.; Biffi, E.; Cuesta-Frau, D.; D’Angelo, M.G.; Aliverti, A. A Novel Acquisition Platform for Long-Term Breathing Frequency Monitoring Based on Inertial Measurement Units. Med. Biol. Eng. Comput. 2020, 58, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 2: Respiratory, Cardiac, Bone Health, and Orthopaedic Management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Norwood, F.; De Visser, M.; Eymard, B.; Lochmüller, H.; Bushby, K. EFNS Guideline on Diagnosis and Management of Limb Girdle Muscular Dystrophies. Eur. J. Neurol. 2007, 14, 1305–1312. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. General Considerations for Lung Function Testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- Aliverti, A.; Dellacà, R.; Pelosi, P.; Chiumello, D.; Gattinoni, L.; Pedotti, A. Compartmental Analysis of Breathing in the Supine and Prone Positions by Optoelectronic Plethysmography. Ann. Biomed. Eng. 2001, 29, 60–70. [Google Scholar] [CrossRef]
- Aliverti, A.; Dellaca, R.; Pelosi, P.; Chiumello, D.; Pedotti, A.; Gattinoni, L. Optoelectronic Plethysmography in Intensive Care Patients. Am. J. Respir. Crit. Care Med. 2000, 161, 1546–1552. [Google Scholar] [CrossRef]
- Cala, S.; Kenyon, C.; Ferrigno, G.; Carnevali, P.; Aliverti, A.; Pedotti, A.; Macklem, P.; Rochester, D. Chest Wall and Lung Volume Estimation by Optical Reflectance Motion Analysis. J. Appl. Physiol. 1996, 81, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Cala, S.; Yan, S.; Aliverti, A.; Scano, G.; Duranti, R.; Pedotti, A.; Macklem, P.T. Rib Cage Mechanics during Quiet Breathing and Exercise in Humans. J. Appl. Physiol. 1997, 83, 1242–1255. [Google Scholar] [CrossRef] [Green Version]
- Vieira, D.S.; Hoffman, M.; Pereira, D.A.; Britto, R.R.; Parreira, V.F. Optoelectronic Plethysmography: Intra-Rater and Inter-Rater Reliability in Healthy Subjects. Respir. Physiol. Neurobiol. 2013, 189, 473–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layton, A.M.; Moran, S.L.; Garber, C.E.; Armstrong, H.F.; Basner, R.C.; Thomashow, B.M.; Bartels, M.N. Optoelectronic Plethysmography Compared to Spirometry during Maximal Exercise. Respir. Physiol. Neurobiol. 2013, 185, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Iandelli, I.; Aliverti, A.; Kayser, B.; Dellacà, R.; Cala, S.J.; Duranti, R.; Kelly, S.; Scano, G.; Sliwinski, P.; Yan, S. Determinants of Exercise Performance in Normal Men with Externally Imposed Expiratory Flow Limitation. J. Appl. Physiol. 2002, 92, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Lo Mauro, A.; D’Angelo, M.G.; Romei, M.; Motta, F.; Colombo, D.; Comi, G.P.; Pedotti, A.; Marchi, E.; Turconi, A.C.; Bresolin, N.; et al. Abdominal Volume Contribution to Tidal Volume as an Early Indicator of Respiratory Impairment in Duchenne Muscular Dystrophy. Eur. Respir. J. 2010, 35, 1118–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesareo, A.; LoMauro, A.; Santi, M.; Biffi, E.; D’Angelo, M.G.; Aliverti, A. Acute Effects of Mechanical Insufflation-Exsufflation on the Breathing Pattern in Stable Subjects with Duchenne Muscular Dystrophy. Respir. Care 2018, 63, 955–965. [Google Scholar] [CrossRef] [Green Version]
- LoMauro, A.; Cesareo, A.; Agosti, F.; Tringali, G.; Salvadego, D.; Grassi, B.; Sartorio, A.; Aliverti, A. Effects of a Multidisciplinary Body Weight Reduction Program on Static and Dynamic Thoraco-Abdominal Volumes in Obese Adolescents. Appl. Physiol. Nutr. Metab. 2016, 41, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Brooke, J. SUS-A Quick and Dirty Usability Scale. Usability Eval. Ind. 1996, 189, 4–7. [Google Scholar]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An Empirical Evaluation of the System Usability Scale. Int. J. Hum. Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Lewis, J.R.; Sauro, J. The Factor Structure of the System Usability Scale. In Proceedings of the International Conference on Human Centered Design, San Diego, CA, USA, 19–24 July 2009; pp. 94–103. [Google Scholar]
- Measuring Usability with the System Usability Scale. Available online: https://www.userfocus.co.uk/articles/measuring-usability-with-the-SUS.html (accessed on 17 September 2020).
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring Agreement in Method Comparison Studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.; Scholtes, V.A.; Dallmeijer, A.J.; Twisk, J.W.; Harlaar, J. The Importance of Addressing Heteroscedasticity in the Reliability Analysis of ratio-scaled Variables: An Example Based on Walking energy-cost Measurements. Dev. Med. Child Neurol. 2012, 54, 267–273. [Google Scholar] [CrossRef] [PubMed]
- How Do I Estimate Limits of Agreement When the Mean or SD of Differences Is not Constant. Available online: https://www-users.york.ac.uk/~mb55/meas/glucose.htm (accessed on 10 December 2009).
- Ludbrook, J. Confidence in Altman–Bland Plots: A Critical Review of the Method of Differences. Clin. Exp. Pharmacol. Physiol. 2010, 37, 143–149. [Google Scholar] [CrossRef]
- Morillo, D.S.; Ojeda, J.L.R.; Foix, L.F.C.; Jiménez, A.L. An Accelerometer-Based Device for Sleep Apnea Screening. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 491–499. [Google Scholar] [CrossRef]
- Lapi, S.; Lavorini, F.; Borgioli, G.; Calzolai, M.; Masotti, L.; Pistolesi, M.; Fontana, G.A. Respiratory Rate Assessments using a Dual-Accelerometer Device. Respir. Physiol. Neurobiol. 2014, 191, 60–66. [Google Scholar] [CrossRef]




| Parameter | DMD (n = 13) | LGMD (n = 2) | |
|---|---|---|---|
| Age (years) | 23.99 ± 5.94 | 53 ± 0.04 | |
| Weight (kg) | 54.77 ± 14.96 | 86 ± 45.96 | |
| Height (cm) | 166 ± 10 | 180 ± 7 | |
| BMI (km∗m−2) | 19.77 ± 4.64 | 26.41 ± 11.41 | |
| Gene mutation (N) | Deletion | 9 | - |
| Duplication | 1 | - | |
| Point mutation | 3 | - | |
| Scoliosis (N) | No | 0 | 1 |
| Mild | 1 | 1 | |
| Moderate | 2 | 0 | |
| Severe | 7 | 0 | |
| Spinal fusion | 2 | 0 | |
| NIV (N) | 10 | 1 | |
| Heart dysfunction (N) | 12 | 1 | |
| Use of M-IE (N) | 11 | 0 |
| Parameter | DMD (n = 13) | LGMD (n = 2) | |
|---|---|---|---|
| Spirometry | FVC [L] | 1.18 ± 0.90 | 3.45 ± 0.99 |
| FVC (% pred) | 28.44 ± 27.34 | 78.00 ± 33.94 | |
| FEV1 [L] | 1.10 ± 0.87 | 2.65 ± 0.85 | |
| FEV1 (% pred) | 31.44 ± 31.87 | 75 ± 33.94 | |
| FEF25–75% [L/sec] | 1.68 ± 1.37 | 2.32 ± 1.12 | |
| FEF 25–75% (% pred) | 35.11 ± 29.19 | 61.00 ± 32.53 | |
| FEF50 [L/sec] | 2.07 ± 1.63 | 3.71 ± 1.14 | |
| FEF50 (% pred) | 43.67 ± 39.70 | 79.00 ± 31.11 | |
| PEF [L/sec] | 2.75 ± 1.72 | 6.33 ± 0.46 | |
| PEF (% pred) | 33.44 ± 27.74 | 72.00 ± 11.31 | |
| Lung volumes | TLC [L] | 4.52 ± 1.42 | 6.41 ± 0.36 |
| TLC (% pred) | 77.40 ± 25.91 | 90 ± 7.07 | |
| RV [L] | 2.80 ± 1.10 | 2.96 ± 1.35 | |
| RV (% pred) | 189.80 ± 52.45 | 128.5 ± 50.20 | |
| FRC [L] | 3.54 ± 1.22 | 4.26 ± 0.35 | |
| FRC (% pred) | 120.63 ± 36.55 | 119.5 ± 0.71 |
| Parameter | Position | Compartment | E | E% |
|---|---|---|---|---|
| fB | Supine | thorax | 0.79 (0.55; 1.11) | 4.53 (2.86; 6.17) |
| abdomen | 1.08 (0.53; 1.56) | 3.53 (1.61; 8.29) | ||
| Seated | thorax | 1.09 (0.73; 0.73) | 4.99 (3.11; 0.48) | |
| abdomen | 1.02 (0.60; 1.50) | 5.31 (3.25; 7.06) | ||
| TI | Supine | thorax | 0.24 (0.37; 0.13) | 16.82 (25.43; 9.03) |
| abdomen | 0.279 (0.42; 0.14) | 20.67 (30.15; 9.61) | ||
| Seated | thorax | 0.149 (0.36; 0.08) | 10.79 (24.48; 6.46) | |
| abdomen | 0.349 (0.50; 0.10) | 24.29 (40.30; 7.96) | ||
| TE | Supine | thorax | 0.19 (0.32;0.09) | 11.24 (15.10; 5.15) |
| abdomen | 0.20 (0.20;0.19) | 11.13 (22.84; 6.90) | ||
| Seated | thorax | 0.11 (0.26;0.04) | 6.89 (15.60; 2.85) | |
| abdomen | 0.19 (0.34; 0.13) | 13.21 (22.01; 8.96) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesareo, A.; Nido, S.A.; Biffi, E.; Gandossini, S.; D’Angelo, M.G.; Aliverti, A. A Wearable Device for Breathing Frequency Monitoring: A Pilot Study on Patients with Muscular Dystrophy. Sensors 2020, 20, 5346. https://doi.org/10.3390/s20185346
Cesareo A, Nido SA, Biffi E, Gandossini S, D’Angelo MG, Aliverti A. A Wearable Device for Breathing Frequency Monitoring: A Pilot Study on Patients with Muscular Dystrophy. Sensors. 2020; 20(18):5346. https://doi.org/10.3390/s20185346
Chicago/Turabian StyleCesareo, Ambra, Santa Aurelia Nido, Emilia Biffi, Sandra Gandossini, Maria Grazia D’Angelo, and Andrea Aliverti. 2020. "A Wearable Device for Breathing Frequency Monitoring: A Pilot Study on Patients with Muscular Dystrophy" Sensors 20, no. 18: 5346. https://doi.org/10.3390/s20185346
APA StyleCesareo, A., Nido, S. A., Biffi, E., Gandossini, S., D’Angelo, M. G., & Aliverti, A. (2020). A Wearable Device for Breathing Frequency Monitoring: A Pilot Study on Patients with Muscular Dystrophy. Sensors, 20(18), 5346. https://doi.org/10.3390/s20185346








