Evaluation of a Low-Cost Commercial Actigraph and Its Potential Use in Detecting Cultural Variations in Physical Activity and Sleep
Abstract
:1. Introduction
2. Study 1: Evaluation of Mi Band 3 Performance in Tracking Sleep and Physical Activity
2.1. Methods and Materials
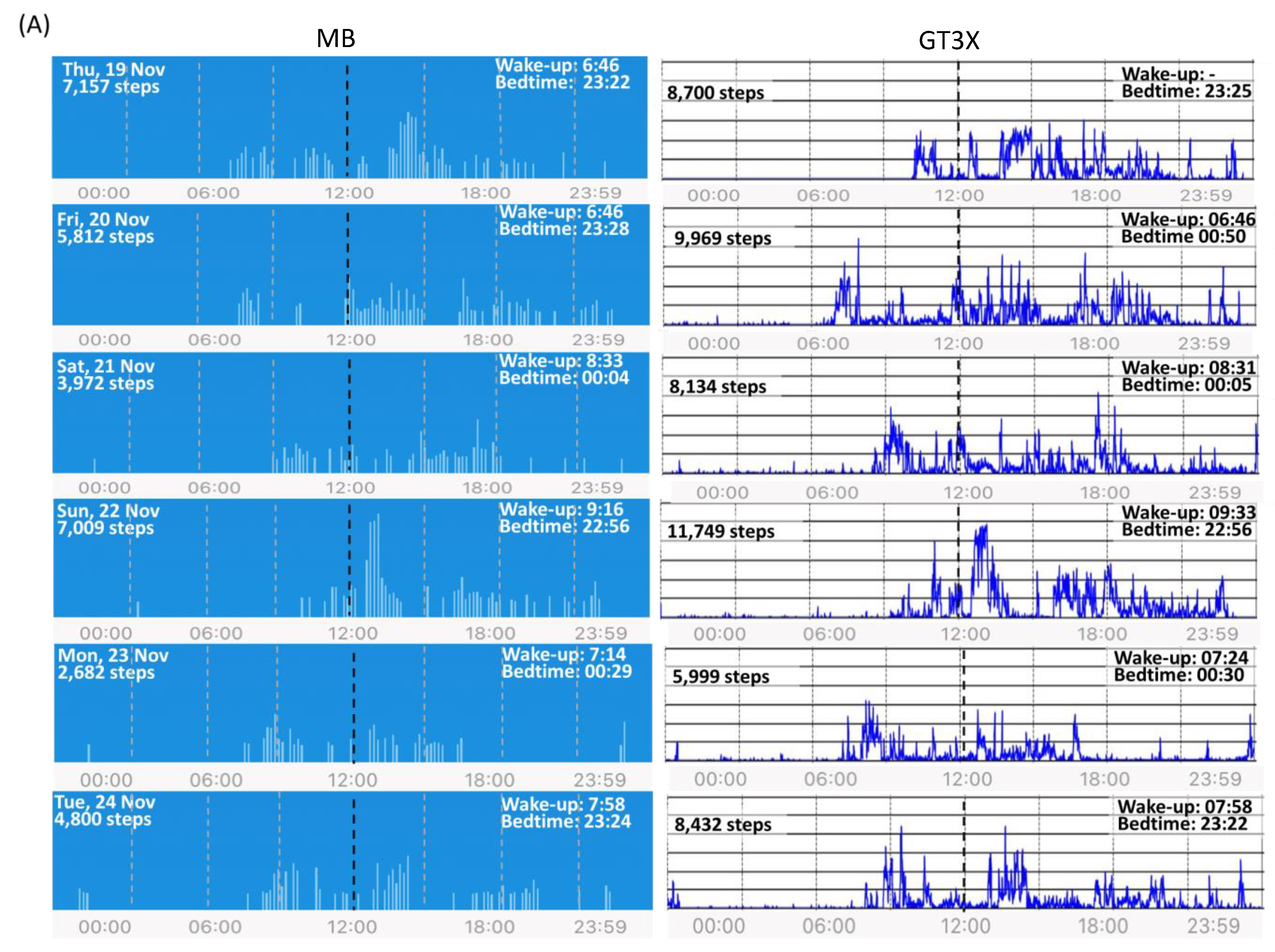
2.2. Results
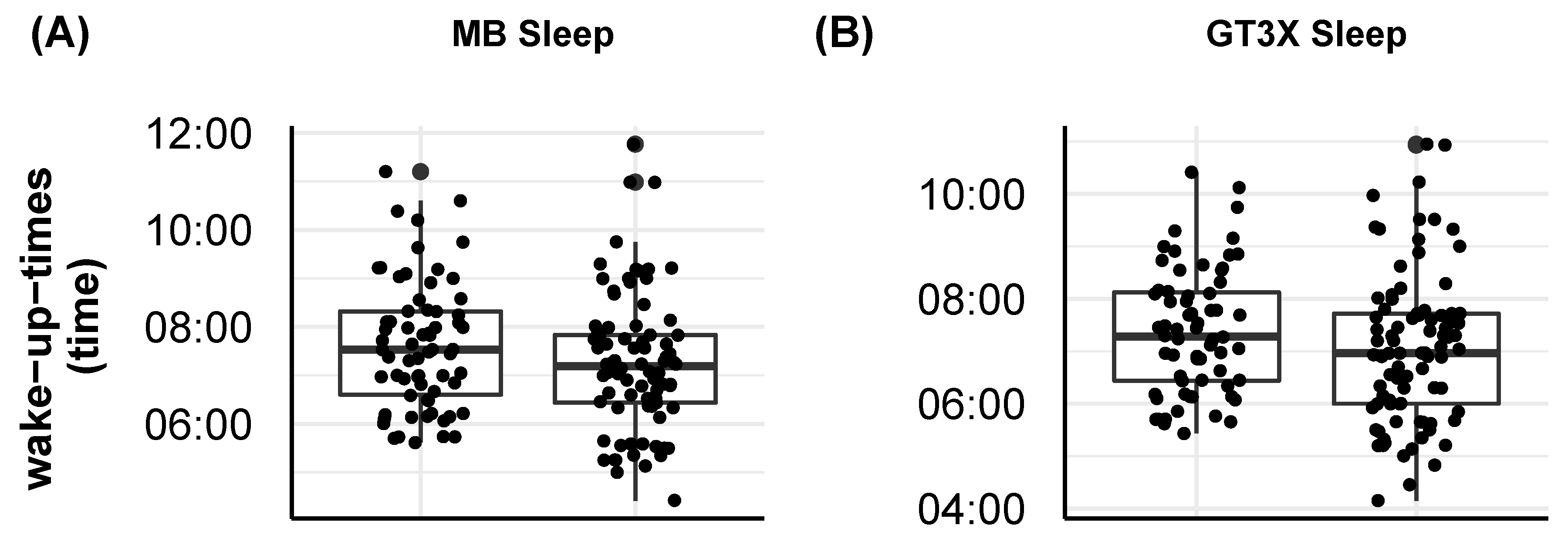
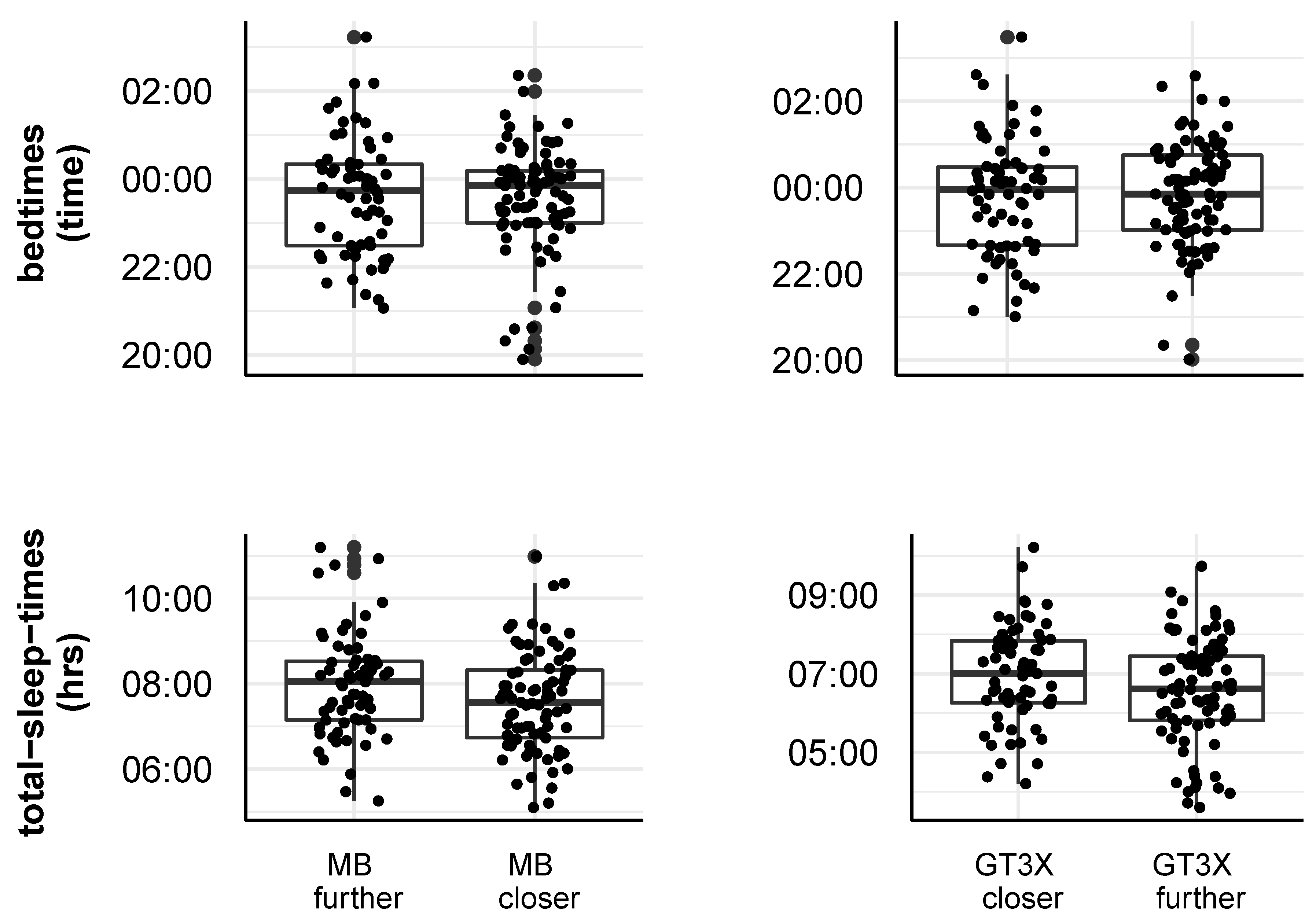
2.2.1. Impact of Actigraph Wrist Position on Step Count and Sleep Measures
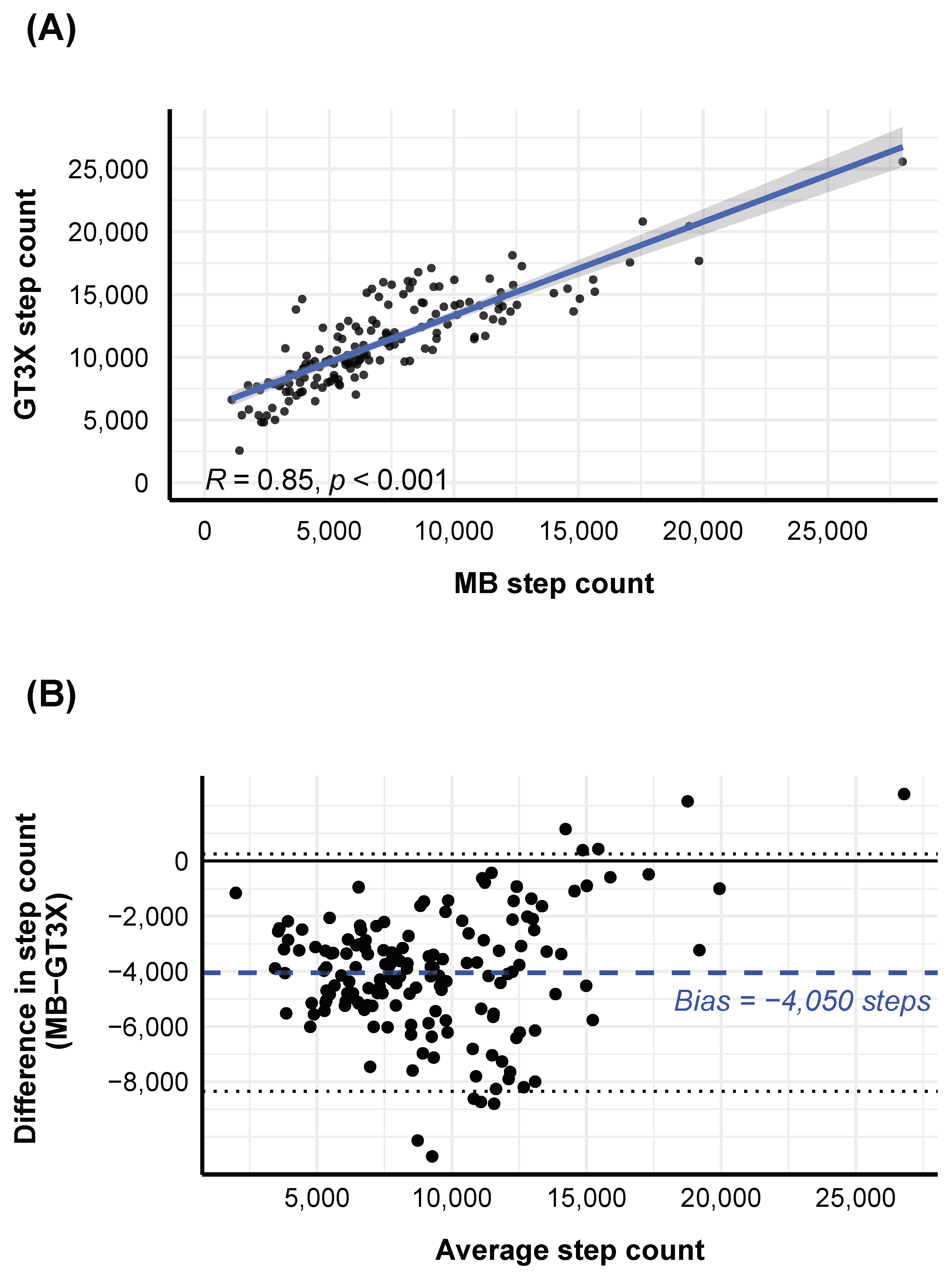
2.2.2. Agreement between MB and GT3X Step Counts
2.2.3. Agreement between Subjective and Actigraphy-Based Sleep Measures
3. Study 2: Cultural Variations in Objective Physical Activity and Sleep
3.1. Methods and Materials
3.2. Results
3.2.1. Subjective Physical Activity and Objective Step Count Differences across Countries
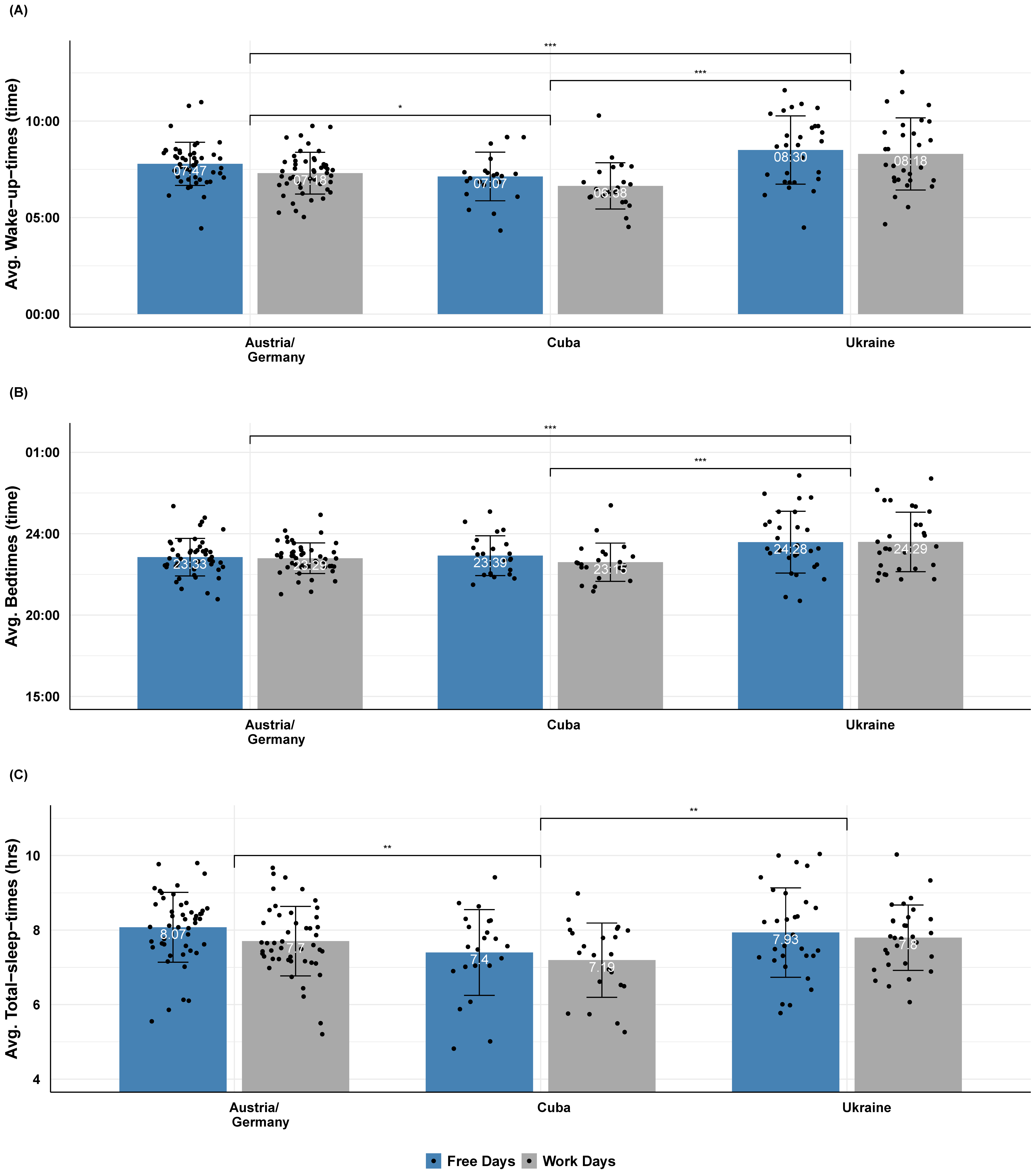
3.2.2. Subjective and Objective Sleep Measure Differences across Countries
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MB | Xiaomi Mi Band |
| GT3X | wGT3X-BT ActiGraph |
Appendix A

References
- IDC. Forecast Unit Shipments of Wrist-Worn Wearables Worldwide from 2019 to 2024 (in Millions). Available online: https://www.statista.com/statistics/296565/wearables-worldwide-shipments/ (accessed on 26 February 2021).
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1–9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Bauman, A.; Phongsavan, P.; Schoeppe, S.; Owen, N. Physical activity measurement-a primer for health promotion. Promot. Educ. 2006, 13, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.; Webster, J.G.; Montoye, H.J.; Washburn, R. Portable accelerometer device for measuring human energy expenditure. IEEE Trans. Biomed. Eng. 1981, 28, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Servais, S.B.; Webster, J.G. Estimating human energy expenditure using an accelerometer device. J. Clin. Eng. 1984, 9, 159–170. [Google Scholar] [CrossRef]
- Ward, D.S.; Evenson, K.R.; Vaughn, A.; Rodgers, A.B.; Troiano, R.P. Accelerometer use in physical activity: Best practices and research recommendations. Med. Sci. Sport. Exerc. 2005, 37, S582–S588. [Google Scholar] [CrossRef] [PubMed]
- Strath, S.J.; Kaminsky, L.A.; Ainsworth, B.E.; Ekelund, U.; Freedson, P.S.; Gary, R.A.; Richardson, C.R.; Smith, D.T.; Swartz, A.M. Guide to the assessment of physical activity: Clinical and research applications: A scientific statement from the American Heart Association. Circulation 2013, 128, 2259–2279. [Google Scholar] [CrossRef]
- Silfee, V.J.; Haughton, C.F.; Jake-Schoffman, D.E.; Lopez-Cepero, A.; May, C.N.; Sreedhara, M.; Rosal, M.C.; Lemon, S.C. Objective measurement of physical activity outcomes in lifestyle interventions among adults: A systematic review. Prev. Med. Rep. 2018, 11, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.; Hale, L. Longitudinal associations between sleep duration and subsequent weight gain: A systematic review. Sleep Med. Rev. 2012, 16, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Guallar-Castillón, P.; Bayán-Bravo, A.; León-Muñoz, L.M.; Balboa-Castillo, T.; López-García, E.; Gutierrez-Fisac, J.L.; Rodríguez-Artalejo, F. The association of major patterns of physical activity, sedentary behavior and sleep with health-related quality of life: A cohort study. Prev. Med. 2014, 67, 248–254. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [Green Version]
- de Zambotti, M.; Claudatos, S.; Inkelis, S.; Colrain, I.M.; Baker, F.C. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol. Int. 2015, 32, 1024–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantua, J.; Gravel, N.; Spencer, R.M.C. Reliability of Sleep Measures from Four Personal Health Monitoring Devices Compared to Research-Based Actigraphy and Polysomnography. Sensors 2016, 16, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüdtke, S.; Hermann, W.; Kirste, T.; Beneš, H.; Teipel, S. An algorithm for actigraphy-based sleep/wake scoring: Comparison with polysomnography. Clin. Neurophysiol. 2021, 132, 137–145. [Google Scholar] [CrossRef]
- Stone, J.D.; Rentz, L.E.; Forsey, J.; Ramadan, J.; Markwald, R.R.; Finomore, V.S.; Galster, S.M.; Rezai, A.; Hagen, J.A. Evaluations of Commercial Sleep Technologies for Objective Monitoring During Routine Sleeping Conditions. Nat. Sci. Sleep 2020, 12, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.K.; Chee, N.I.Y.N.; Ong, J.L.; Teo, T.B.; van Rijn, E.; Lo, J.C.; Chee, M.W.L. Validation of a Consumer Sleep Wearable Device With Actigraphy and Polysomnography in Adolescents Across Sleep Opportunity Manipulations. J. Clin. Sleep Med. 2019, 15, 1337–1346. [Google Scholar] [CrossRef]
- Hickey, A.M.; Freedson, P.S. Utility of consumer physical activity trackers as an intervention tool in cardiovascular disease prevention and treatment. Prog. Cardiovasc. Dis. 2016, 58, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Centurion, V.; Bracken, N.; Norwick, L.; Zaidi, F.; Mutso, A.A.; Morken, V.; Coultas, D.B.; Rand, C.S.; Marquez, D.X.; Krishnan, J.A. Can commercially available pedometers be used for physical activity monitoring in patients with COPD following exacerbations? Chronic Obstr. Pulm. Dis. J. Copd Found. 2016, 3, 636. [Google Scholar] [CrossRef]
- Wahl, Y.; Düking, P.; Droszez, A.; Wahl, P.; Mester, J. Criterion-Validity of Commercially Available Physical Activity Tracker to Estimate Step Count, Covered Distance and Energy Expenditure during Sports Conditions. Front. Physiol. 2017, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wen, D.; Liang, L.; Jia, Y.; Gao, L.; Lei, J. Evaluating the Validity of Current Mainstream Wearable Devices in Fitness Tracking Under Various Physical Activities: Comparative Study. JMIR mHealth uHealth 2018, 6, e94. [Google Scholar] [CrossRef] [Green Version]
- Degroote, L.; Hamerlinck, G.; Poels, K.; Maher, C.; Crombez, G.; de Bourdeaudhuij, I.; Vandendriessche, A.; Curtis, R.G.; DeSmet, A. Low-Cost Consumer-Based Trackers to Measure Physical Activity and Sleep Duration Among Adults in Free-Living Conditions: Validation Study. JMIR mHealth uHealth 2020, 8, e16674. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Stone, M.R.; Eston, R.G. Influence of speed and step frequency during walking and running on motion sensor output. Med. Sci. Sport. Exerc. 2007, 39, 716–727. [Google Scholar] [CrossRef]
- Le Masurier, G.C.; Lee, S.M.; Tudor-Locke, C. Motion sensor accuracy under controlled and free-living conditions. Med. Sci. Sport. Exerc. 2004, 36, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Le Masurier, G.C.; Tudor-Locke, C. Comparison of pedometer and accelerometer accuracy under controlled conditions. Med. Sci. Sport. Exerc. 2003, 35, 867–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.; Swan, P.; Chow, C.M. The validity of Actiwatch2 and SenseWear armband compared against polysomnography at different ambient temperature conditions. Sleep Sci. 2015, 8, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartung, V.; Sarshar, M.; Karle, V.; Shammas, L.; Rashid, A.; Roullier, P.; Eilers, C.; Mäurer, M.; Flachenecker, P.; Pfeifer, K.; et al. Validity of Consumer Activity Monitors and an Algorithm Using Smartphone Data for Measuring Steps during Different Activity Types. Int. J. Environ. Res. Public Health 2020, 17, 9314. [Google Scholar] [CrossRef]
- Ameen, M.S.; Cheung, L.M.; Hauser, T.; Hahn, M.A.; Schabus, M. About the Accuracy and Problems of Consumer Devices in the Assessment of Sleep. Sensors 2019, 19, 4160. [Google Scholar] [CrossRef] [Green Version]
- Worthman, C.M. Sleep in different cultures. Front. Neurosci 2009, 3, 1–2. [Google Scholar]
- Henrich, J.; Heine, S.J.; Norenzayan, A. The weirdest people in the world? Behav. Brain Sci. 2010, 33, 61–83. [Google Scholar] [CrossRef]
- Yetish, G.; Kaplan, H.; Gurven, M.; Wood, B.; Pontzer, H.; Manger, P.R.; Wilson, C.; McGregor, R.; Siegel, J.M. Natural sleep and its seasonal variations in three pre-industrial societies. Curr. Biol. 2015, 25, 2862–2868. [Google Scholar] [CrossRef] [Green Version]
- de La Iglesia, H.O.; Fernández-Duque, E.; Golombek, D.A.; Lanza, N.; Duffy, J.F.; Czeisler, C.A.; Valeggia, C.R. Access to Electric Light Is Associated with Shorter Sleep Duration in a Traditionally Hunter-Gatherer Community. J. Biol. Rhythm. 2015, 30, 342–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.A.; Jackson, C.L.; Williams, N.J.; Alcántara, C. Are sleep patterns influenced by race/ethnicity—A marker of relative advantage or disadvantage? Evidence to date. Nat. Sci. Sleep 2019, 11, 79–95. [Google Scholar] [CrossRef] [Green Version]
- Petrov, M.E.; Lichstein, K.L. Differences in sleep between black and white adults: An update and future directions. Sleep Med. 2016, 18, 74–81. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Allaert, F.A.; Ohta, T.; Dikeos, D.G. How do individuals sleep around the world? Results from a single-day survey in ten countries. Sleep Med. 2005, 6, 5–13. [Google Scholar] [CrossRef]
- Florea, C.; Topalidis, P.; Hauser, T.; Angerer, M.; Kurapov, A.; Beltran Leon, C.A.; Soares Brandão, D.; Schabus, M. Sleep during COVID-19 lockdown: A cross-cultural study investigating job system relevance. Biochem. Pharmacol. 2021, 114463. [Google Scholar] [CrossRef]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Fuller, K.L.; Juliff, L.; Gore, C.J.; Peiffer, J.J.; Halson, S.L. Software thresholds alter the bias of actigraphy for monitoring sleep in team-sport athletes. J. Sci. Med. Sport 2017, 20, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Buonani, C.; Rosa, C.S.d.C.; Diniz, T.A.; Christofaro, D.G.D.; Monteiro, H.L.; Rossi, F.E.; Freitas Júnior, I.F. Prática de atividade física e composição corporal em mulheres na menopausa. Rev. Bras. Ginecol. Obs. 2013, 35, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, R.J.; Kripke, D.F.; Gruen, W.; Mullaney, D.J.; Gillin, J.C. Automatic sleep/wake identification from wrist activity. Sleep 1992, 15, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.; Filon, L.N.G. Mathematical contributions to the theory of evolution. IV. On the probable errors of frequency constants and on the influence of random selection on variation and correlation. Proc. R. Soc. Lond. 1898, 62, 173–176. [Google Scholar]
- Diedenhofen, B.; Musch, J. cocor: A comprehensive solution for the statistical comparison of correlations. PLoS ONE 2015, 10, e0121945. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. J. R. Stat. Soc. Ser. D 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding bland altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Myles, P.S.; Cui, J. Using the Bland–Altman method to measure agreement with repeated measures. Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, D. Blandr: A Bland–Altman Method Comparison Package for R. 2017. Available online: https://cran.r-project.org/web/packages/blandr/vignettes/introduction.html (accessed on 26 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Limesurvey. LimeSurvey: The Open Source Survey Application. 2011. Available online: https://www.limesurvey.org/ (accessed on 26 May 2021).
- Pilz, L.K.; Keller, L.K.; Lenssen, D.; Roenneberg, T. Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep 2018, 41, zsy029. [Google Scholar] [CrossRef] [PubMed]
- Höchsmann, C.; Knaier, R.; Infanger, D.; Schmidt-Trucksäss, A. Validity of smartphones and activity trackers to measure steps in a free-living setting over three consecutive days. Physiol. Meas. 2020, 41, 015001. [Google Scholar] [CrossRef]
- Kubala, A.G.; Barone Gibbs, B.; Buysse, D.J.; Patel, S.R.; Hall, M.H.; Kline, C.E. Field-based Measurement of Sleep: Agreement between Six Commercial Activity Monitors and a Validated Accelerometer. Behav. Sleep Med. 2020, 18, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Griessenberger, H.; Heib, D.P.J.; Kunz, A.; Hödlmoser, K.; Schabus, M. Assessment of a wireless headband for automatic sleep scoring. Sleep Breath. 2013, 17, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkley, J.E.; Lepp, A.; Glickman, E.; Farnell, G.; Beiting, J.; Wiet, R.; Dowdell, B. The acute effects of the COVID-19 pandemic on physical activity and sedentary behavior in university students and employees. Int. J. Exerc. Sci. 2020, 13, 1326. [Google Scholar]
- Chaudhry, F.F.; Danieletto, M.; Golden, E.; Scelza, J.; Botwin, G.; Shervey, M.; de Freitas, J.K.; Paranjpe, I.; Nadkarni, G.N.; Miotto, R.; et al. Sleep in the Natural Environment: A Pilot Study. Sensors 2020, 20, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Country | N | Males | Femeales | Age Mean | Age AD |
|---|---|---|---|---|---|
 | 50 | 20 | 30 | 32.8 | 8.66 |
 | 20 | 10 | 10 | 41.1 | 8.41 |
 | 29 | 9 | 20 | 35.34 | 10.28 |
 | 99 | 39 | 60 | 35.34 | 8.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topalidis, P.; Florea, C.; Eigl, E.-S.; Kurapov, A.; Leon, C.A.B.; Schabus, M. Evaluation of a Low-Cost Commercial Actigraph and Its Potential Use in Detecting Cultural Variations in Physical Activity and Sleep. Sensors 2021, 21, 3774. https://doi.org/10.3390/s21113774
Topalidis P, Florea C, Eigl E-S, Kurapov A, Leon CAB, Schabus M. Evaluation of a Low-Cost Commercial Actigraph and Its Potential Use in Detecting Cultural Variations in Physical Activity and Sleep. Sensors. 2021; 21(11):3774. https://doi.org/10.3390/s21113774
Chicago/Turabian StyleTopalidis, Pavlos, Cristina Florea, Esther-Sevil Eigl, Anton Kurapov, Carlos Alberto Beltran Leon, and Manuel Schabus. 2021. "Evaluation of a Low-Cost Commercial Actigraph and Its Potential Use in Detecting Cultural Variations in Physical Activity and Sleep" Sensors 21, no. 11: 3774. https://doi.org/10.3390/s21113774
APA StyleTopalidis, P., Florea, C., Eigl, E.-S., Kurapov, A., Leon, C. A. B., & Schabus, M. (2021). Evaluation of a Low-Cost Commercial Actigraph and Its Potential Use in Detecting Cultural Variations in Physical Activity and Sleep. Sensors, 21(11), 3774. https://doi.org/10.3390/s21113774








