Validation of a New Contactless and Continuous Respiratory Rate Monitoring Device Based on Ultra-Wideband Radar Technology
Abstract
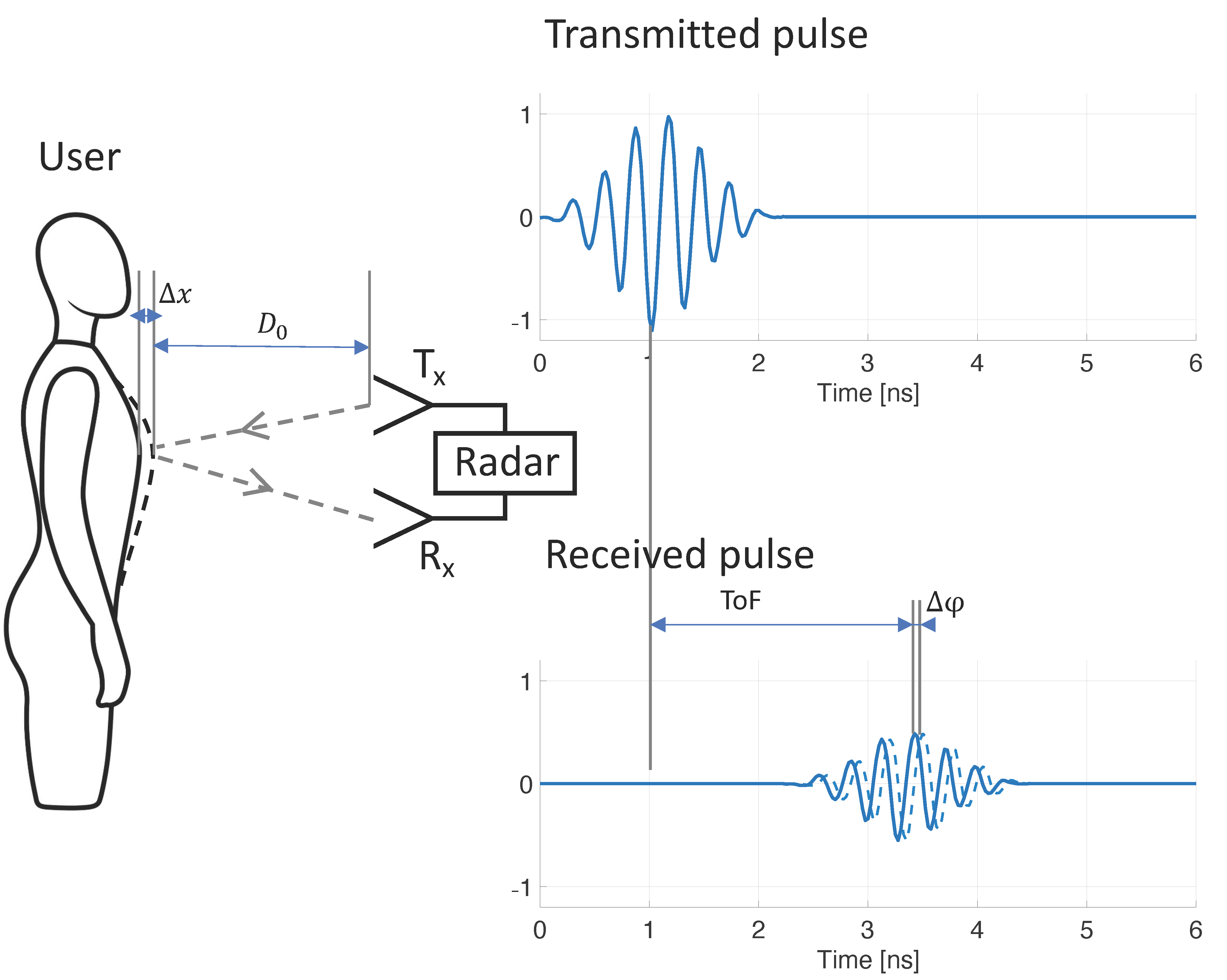
1. Introduction
2. Materials and Methods
2.1. Study Population and Procedures
2.1.1. Study 1
- Seated, at 0.5 m distance D0;
- Seated, at 1.0 m distance D0;
- Seated, at 1.0 m distance D0, subject covered by blanket;
- Seated, at 1.5 m distance D0;
- Lying down in supine position, at 0.5 m distance D0;
- Lying down in supine position, at 0.5 m distance D0, subject covered by blanket;
- Lying down on the side, facing towards the device, at 0.5 m distance D0;
- Lying down on the side, facing away from the device, at 0.5 m distance D0.A 9 min, continuous recording was obtained for the following condition:
- Lying down in supine position, at 0.5 m distance D0.Thoracic effort, abdominal effort, nasal pressure, and nasal flow were obtained using the Natus Embletta MPR PG (Pleasanton, CA, USA) polygraphy device.
2.1.2. Study 2
2.1.3. Study 3
2.2. Manual Scoring of Reference Data
2.3. Statistical Analysis
3. Results
3.1. Overall Analysis of Agreement
3.2. Analysis of Agreement per Condition
3.3. Respiratory Waveform Correspondence
4. Discussion
4.1. Methodology and Sources of Error
4.2. Study Samples and Generalizability
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.P.; Price, R.C.; Eastwood, H.D.; Briggs, R.S. Raised respiratory rate in elderly patients: A valuable physical sign. Br. Med. J. Clin. Res. Ed. 1982, 284, 626–627. [Google Scholar] [CrossRef]
- Fieselmann, J.F.; Hendryx, M.S.; Helms, C.M.; Wakefield, D.S. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J. Gen. Intern. Med. 1993, 8, 354–360. [Google Scholar] [CrossRef]
- Yañez, A.M.; Guerrero, D.; De Alejo, R.P.; Garcia-Rio, F.; Alvarez-Sala, J.L.; Calle-Rubio, M.; De Molina, R.M.; Falcones, M.V.; Ussetti, P.; Sauleda, J.; et al. Monitoring Breathing Rate at Home Allows Early Identification of COPD Exacerbations. Chest 2012, 142, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, L.G.; Page, C.P.; Celli, B.R.; Cazzola, M.; Walker, M.J.; Danhof, M.; Rabe, K.F.; E Della Pasqua, O. Markers of exacerbation severity in chronic obstructive pulmonary disease. Respir. Res. 2006, 7, 74. [Google Scholar] [CrossRef]
- Churpek, M.; Yuen, T.C.; Huber, M.T.; Park, S.Y.; Hall, J.B.; Edelson, D.P. Predicting Cardiac Arrest on the Wards: A Nested Case-Control Study. Chest 2012, 141, 1170–1176. [Google Scholar] [CrossRef]
- Rosenberg, A.L.; Watts, C. Patients readmitted to ICUs: A systematic review of risk factors and outcomes. Chest 2000, 118, 492–502. [Google Scholar] [CrossRef]
- Goldhill, D.R.; White, S.A.; Sumner, A. Physiological values and procedures in the 24 h before ICU admission from the ward. Anaesthesia 1999, 54, 529–534. [Google Scholar] [CrossRef]
- Goldhill, D.R.; McNarry, A.F.; Mandersloot, G.; McGinley, A. A physiologically-based early warning score for ward patients: The association between score and outcome. Anaesthesia 2005, 60, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.B.; Fischer, D.; Arvind, D. Current clinical methods of measurement of respiratory rate give imprecise values. ERJ Open Res. 2020, 6, 00023-2020. [Google Scholar] [CrossRef] [PubMed]
- A Cretikos, M.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory rate: The neglected vital sign. Med. J. Aust. 2008, 188, 657–659. [Google Scholar] [CrossRef]
- Jonsson, T.; Jonsdottir, H.; Möller, A.D.; Baldursdottir, L. Nursing documentation prior to emergency admissions to the in-tensive care unit. Nurs. Crit. Care 2011, 16, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gennings, C.; Wenzel, R.P. R = 20: Bias in the reporting of respiratory rates. Am. J. Emerg. Med. 2008, 26, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Latten, G.H.P.; Spek, M.; Muris, J.W.M.; Cals, J.W.L.; Stassen, P.M. Accuracy and interobserver-agreement of respiratory rate measurements by healthcare professionals, and its effect on the outcomes of clinical prediction/diagnostic rules. PLoS ONE 2019, 14, e0223155. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Carty, S.; Macfarlane, J.; Anthony, R.; Christian, J.; Dakin, K.; Dennis, P. Respiratory rate measurement in adults—how reliable is it? Respir. Med. 2002, 96, 31–33. [Google Scholar] [CrossRef][Green Version]
- Sun, Z.; Sessler, D.I.; Dalton, J.E.; Devereaux, P.J.; Shahinyan, A.; Naylor, A.J.; Hutcherson, M.T.; Finnegan, P.S.; Tandon, V.; Darvish-Kazem, S. Postoperative hypoxemia is common and persistent: A prospective blinded observational study. Anesth. Analg. 2015, 121, 709. [Google Scholar] [CrossRef]
- Bergese, S.D.; Mestek, M.L.; Kelley, S.D.; McIntyre, R.; Uribe, A.A.; Sethi, R.; Watson, J.N.; Addison, P.S. Multicenter study validating accuracy of a continuous respiratory rate measurement derived from pulse oximetry: A comparison with capnography. Anesth. Analg. 2017, 124, 1153–1159. [Google Scholar] [CrossRef]
- Subbe, C.P.; Kinsella, S. Continuous Monitoring of Respiratory Rate in Emergency Admissions: Evaluation of the RespiraSense™ Sensor in Acute Care Compared to the Industry Standard and Gold Standard. Sensors 2018, 18, 2700. [Google Scholar] [CrossRef]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef]
- Jeffs, E.; Vollam, S.; Young, J.D.; Horsington, L.; Lynch, B.; Watkinson, P.J. Wearable monitors for patients following discharge from an intensive care unit: Practical lessons learnt from an observational study. J. Adv. Nurs. 2016, 72, 1851–1862. [Google Scholar] [CrossRef]
- Nowogrodzki, M.; Mawhinney, D.D.; Milgazo, H.F. Non-invasive microwave instruments for the measurement of respiration and heart rates. In Proceedings of the 1984 National Aerospace and Electronics Conference (NAECON), Dayton, OH, USA, 21–25 May 1984; pp. 958–960. [Google Scholar]
- Sharpe, S.M.; Seals, J.; MacDonald, A.H.; Crowgey, S.R. Non-Contact Vital Signs Monitor. U.S. Patent 4,958,638, 25 September 1990. [Google Scholar]
- Lin, J.C. Microwave sensing of physiological movement and volume change: A review. Bioelectromagnetics 1992, 13, 557–565. [Google Scholar] [CrossRef]
- Gu, C. Short-Range Noncontact Sensors for Healthcare and Other Emerging Applications: A Review. Sensors 2016, 16, 1169. [Google Scholar] [CrossRef]
- Droitcour, A.D.; Boric-Lubecke, O.; Lubecke, V.M.; Lin, J.; Kovacs, G.T.A. Range correlation and I/Q performance benefits in single-chip silicon Doppler radars for noncontact cardiopulmonary monitoring. IEEE Trans. Microw. Theory Tech. 2004, 52, 838–848. [Google Scholar] [CrossRef]
- Lazaro, A.; Girbau, D.; Villarino, R. Techniques for Clutter Suppression in the Presence of Body Movements during the Detection of Respiratory Activity through UWB Radars. Sensors 2014, 14, 2595–2618. [Google Scholar] [CrossRef] [PubMed]
- Kebe, M.; Gadhafi, R.; Mohammad, B.; Sanduleanu, M.; Saleh, H.; Al-Qutayri, M. Human Vital Signs Detection Methods and Potential Using Radars: A Review. Sensors 2020, 20, 1454. [Google Scholar] [CrossRef] [PubMed]
- Sacco, G.; Piuzzi, E.; Pittella, E.; Pisa, S. An FMCW Radar for Localization and Vital Signs Measurement for Different Chest Orientations. Sensors 2020, 20, 3489. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, K.; Breteler, M.J.; Van Wolfwinkel, L.; Leyssius, A.R.; Kossen, S.; Kalkman, C.J.; van Zaane, B.; Peelen, L.M. Wireless non-invasive continuous respiratory monitoring with FMCW radar: A clinical validation study. J. Clin. Monit. Comput. 2016, 30, 797–805. [Google Scholar] [CrossRef]
- Alizadeh, M.; Shaker, G.; De Almeida, J.C.; Morita, P.P.; Safavi-Naeini, S. Remote Monitoring of Human Vital Signs Using mm-Wave FMCW Radar. IEEE Access 2019, 7, 54958–54968. [Google Scholar] [CrossRef]
- Fontana, R. Recent System Applications of Short-Pulse Ultra-Wideband (UWB) Technology. IEEE Trans. Microw. Theory Tech. 2004, 52, 2087–2104. [Google Scholar] [CrossRef]
- Ossberger, G.; Buchegger, T.; Schimback, E.; Stelzer, A.; Weigel, R. Non-invasive respiratory movement detection and mon-itoring of hidden humans using ultra wideband pulse radar. In Proceedings of the 2004 International Workshop on Ultra Wideband Systems Joint with Conference on Ultra Wideband Systems and Technologies, Joint UWBST & IWUWBS 2004 (IEEE Cat. No. 04EX812), Kyoto, Japan, 18–21 May 2004; pp. 395–399. [Google Scholar]
- Li, J.; Zeng, Z.; Sun, J.; Liu, F. Through-Wall Detection of Human Being’s Movement by UWB Radar. IEEE Geosci. Remote. Sens. Lett. 2012, 9, 1079–1083. [Google Scholar] [CrossRef]
- Yarovoy, A.; Ligthart, L.; Matuzas, J.; Levitas, B. UWB Radar for Human Being Detection. IEEE Aerosp. Electron. Syst. Mag. 2006, 21, 22–26. [Google Scholar] [CrossRef]
- Immoreev, I.; Tao, T.-H. UWB radar for patient monitoring. IEEE Aerosp. Electron. Syst. Mag. 2008, 23, 11–18. [Google Scholar] [CrossRef]
- Lazaro, A.; Girbau, D.; Villarino, R. Analysis of vital signs monitoring using an ir-uwb radar. Prog. Electromagn. Res. 2010, 100, 265–284. [Google Scholar] [CrossRef]
- Zito, D.; Pepe, D.; Mincica, M.; Zito, F.; Tognetti, A.; Lanata, A.; De Rossi, D. SoC CMOS UWB Pulse Radar Sensor for Contactless Respiratory Rate Monitoring. IEEE Trans. Biomed. Circuits Syst. 2011, 5, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Maalik, S.; Yang, J.; McKelvey, T.; Malmström, K.; Landén, L.; Stoew, B. A new UWB radar system using UWB CMOS chip. In Proceedings of the 5th European Conference on Antennas and Propagation (EUCAP), Rome, Italy, 11–15 April 2011; pp. 771–775. [Google Scholar]
- Chu, T.-S.; Roderick, J.; Chang, S.; Mercer, T.; Du, C.; Hashemi, H. A short-range UWB impulse-radio CMOS sensor for human feature detection. In Proceedings of the 2011 IEEE International Solid-State Circuits Conference, San Francisco, CA, USA, 20–24 February 2011; pp. 294–296. [Google Scholar]
- Park, J.-Y.; Lee, Y.; Choi, Y.-W.; Heo, R.; Park, H.-K.; Cho, S.-H.; Cho, S.H.; Lim, Y.-H. Preclinical Evaluation of a Noncontact Simultaneous Monitoring Method for Respiration and Carotid Pulsation Using Impulse-Radio Ultra-Wideband Radar. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Regev, N.; Wulich, D. Multi-Modal, Remote Breathing Monitor. Sensors 2020, 20, 1229. [Google Scholar] [CrossRef]
- Khan, F.; Choi, J.W.; Cho, S.H. Vital sign monitoring of a non-stationary human through IR-UWB radar. In Proceedings of the 2014 4th IEEE International Conference on Network Infrastructure and Digital Content, Beijing, China, 19–21 September 2014; pp. 511–514. [Google Scholar]
- Zou, G.Y. Confidence interval estimation for the Bland–Altman limits of agreement with multiple observations per individual. Stat. Methods Med. Res. 2011, 22, 630–642. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolo, A.; Sacchetti, M.; Schena, E. Contactless Methods for Measuring Respiratory Rate: A Review. IEEE Sens. J. 2021, 21, 12821–12839. [Google Scholar] [CrossRef]
- Van Loon, K.; Peelen, L.M.; Van De Vlasakker, E.C.; Kalkman, C.J.; Van Wolfswinkel, L.; Van Zaane, B. Accuracy of remote continuous respiratory rate monitoring technologies intended for low care clinical settings: A prospective observational study. Can. J. Anesth. J. Can. D’anesthésie 2018, 65, 1324–1332. [Google Scholar] [CrossRef]
- Droitcour, A.D.; Seto, T.B.; Park, B.-K.; Yamada, S.; Vergara, A.; El Hourani, C.; Shing, T.; Yuen, A.; Lubecke, V.M.; Boric-Lubecke, O. Non-contact respiratory rate measurement validation for hospitalized patients. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 2–6 September 2009; pp. 4812–4815. [Google Scholar] [CrossRef]





| Study 1 | Study 2 | Study 3 | |
|---|---|---|---|
| Sample | Nonprobability sample of volunteers, wide range of age, BMI, and sex | Patients in an overnight OSA diagnostic assessment, suspicion of OSA, various health conditions * | Healthy volunteers in an overnight sleep–memory study |
| n (of which women) | 26 (10) | 12 (4) | 12 (11) |
| Age | 23–73 (uniform) | Mean 55.0 (±10.6 SD) | Mean 23.7 (±3.2 SD) |
| BMI | 19.6–60.7 (uniform) | Mean 33.3 (±7.8 SD) | Not recorded |
| Reference method | Manually scored ventilatory effort (nasal pressure, thoracic and abdominal RIP belts) | Manually scored ETCO2 capnography | Manually scored abdominal effort belt |
| Recording conditions | Seated (1 min, D0: 0.5 m) Seated (1 min, D0: 1.0 m) Seated, with blanket (1 min, D0: 1.0 m) Seated (1 min, D0: 1.5 m) Lying Supine (1 min, D0: 0.5 m) Lying Supine, with blanket (1 min, D0: 0.5 m) Lying Side, facing device (1 min, D0: 0.5 m) Lying Side, back to device (1 min, D0: 0.5 m) Lying Supine (9 min, D0: 0.5 m) | 10 min recording, while asleep, with blanket. D0: 0.5–1.0 m | 10 min recording, while asleep, with blanket. D0: 0.5–1.0 m |
| Study 1 (n = 26) | Study 2 (n = 12) | Study 3 (n = 12) | |
|---|---|---|---|
| Total Number of Epochs | 468 | 120 | 150 |
| Number of Technician Excluded Epochs | 69 | 12 | 6 |
| Mean Circadia Success Rate (±SD) | 98.5% (±3.0 pp) | 91.6% (±12.7 pp) | 100.0% (±0.0 pp) |
| Mean MAE (±SD) | 0.95 (±0.65) BrPM | 0.81 (±0.35) BrPM | 0.44 (±0.13) BrPM |
| Mean Accuracy Rate (±SD): Error <2 BrPM | 89.7% (±16.6 pp) | 94.1% (±9.8 pp) | 100.0% (±0.0 pp) |
| Agreement: Bias | −0.85 BrPM | −0.29 BrPM | −0.27 BrPM |
| 95% LOA Lower (95% CI) | −3.03 (−3.5 to −2.7) BrPM | −2.31 (−2.7 to −2.0) BrPM | −1.21 (−1.4 to −1.1) BrPM |
| 95% LOA Upper (95% CI) | 1.32 (1.0 to 1.8) BrPM | 1.73 (1.4 to 2.2) BrPM | 0.66 (0.5 to 0.8) BrPM |
| Condition | Num Epochs | Num Excluded Epochs | Circadia Success Rate (%) | MAE (BrPM) | Accuracy Rate (%) | Bias (BrPM) | LOA (BrPM) | |
|---|---|---|---|---|---|---|---|---|
| A | Seated, D0: 0.5 m | 52 | 21 | 100.0 | 0.85 | 90.3 | −0.67 | −2.25 to 0.91 |
| B | Seated, D0: 1.0 m | 52 | 6 | 100.0 | 0.89 | 93.5 | −0.89 | −2.73 to 0.95 |
| C | Seated, D0: 1.0 m, blanket | 52 | 6 | 100.0 | 0.93 | 91.3 | −0.89 | −3.00 to 1.22 |
| D | Seated, D0: 1.5 m | 52 | 5 | 100.0 | 0.75 | 95.7 | −0.72 | −2.76 to 1.31 |
| E | Lying supine, D0: 0.5 m | 52 | 5 | 97.9 | 1.04 | 84.8 | −0.85 | −3.74 to 2.04 |
| F | Lying supine, D0: 0.5 m, blanket | 52 | 8 | 90.9 | 1.04 | 85.0 | −0.99 | −3.37 to 1.39 |
| G | Lying side, facing device, D0: 0.5 m | 52 | 6 | 100.0 | 0.63 | 97.8 | −0.62 | −1.52 to 0.29 |
| H | Lying side, facing away, D0: 0.5 m | 52 | 6 | 100.0 | 0.94 | 91.3 | −0.99 | −3.66 to 1.68 |
| I | Lying supine, D0: 0.5 m, 9 min | 234 | 30 | 95.6 | 0.98 | 88.2 | −0.89 | −3.22 to 1.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauteslager, T.; Maslik, M.; Siddiqui, F.; Marfani, S.; Leschziner, G.D.; Williams, A.J. Validation of a New Contactless and Continuous Respiratory Rate Monitoring Device Based on Ultra-Wideband Radar Technology. Sensors 2021, 21, 4027. https://doi.org/10.3390/s21124027
Lauteslager T, Maslik M, Siddiqui F, Marfani S, Leschziner GD, Williams AJ. Validation of a New Contactless and Continuous Respiratory Rate Monitoring Device Based on Ultra-Wideband Radar Technology. Sensors. 2021; 21(12):4027. https://doi.org/10.3390/s21124027
Chicago/Turabian StyleLauteslager, Timo, Michal Maslik, Fares Siddiqui, Saad Marfani, Guy D. Leschziner, and Adrian J. Williams. 2021. "Validation of a New Contactless and Continuous Respiratory Rate Monitoring Device Based on Ultra-Wideband Radar Technology" Sensors 21, no. 12: 4027. https://doi.org/10.3390/s21124027
APA StyleLauteslager, T., Maslik, M., Siddiqui, F., Marfani, S., Leschziner, G. D., & Williams, A. J. (2021). Validation of a New Contactless and Continuous Respiratory Rate Monitoring Device Based on Ultra-Wideband Radar Technology. Sensors, 21(12), 4027. https://doi.org/10.3390/s21124027






