Cybersickness and Its Severity Arising from Virtual Reality Content: A Comprehensive Study
Abstract
:1. Introduction
1.1. Motivation
1.2. Related Work
1.2.1. Content Factor-Based Cybersickness Analysis
1.2.2. Machine Learning for Quantifying Cybersickness
1.2.3. Quantitative Cybersickness Analysis via Biological Signal
1.3. Contributions
- We generated synthetic CYRE content: 52 VR scenes that represent different content factors associated with causing cybersickness.
- We designed a cybersickness evaluation protocol and obtained a number of subjective opinions from 154 participants in conjunction with objective data (e.g., rendered videos of VR scenes and biological signals) to construct a database.
- We quantitatively analyzed how various factors (e.g., content attributes, physiological responses, and individual characteristics including sex, age, and susceptibility to motion sickness) influence the severity of cybersickness.
- We constructed a number of data-label pairs (i.e., the number of scenes × the number of participants) for supervised learning.
2. Construction of CYRE Content
2.1. Background of Scene
2.2. Camera Movement
2.2.1. Simple Movement
2.2.2. Complex Movement
2.2.3. Translation Acceleration
2.2.4. Translation Speed
2.3. FOV
2.4. Frame Reference
2.5. Duration (Path Length)
2.6. Controllability
3. Subjective Evaluation and Data Acquisition
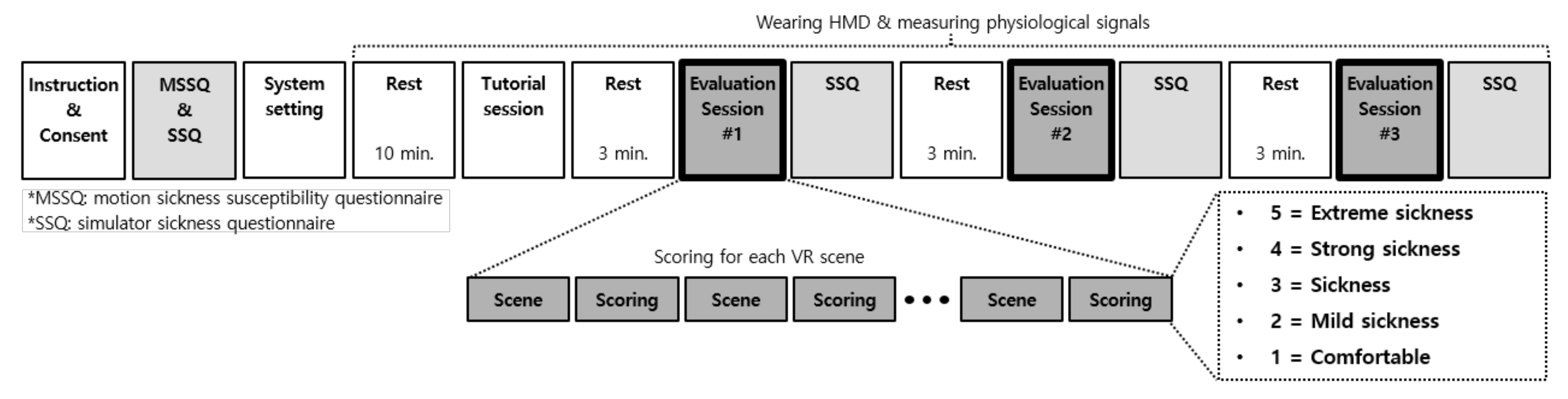
3.1. Protocol Design
3.1.1. Tutorial Session
3.1.2. Evaluation Session
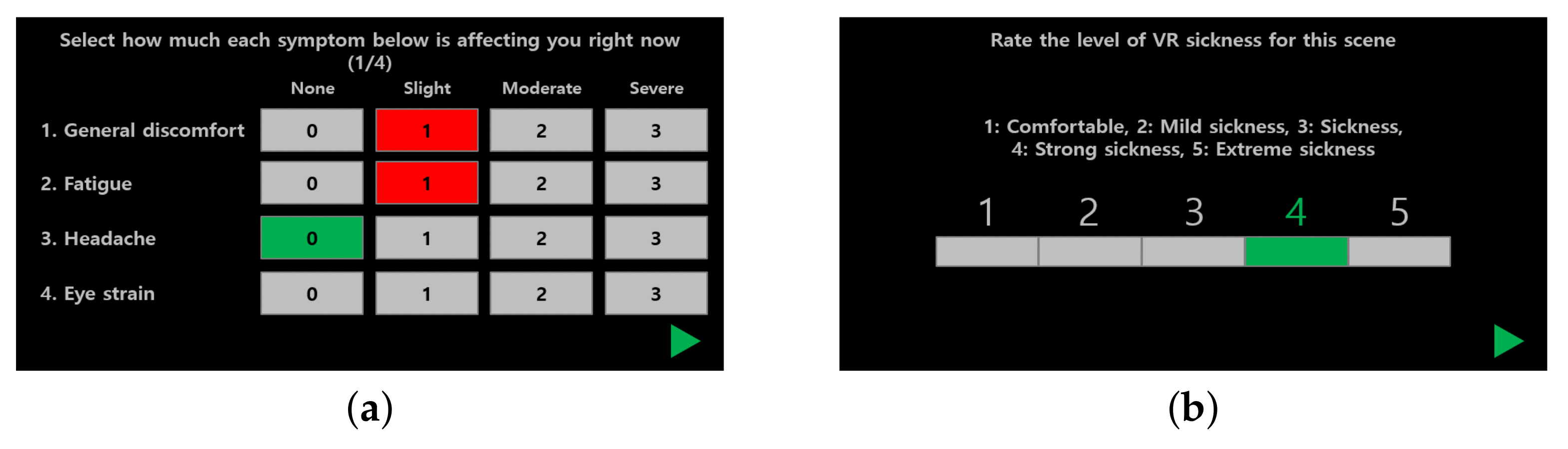
3.1.3. Questionnaires
3.1.4. Scoring Cybersickness
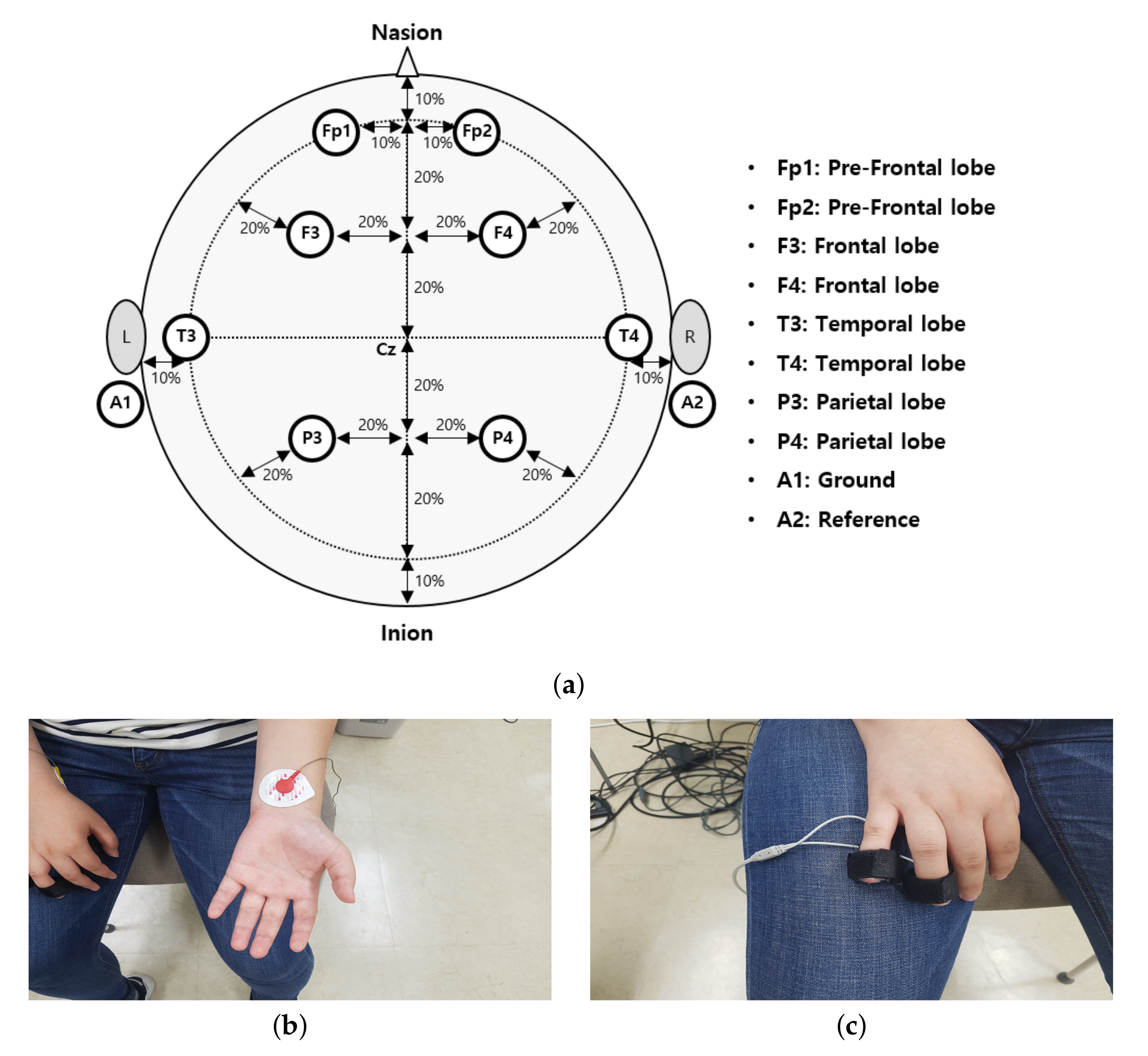
3.2. Acquisition of Physiological Signals
3.3. Participants and Environment
- the same scores for all 116 ratings,
- the discrepancy between the answers in consent and personal information, and
- the eye tracker capture showed the eyes were closed throughout the experiment.
4. Experimental Results
4.1. Scene Factors and Cybersickness
4.1.1. Camera Rotation
4.1.2. Camera Translation
4.1.3. FOV
4.1.4. Translation Acceleration
4.1.5. Translation Speed
4.1.6. Frame Reference
4.1.7. Duration
4.1.8. Controllability
4.2. Physiological Signals and Cybersickness
4.2.1. Feature Processing
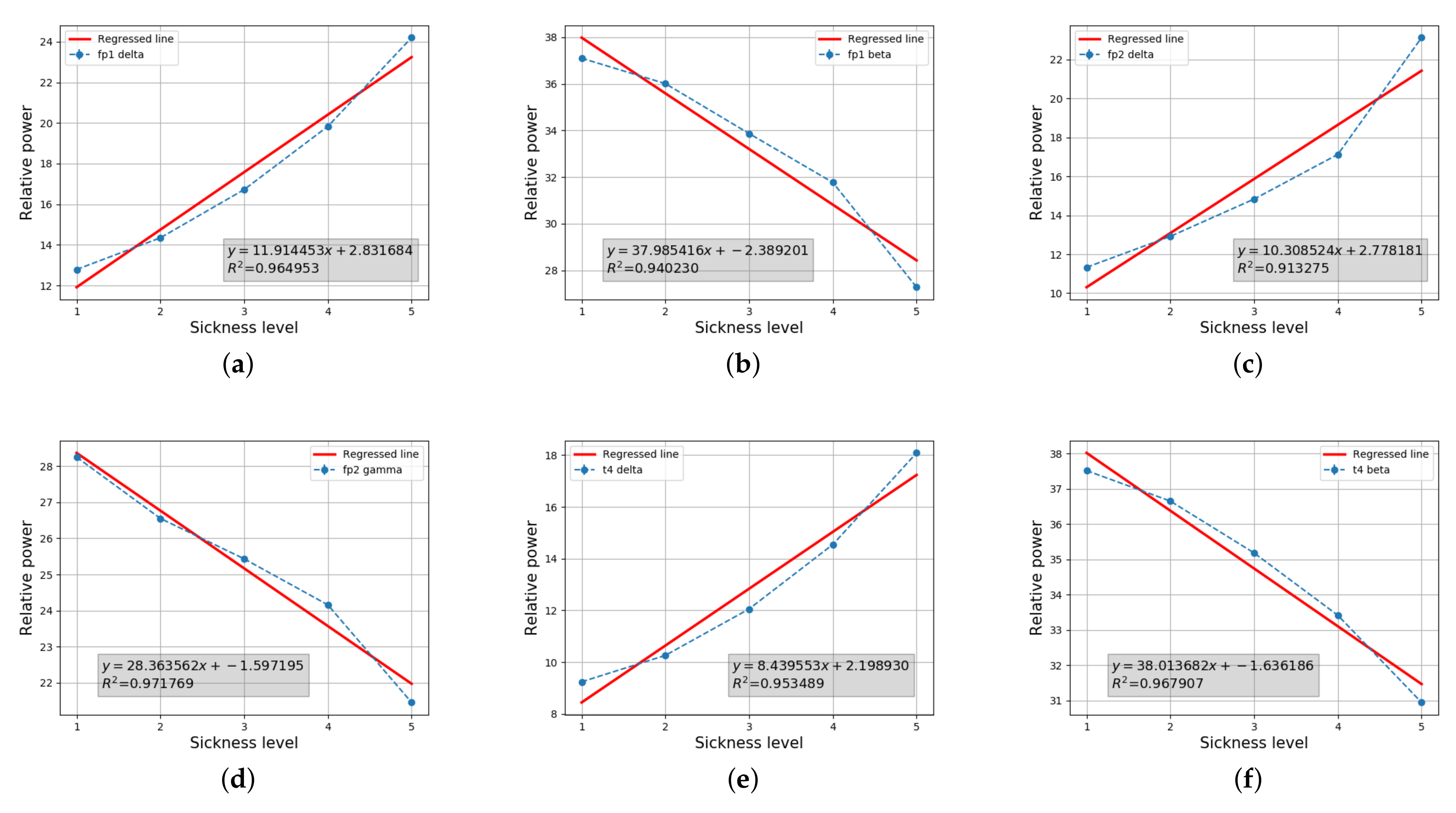
4.2.2. Statistical Analysis
4.2.3. Discussion
4.3. Individual Characteristics and Cybersickness
4.3.1. Sex and Cybersickness
4.3.2. Age and Cybersickness
4.3.3. Susceptibility and Cybersickness
4.4. Cybersickness Prediction
4.5. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.Y.; Kim, H.J.; Kim, E.N.; Ko, H.D.; Kim, H.Y. Characteristic changes in the physiological components of cybersickness. Psychophysiology 2005, 42, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.S.; Lane, N.E.; Berbaum, K.S.; Lilienthal, M.G. Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness. Int. J. Aviat. Psychol. 2009, 3, 203–220. [Google Scholar] [CrossRef]
- Cobb, S.V.G. Measurement of postural stability before and after immersion in a virtual environment. Appl. Ergon. 1999, 30, 47–57. [Google Scholar] [CrossRef]
- Reason, J.T.; Brand, J.J. Motion Sickness; Academic Press: London, UK, 1975; pp. 83–101. [Google Scholar]
- Oman, C.M. A heuristic mathematical model for dynamics of sensory conflict and motion sickness hearing in classical musicians. Acta Oto-Laryngol. 1982, 94, sup392. [Google Scholar] [CrossRef]
- Lewkowicz, R. Modeling motion sickness. Pol. J. Aviat. Bioeng. Psychol. 2019, 22, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Bles, W.; Bos, J.E.; Graaf, B.D.; Groen, E.; Wertheim, A.H. Motion sickness: Only one provocative conflict? Brain Res. Bull. 1998, 47, 481–487. [Google Scholar] [CrossRef]
- Bos, J.E.; Bles, W.; Groen, E.L. A theory on visually induced motion sickness. Displays 2008, 29, 47–57. [Google Scholar] [CrossRef]
- Mittelstaedt, J.; Wacker, J.; Stelling, D. Effects of display type and motion control on cybersickness in a virtual bike simulator. Displays 2018, 51, 43–50. [Google Scholar] [CrossRef]
- Sharples, S.; Cobb, S.; Moody, A.; Wilson, J.R. Virtual reality induced symptoms and effects (VRISE): Comparison of head mounted display (HMD), desktop and projection display systems. Displays 2007, 29, 58–69. [Google Scholar] [CrossRef]
- Moss, J.D.; Austin, J.; Salley, J.; Coats, J.; Williams, K.; Muth, E.R. The effects of display delay on simulator sickness. Displays 2011, 32, 159–168. [Google Scholar] [CrossRef]
- Kawamura, S.; Kijima, R. Effect of head mounted display latency on human stability during quiescent standing on one foot. In Proceedings of the 2016 IEEE Virtual Reality (VR), Greenville, SC, USA, 19–23 March 2016. [Google Scholar]
- Singla, A.; Fremerey, S.; Robitza, W.; Raake, A. Measuring and comparing QoE and simulator sickness of omnidirectional videos in different head mounted displays. In Proceedings of the 9th Int’l Conference Quality of Multimedia Experience (QoMEX), Erfurt, Germany, 31 May–2 June 2017. [Google Scholar]
- Friston, S.; Steed, A.; Tilbury, S.; Gaydadjiev, G. Construction and evaluation of an ultra low latency frameless renderer for VR. IEEE Trans. Vis. Comput. Graph. 2016, 22, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.S.; Harris, L.R.; Jenkin, M.; Jasiobedzka, U.; Zacher, J.E. Tolerance of temporal delay in virtual environments. In Proceedings of the IEEE Virtual Reality (VR), Washington, DC, USA, 13–17 March 2001; pp. 247–254. [Google Scholar]
- Dennison, M.; D’Zmura, M. Effects of unexpected visual motion on postural sway and motion sickness. Appl. Ergon. 2011, 71, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Stauffert, J.-P.; Niebling, F.; Latoschik, M.E. Latency and cybersickness: Impact, causes, and measures: A review. Front. Virtual Real. 2020, 1, 31. [Google Scholar] [CrossRef]
- Hell, S.; Argyriou, V. Machine learning architectures to predict motion sickness using a virtual reality rollercoaster simulation tool. In Proceedings of the 2018 IEEE International Conference on Artificial Intelligence and Virtual Reality (AIVR), Taichung, Taiwan, 10–12 December 2018. [Google Scholar]
- Joseph, J.A.; Griffin, M.J. Motion sickness: Effect of the magnitude of roll and pitch oscillation. Aviat. Space Environ. Med. 2008, 79, 390–396. [Google Scholar] [CrossRef]
- Porcino, T.M.; Clua, E.W.; Vasconcelos, C.N.; Trevisan, D.; Valente, L. Minimizing cyber sickness in head mounted display system: Design guidelines and applications. In Proceedings of the 2017 IEEE 5th International Conference on Serious Games and Applications for Health (SeGAH), Perth, WA, USA, 2–4 April 2017. [Google Scholar]
- Fernandes, A.S.; Feiner, S.K. Combating VR sickness through subtle dynamic field-of-view modification. In Proceedings of the 2016 IEEE Symposium on 3D User Interfaces (3DUI), Greenville, SC, USA, 19–20 March 2016; pp. 201–210. [Google Scholar]
- So, R.H.Y.; Lo, W.T.; Ho, A.T.K. Effects of navigation speed on motion sickness caused by an immersive virtual environment. Hum. Factors J. Hum. Factors Ergon. Soc. 2001, 43, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Chardonnet, J.-R.; Mirzaei, M.A.; Merienne, F. Influence of navigation parameters on cybersickness in virtual reality. Virtual Real. 2021, 25, 565–574. [Google Scholar] [CrossRef]
- Duh, H.B.; Parker, D.E.; Furness, T.A. An “independent visual backgrond” reduced balance disturbance evoked by visual scene motion: Implication for alleviating simulator sickness. In Proceedings of the CHI 2001 Conference on Human Factors in Computing Systems, Seattle, WA, USA, 31 March–5 April 2001. [Google Scholar]
- Lin, J.J.; Rached, H.A.; Kim, D.H.; Parker, D.E.; Furness, T.A. A “natural” independent visual background reduced simulator sickness. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Baltimore, MD, USA, 30 September–4 October 2002. [Google Scholar]
- Padmanaban, N.; Ruban, T.; Sitzmann, V.; Norcia, A.M.; Wetzstein, G. Towards a machine learning approach for sickness prediction in 360∘ stereoscopic video. IEEE Trans. Vis. Comput. Graph. 2018, 24, 1594–1603. [Google Scholar] [CrossRef]
- Kim, H.G.; Lim, H.T.; Lee, S.; Ro, Y.M. VRSA Net: VR sickness assessment considering exceptional motion for 360∘ VR video. IEEE Trans. Image Process. 2019, 28, 1646–1660. [Google Scholar] [CrossRef]
- Kim, H.G.; Baddar, W.J.; Lim, H.T.; Jeong, H.; Ro, Y.M. Measurement of exceptional motion in VR video contents for VR sickness assessment using deep convolutional autoencoder. In Proceedings of the 23rd ACM Symposium on Virtual Reality Software and Technology, Gothenburg, Sweden, 8–10 November 2017. [Google Scholar]
- Lee, T.M.; Yoon, J.C.; Lee, I.K. Motion sickness prediction in stereoscopic videos using 3D convolutional neural networks. IEEE Trans. Vis. Comput. Graph. 2019, 25, 1919–1927. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Soundararajan, R. Prediction of discomfort due to egomotion in immersive videos for virtual reality. In Proceedings of the 2019 IEEE International Symposium on Mixed and Augmented Reality (ISMAR), Beijing, China, 10–18 October 2019. [Google Scholar]
- Guna, J.; Gersak, G. Influence of video content type on user’s virtual reality sickness perception and physiologial response. Future Gener. Comput. Syst. 2019, 91, 263–276. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chien, Y.Y.; Wang, H.H.; Lin, F.C.; Huang, Y.P. The quantization of cybersickness level using EEG and ECG for virtual reality head-mounted display. In SID Symposium Digest of Technical Papers; SID: Tehran, Iran, 2018. [Google Scholar]
- Islam, R.; Lee, Y.; Jaloli, M.; Muhammad, I.; Zhu, D.; Rad, P.; Huang, Y.; Quarles, J. Automatic detection and prediction of cybersickness severity using deep neural networks from user’s physiological signals. In Proceedings of the 2020 IEEE International Symposium on Mixed and Augmented Reality (ISMAR), Galinhas, Brazil, 9–13 November 2020. [Google Scholar]
- Lin, C.T.; Tsai, S.F.; Ko, L.W. EEG-based learning system for online motion sickness level estimation in a dynamic vehicle environment. IEEE Trans. Neural Netw. Learn. Syst. 2013, 24, 1689–1700. [Google Scholar] [PubMed]
- Whittinghill, D.M.; Ziegler, B.; Moore, J.; Case, T. Nasum virtualis: A simple technique for reducing simulator sickness in head mounted VR. In Proceedings of the Game Developers Conference, San Francisco, CA, USA, 2–6 March 2015. [Google Scholar]
- Kim, J.; Kim, W.; Ahn, S.; Kim, J.; Lee, S. Virtual reality sickness predictor: Analysis of visual-vestibular conflict and VR contents. In Proceedings of the 10th Int’l Conference Quality of Multimedia Experience (QoMEX), Cagliari, Italy, 29 May–1 June 2018. [Google Scholar]
- McCauley, M.E.; Sharkey, T.J. Cybersickness: Perception of self-motion in virtual environments. Presence Teleoper. Virtual Environ. 1992, 1, 311–318. [Google Scholar] [CrossRef]
- Stanney, K.M.; Mourant, R.R.; Kennedy, R.S. Human factors issues in virtual environments: A review of the literature. Presence Teleoper. Virtual Environ. 1998, 1, 327–351. [Google Scholar] [CrossRef]
- Keshavarz, B.; Riecke, B.E.; Hettinger, L.J.; Campos, J.L. Vection and visually induced motion sickness: How are they related? Front. Psychol. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- So, R.H.Y.; Lo, W.T. Cybersickness: An experimental study to isolate the effects of rotational scene oscillations. In Proceedings of the IEEE Virtual Reality (VR), Houston, TX, USA, 13–17 March 1999. [Google Scholar]
- Terziman, L.; Lecuyer, A.; Hillaire, S.; Wiener, J.M. Can camera motions improve the perception of traveled distance in virtual environment? In Proceedings of the IEEE Virtual Reality (VR), Lafayette, LA, USA, 14–18 March 2009. [Google Scholar]
- Keshavarz, B.; Hecht, H. Axis rotation and visually induced motion sickness: The role of combined roll, pitch, and yaw motion. Aviat. Space Environ. Med. 2011, 82, 1023–1029. [Google Scholar] [CrossRef]
- Bertolini, G.; Straumann, D. Moving in a moving world: A review on vestibular motion sickness. Front. Neurol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bos, J.E.; Bles, W. Modeling motion sickness and subjective vertical mismatch detailed for vertical motions. Brain Res. Bull. 1998, 47, 537–542. [Google Scholar] [CrossRef]
- Bos, J.E.; Vries, S.C.D.; Emmerik, M.L.V.; Groen, E.L. The effect of internal and external fields of view on visually induced motion sickness. Appl. Ergon. 2010, 41, 516–521. [Google Scholar] [CrossRef]
- Draper, M.H.; Viirre, E.S.; Furness, T.A.; Gawron, V.J. Effects of image scale and system time delay on simulator sickness within head-coupled virtual environments. Hum. Factors J. Hum. Factors Ergon. Soc. 2001, 43, 129–146. [Google Scholar] [CrossRef]
- Prothero, J.D.; Draper, M.H.; Furness, T.A.; Parker, D.E.; Wells, M.J. The use of an independent visual background to reduce simulator side-effects. Aviat. Space Environ. Med. 1999, 70, 277–283. [Google Scholar]
- Liu, C.L.; Uang, S.T. A study of sickness induced within an 3D virtual store and combated with fuzzy control in the elderly. In Proceedings of the 2012 9th International Conference on Fuzzy Systems and Knowledge Discovery, Chongqing, China, 29–31 May 2012; pp. 334–338. [Google Scholar]
- Lo, W.T.; So, R.H. Cybersickness in the presence of scene rotational movements along different axes. Appl. Ergon. 2001, 32, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ujiake, H.; Yokoi, T.; Saida, S. Effects of virtual body motion on visually-induced motion sickness. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar]
- Kim, W.; Lee, S.; Bovik, A.C. VR sickness versus VR presence. IEEE Trans. Image Process. 2020, 30, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yoshida, K.; Stoffregen, T.A. Control of a virtual vehicle influences postural activity and motion sickness. J. Exp. Psychol. Appl. 2011, 17, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S.D.; Adelstein, B.D.; Ellis, S.R. Demand characteristics in assessing motion sickness in a virtual environment: Or does taking a motion sickness questionnaire make you sick? IEEE Trans. Vis. Comput. Graph. 2007, 13, 422–428. [Google Scholar] [CrossRef]
- Duzmanska, N.; Strojny, P.; Strojny, A. Can simulator sickness be avoided? a review on temporal aspects of simulator sickness. Front. Psychol. 2018, 9, 2132. [Google Scholar] [CrossRef]
- Palmisano, S.; Mursic, R.; Kim, J. Vection and cybersickness generated by head-and-display motion in the Oculus Rift. Displays 2018, 48, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jesse, L. Reducing Cybersickness in VR on an Omnidirectional Treadmill. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2021. [Google Scholar]
- Wibirama, S.; Nugroho, H.A.; Hamamoto, K. Depth gaze and ECG based frequency dynamics during motion sickness in stereoscopic 3D movie. Entertain. Comput. 2018, 26, 117–127. [Google Scholar] [CrossRef]
- Golding, J.F. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res. Bull. 1998, 37, 507–516. [Google Scholar] [CrossRef]
- Golding, J.F. Predicting individual differences in motion sickness susceptibility by questionnaire. Personal. Individ. Differ. 2006, 41, 237–248. [Google Scholar] [CrossRef]
- Curry, C.; Li, R.; Peterson, N.; Storffregen, T.A. Cybersickness ini virtual reality head-mounted displays: Examining the influence of sex differences and vehicle control. Int. J. Hum. Comput. Interact. 2020, 36, 1161–1167. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, J.; Choi, Y.; Choe, M. Virtual reality sickness questionnaire (VRSQ): Motion sickness measurement index in a virtual reality environment. Appl. Ergon. 2018, 69, 66–73. [Google Scholar] [CrossRef] [PubMed]
- ITU-T P.800. Methods for Subjective Determination of Transmission Quality; International Telecommunication Union: Geneva, Switzerland, 1996. [Google Scholar]
- Jo, H.; Jo, G. Electroencephalogram activity induced by magnetic stimulation on heart meridian. Neurosci. Lett. 2011, 495, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.A.; Chia, W.C.; Chin, S.W. A mobile driver safety system: Analysis of single-channel EEG on drowsiness detection. In Proceedings of the 2014 International Conference on Computational Science and Technology, Kota Kinabalu, Malaysia, 27–28 August 2014. [Google Scholar]
- American Electroencephalographic Society, Guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 1991, 8, 200–202. [CrossRef]
- Munafo, J.; Diedrick, M.; Stoffregen, T.A. The virtual reality head-mounted display Oculus Rift induces motion sickness and is sexist in its effects. Exp. Brain Res. 2017, 235, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Koslucher, F.C.; Haaland, E.; Malsch, A.; Webeler, J.; Stoffregen, T.A. Sex differences in the incidence of motion sickness induced by linear visual oscillation. Aviat. Med. Hum. Perform. 2015, 86, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Arns, L.L.; Cerney, M.M. The relationship between age and incidence of cybersickness among immersive environment users. In Proceedings of the IEEE Virtual Reality (VR), Bonn, Germany, 12–16 March 2005. [Google Scholar]
- Delorme, A.; Sejnowski, T.; Makeig, S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage 2007, 34, 1443–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahnev, D.; Lau, H.; de Lange, F.P. Prior expectation modulates the interaction between sensory and prefrontal regions in the human brain. J. Neurosci. 2011, 3, 10741–10748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.C.; Dworkin, S.F.; Haug, J.; Gehrig, J. Topographic brain measures of human pain and pain responsivity. Pain 1989, 37, 129–141. [Google Scholar] [CrossRef]
- Smith, E.E.; Kosslyn, S.M. Cognitie Psychology: Mind and Brain; Pearson Prentice Hall: Hoboken, NJ, USA, 2008. [Google Scholar]
- Valentino, D.A.; Dufresne, R.L. Attention tasks and EEG power spectra. Int. J. Psychophysiol. 1991, 11, 299–302. [Google Scholar] [CrossRef]
- Islam, R.; Lee, Y.; Jaloli, M.; Muhammad, I.; Zhu, D.; Quarles, J. Automatic detection of cybersickness from physiological signal in a virtual roller coaster simulation. In Proceedings of the IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops, Atlanta, GA, USA, 22–26 March 2020. [Google Scholar]
- Kim, K.; Lee, S.; Kim, H.G.; Park, M.; Ro, Y.M. Deep objective assessment model based on spatio-temporal perception of 360-degree video for VR sickness prediction. In Proceedings of the 2019 IEEE International Conference on Image Processing, Taipei, Taiwan, 22–25 September 2019. [Google Scholar]
- So, R.H.Y.; Ho, A.; Lo, W.T. A metric to quantify virtual scene movement for the study of cybersickness: Definition, implementation, and verification. Presence 2001, 10, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Farnebäck, G. Two-frame motion estimation based on polynomial expansion. In Proceedings of the Scandinavia Conference on Image Analysis, Halmstad, Sweden, 29 June–2 July 2003; pp. 363–370. [Google Scholar]
- Moorthy, A.K.; Bovik, A.C. Visual importance pooling for image quality assessment. IEEE J. Sel. Topics Signal Process. 2009, 3, 193–201. [Google Scholar] [CrossRef]
- Oh, H.; Ahn, S.; Lee, S.; Bovik, A.C. Deep visual discomfort predictor for stereoscopic 3D images. IEEE Trans. Image Process. 2018, 27, 5420–5432. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Ahn, S.; Kim, J.; Lee, S. Blind deep S3D image quality evaluation via local to global feature aggregation. IEEE Trans. Image Process. 2017, 26, 4923–4936. [Google Scholar] [CrossRef] [PubMed]
- Izadi, C.M.; Kokaram, A. A perceptual quality metric for video distorted by spatially correlated noise. In Proceedings of the 24th ACM International Conference on Multimedia, Amsterdam, The Netherlands, 15–19 October 2016; pp. 1277–1285. [Google Scholar]
- Oh, H.; Lee, S.; Bovik, A.C. Stereoscopic 3D visual discomfort prediction: A dynamic accommodation and vergence interaction model. IEEE Trans. Image Process. 2016, 25, 615–629. [Google Scholar] [CrossRef] [PubMed]
- IEEE Standard 3079; IEEE Standard for Head-Mounted Display (HMD) Based Virtual Reality (VR) Sickness Reduction Technology. IEEE: Piscataway, NJ, USA, 2020.






| Scene Index | Background | Camera | FoV | Frame Reference | Duration | Controllability | Scene Category | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Movement | Rotation and Translation 1 | Translation Acceleration | Translation Speed | |||||||
| S001 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (∼ s) | Controllable | (∼ s) | |
| S002 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (∼ s) | Controllable | ||
| S003 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (∼ s) | Controllable | ||
| S004 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (∼ s) | Controllable | ||
| S005 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (∼ s) | Controllable | ||
| S006 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (∼ s) | Controllable | ||
| S007 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Long (∼ s) | Controllable | ||
| S008 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Long (∼ s) | Controllable | ||
| S101 | Astrospace | Simple | No | No translation | Large () | No | Short (13 s) | Uncontrollable | ||
| S102 | Astrospace | Simple | No | No translation | Large () | No | Short (13 s) | Uncontrollable | (234 s) | |
| S103 | Astrospace | Simple | No | No translation | Large () | No | Short (13 s) | Uncontrollable | ||
| S104 | Astrospace | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S105 | Astrospace | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S106 | Astrospace | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S107 | Astrospace | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S108 | Astrospace | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S109 | Astrospace | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S110 | Astrospace | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S111 | Astrospace | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S112 | Astrospace | Complex | No | Fast ( m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S113 | Astrospace | Complex | No | Fast ( m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S114 | Astrospace | Complex | No | Moderate (4 m/s) | Middle () | No | Short (13 s) | Uncontrollable | ||
| S115 | Astrospace | Complex | No | Moderate (4 m/s) | Small () | No | Short (13 s) | Uncontrollable | ||
| S116 | Astrospace | Complex | Yes () 2 | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S117 | Astrospace | Complex | Yes () | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S118 | Astrospace | Complex | Yes () | Moderate (4 m/s) | Large () | Yes | Short (13 s) | Uncontrollable | ||
| S201 | Urban | Simple | No | No translation | Large () | No | Short (13 s) | Uncontrollable | ||
| S202 | Urban | Simple | No | No translation | Large () | No | Short (13 s) | Uncontrollable | (371 s) | |
| S203 | Urban | Simple | No | No translation | Large () | No | Short (13 s) | Uncontrollable | ||
| S204 | Urban | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S205 | Urban | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S206 | Urban | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S207 | Urban | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S208 | Urban | Simple | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S209 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S210 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S211 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S212 | Urban | Complex | No | Fast ( m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S213 | Urban | Complex | No | Fast ( m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S214 | Urban | Complex | No | Moderate (4 m/s) | Middle () | No | Short (13 s) | Uncontrollable | ||
| S215 | Urban | Complex | No | Moderate (4 m/s) | Small () | No | Short (13 s) | Uncontrollable | ||
| S216 | Urban | Complex | Yes () | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S217 | Urban | Complex | Yes () | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S218 | Urban | Complex | Yes () | Moderate (4 m/s) | Large () | Yes | Short (13 s) | Uncontrollable | ||
| S219 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S220 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S221 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S222 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S223 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Short (13 s) | Uncontrollable | ||
| S224 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Long (24 s) | Uncontrollable | ||
| S225 | Urban | Complex | No | Moderate (4 m/s) | Large () | No | Long (24 s) | Uncontrollable | ||
| S226 | Urban | Complex | Yes () | Moderate (4 m/s) | Large () | No | Long (24 s) | Uncontrollable | ||
| Men | Women | |
|---|---|---|
| Young | 43 | 63 |
| (under 30 yr) | (mean age 22.98 yr) | (mean age 21.02 yr) |
| Middle-aged | 28 | 20 |
| (upper 30 yr) | (mean age 38.82 yr) | (mean age 42.40 yr) |
| Data | Feature | p-Values of Statistical Test | Data | Feature | p-Values of Statistical Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA | t-test (1, 2) | t-Test (2, 3) | t-Test (3, 4) | t-Test (4, 5) | ANOVA | t-Test (1, 2) | t-Test (2, 3) | t-Test (3, 4) | t-Test (4, 5) | ||||
| EEG Fp1 | Delta | < *** | < *** | < *** | < *** | < * | EEG T3 | Delta | < *** | < *** | < *** | 0.2240 | < ** |
| Theta | < *** | < ** | < * | 0.8881 | 0.1415 | Theta | < *** | < ** | < ** | 0.5028 | 0.3645 | ||
| Alpha | < *** | < ** | 0.0700 | 0.5406 | 0.2676 | Alpha | < *** | < *** | 0.0848 | 0.9419 | 0.3362 | ||
| Beta | < *** | < ** | < *** | < * | < ** | Beta | < *** | < *** | < *** | 0.1927 | < * | ||
| Gamma | < *** | < *** | < ** | < ** | 0.2268 | Gamma | < *** | < *** | < *** | 0.5954 | 0.2294 | ||
| Mu | < *** | < ** | 0.0793 | 0.4439 | < * | Mu | < *** | < *** | 0.0790 | 0.8074 | 0.5842 | ||
| EEG Fp2 | Delta | < *** | < *** | < ** | < * | < *** | EEG T4 | Delta | < *** | < ** | < *** | < ** | < * |
| Theta | < *** | < *** | < * | 0.4147 | < ** | Theta | < *** | 0.0531 | 0.0991 | 0.0718 | 0.1780 | ||
| Alpha | < *** | < *** | < * | 0.4755 | 0.1357 | Alpha | < *** | < ** | 0.1741 | 0.9474 | 0.6311 | ||
| Beta | < *** | < *** | < ** | 0.0625 | < *** | Beta | < *** | < ** | < *** | < * | < * | ||
| Gamma | < *** | < *** | < ** | < * | < ** | Gamma | < *** | < *** | < ** | 0.1085 | 0.0813 | ||
| Mu | < *** | < *** | 0.1113 | 0.2698 | < * | Mu | < *** | < * | 0.1957 | 0.6509 | 0.3801 | ||
| EEG F3 | Delta | < *** | < ** | < *** | < * | 0.1582 | EEG P3 | Delta | < *** | < ** | < *** | 0.3325 | < ** |
| Theta | < ** | 0.9996 | 0.0539 | 0.1751 | 0.9101 | Theta | < * | 0.7200 | < * | 0.2063 | 0.4868 | ||
| Alpha | < ** | 0.0582 | 0.7701 | 0.3810 | < ** | Alpha | 0.2211 | 0.2263 | 0.5675 | 0.6228 | 0.8228 | ||
| Beta | < *** | 0.4779 | < ** | < * | < * | Beta | < *** | 0.0578 | < ** | 0.1048 | < ** | ||
| Gamma | < *** | < ** | < * | 0.1821 | 0.1738 | Gamma | < *** | < * | < ** | 0.3226 | 0.7697 | ||
| Mu | < * | 0.3330 | 0.4663 | 0.5991 | < ** | Mu | 0.1596 | 0.3706 | 0.5515 | 0.2886 | 0.9481 | ||
| EEG F4 | Delta | < *** | < *** | < *** | 0.1333 | < ** | EEG P4 | Delta | < *** | 0.1651 | < *** | 0.1990 | < ** |
| Theta | < *** | 0.0537 | 0.0663 | 0.4235 | 0.3859 | Theta | 0.0510 | 0.1515 | 0.0258 | 0.8868 | 0.3862 | ||
| Alpha | < *** | < ** | 0.3938 | 0.5183 | 0.1883 | Alpha | < * | 0.1066 | 0.6538 | 0.5482 | 0.1261 | ||
| Beta | < *** | < * | < *** | 0.1960 | < ** | Beta | < *** | 0.3294 | < ** | 0.3998 | < *** | ||
| Gamma | < *** | < *** | < * | 0.2301 | 0.0701 | Gamma | < *** | 0.1040 | < * | 0.2021 | 0.1609 | ||
| Mu | < *** | < * | 0.1889 | 0.6718 | < * | Mu | < ** | 0.4021 | 0.8191 | 0.3378 | 0.0573 | ||
| ECG | BPM | < ** | 0.1308 | 0.2590 | 0.9342 | 0.0910 | GSR | Mean | < ** | 0.1110 | 0.1162 | 0.6136 | 0.2411 |
| SDNN | < *** | < *** | 0.7875 | 0.8688 | < ** | ||||||||
| RMSSD | < *** | < *** | 0.7840 | 0.9575 | < ** | ||||||||
| Rest | Men | 7.325 | 17.190 | 13.423 | 14.960 |
| Women | 9.817 | 14.630 | 18.358 | 19.947 | |
| p-value | 0.336 | 0.138 | 0.311 | 0.191 | |
| Men | 14.140 | 19.627 | 35.794 | 24.844 | |
| Women | 12.582 | 15.490 | 27.840 | 20.055 | |
| p-value | 0.745 | 0.353 | 0.352 | 0.432 | |
| Men | 20.273 | 26.395 | 44.991 | 33.059 | |
| Women | 17.974 | 20.763 | 32.480 | 26.017 | |
| p-value | 0.631 | 0.156 | 0.081 | 0.183 | |
| Men | 35.945 | 34.110 | 63.386 | 47.952 | |
| Women | 29.450 | 29.551 | 52.856 | 40.327 | |
| p-value | 0.356 | 0.429 | 0.345 | 0.351 |
| Rest | Under 30 | ||||
| Upper 30 | |||||
| p-value | |||||
| Under 30 | |||||
| Upper 30 | |||||
| p-value | <0.001 *** | <0.01 ** | <0.001 *** | <0.001 *** | |
| Under 30 | |||||
| Upper 30 | |||||
| p-value | |||||
| Under 30 | |||||
| Upper 30 | |||||
| p-value |
| Visual Features | SROCC | PLCC |
|---|---|---|
| 0.635 | 0.602 | |
| 0.665 | 0.642 | |
| 0.603 | 0.574 | |
| 0.606 | 0.602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.; Son, W. Cybersickness and Its Severity Arising from Virtual Reality Content: A Comprehensive Study. Sensors 2022, 22, 1314. https://doi.org/10.3390/s22041314
Oh H, Son W. Cybersickness and Its Severity Arising from Virtual Reality Content: A Comprehensive Study. Sensors. 2022; 22(4):1314. https://doi.org/10.3390/s22041314
Chicago/Turabian StyleOh, Heeseok, and Wookho Son. 2022. "Cybersickness and Its Severity Arising from Virtual Reality Content: A Comprehensive Study" Sensors 22, no. 4: 1314. https://doi.org/10.3390/s22041314
APA StyleOh, H., & Son, W. (2022). Cybersickness and Its Severity Arising from Virtual Reality Content: A Comprehensive Study. Sensors, 22(4), 1314. https://doi.org/10.3390/s22041314






