A Systematic Review of Wearable Sensor-Based Technologies for Fall Risk Assessment in Older Adults
Abstract
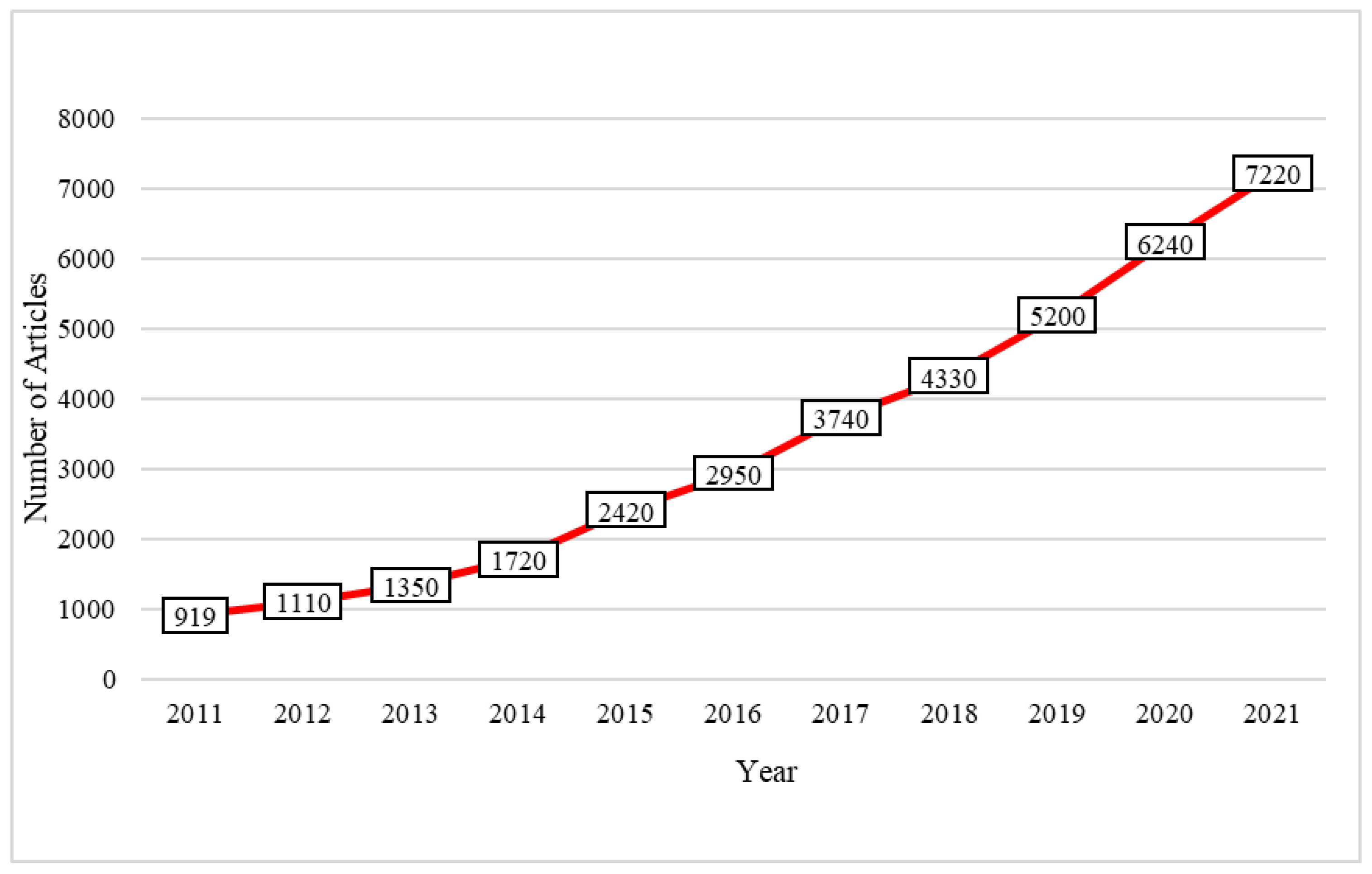
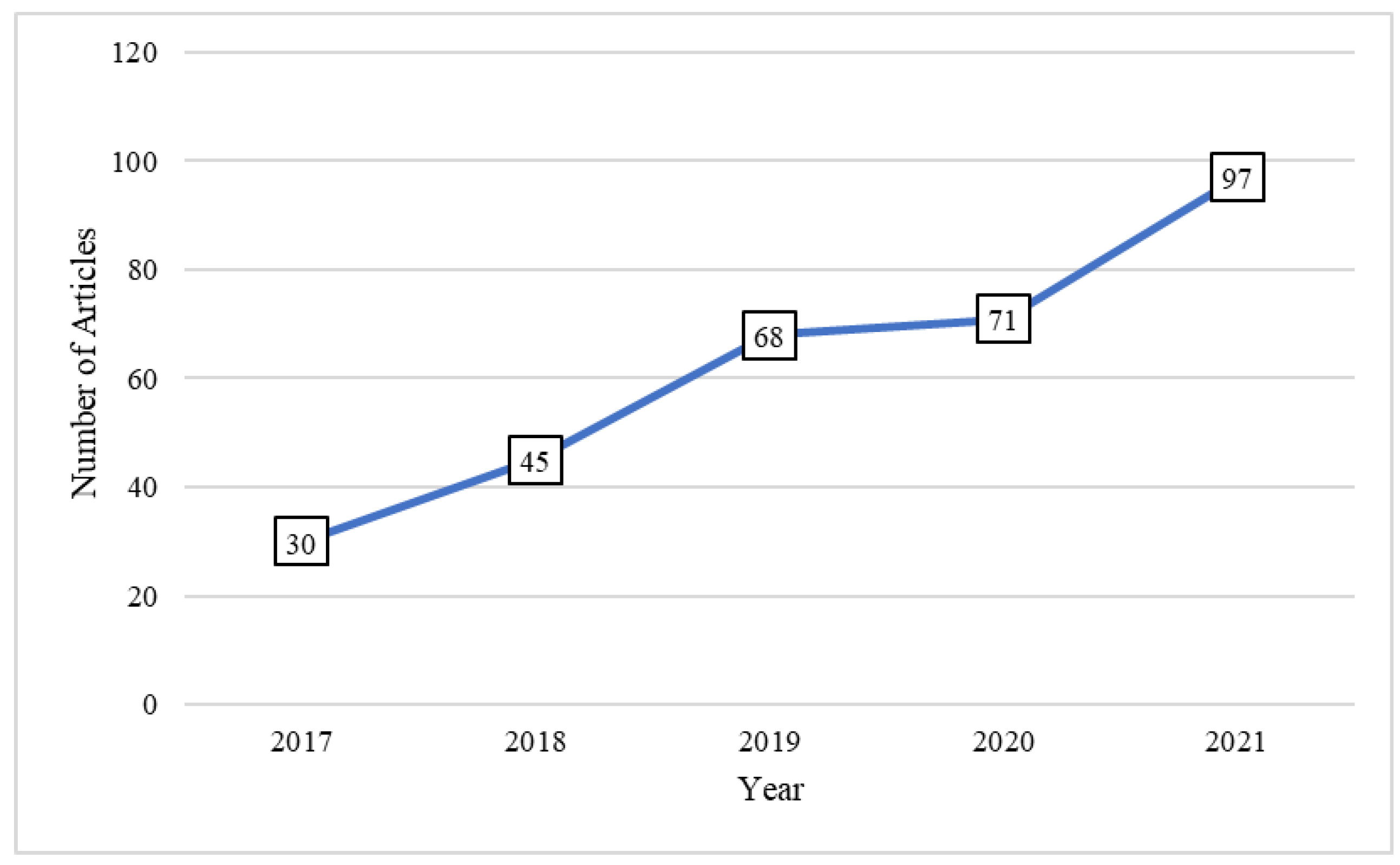
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
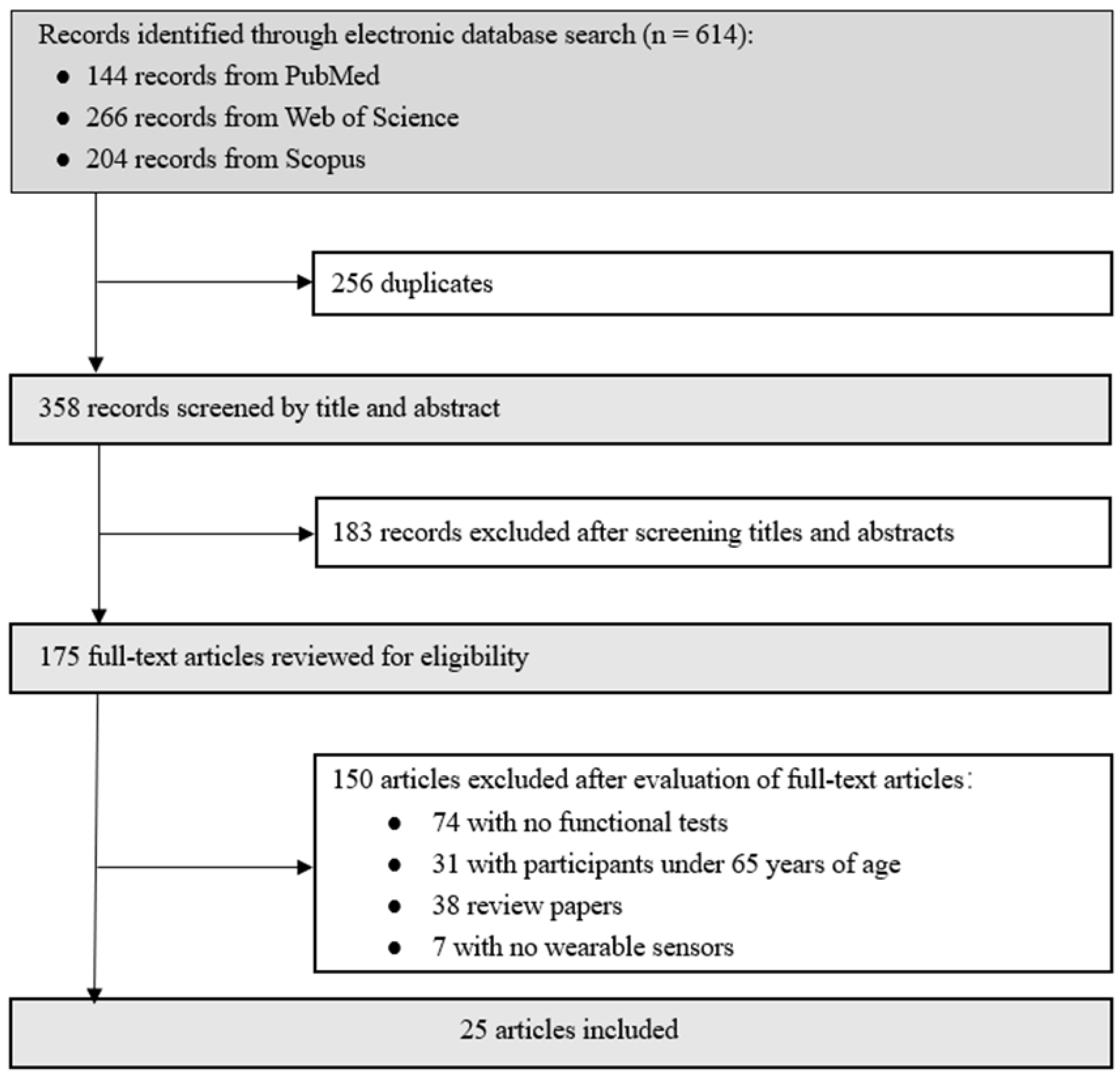
2.3. Study Selection
2.4. Data Extraction
3. Results
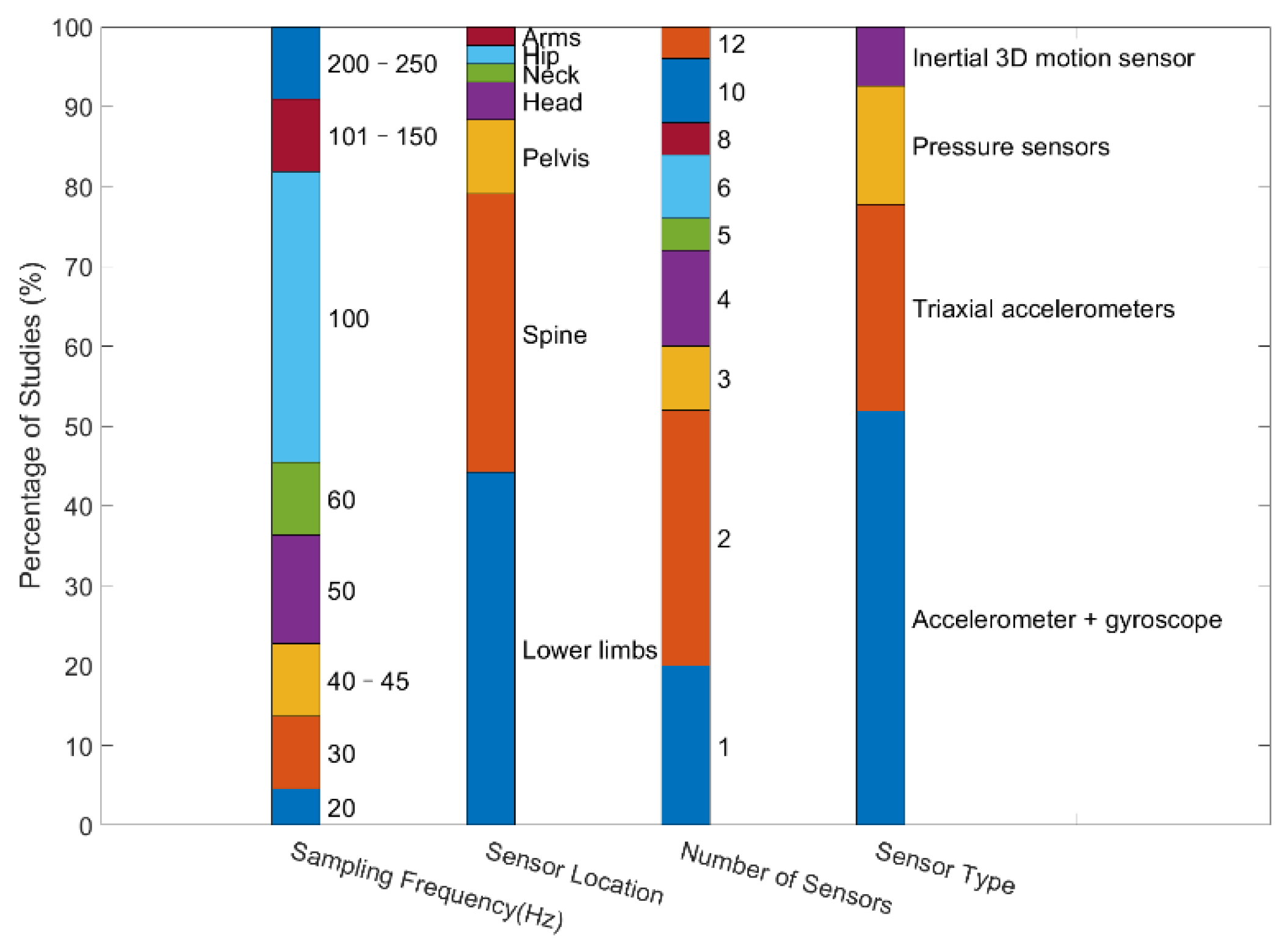
3.1. Wearable Sensors
3.2. Functional Tests
3.3. Data Processing and Feature Construction
3.4. Predictive Method for Fall Risk Assessment
3.5. Features Used for Modeling
3.6. Evaluation Metrics
4. Discussion
4.1. Participants
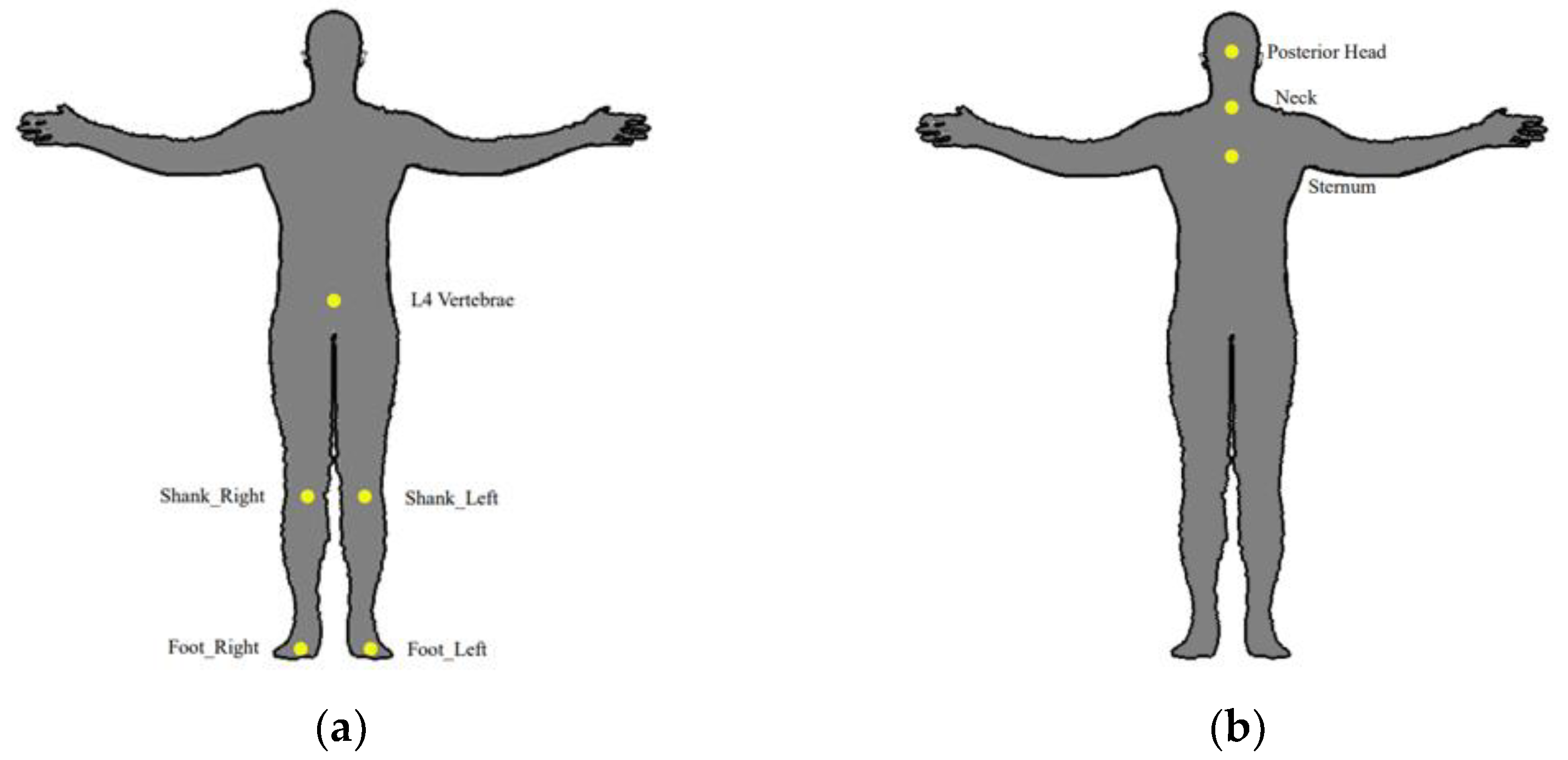
4.2. Sensor Location
4.3. Response Variables
4.4. Fall Risk Modelling Method
4.5. Extracted Features
4.6. Non-Wearable Sensors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC Older Adult Fall Prevention. Available online: https://www.cdc.gov/falls/ (accessed on 10 June 2022).
- Population Ageing: An Inescapable Future. Available online: https://www.globalissues.org/news/2022/01/05/29746 (accessed on 5 June 2022).
- Ravindran, R.M.; Kutty, V.R. Risk Factors for Fall-Related Injuries Leading to Hospitalization Among Community-Dwelling Older Persons: A Hospital-Based Case-Control Study in Thiruvananthapuram, Kerala, India. Asia Pac. J. Public Health 2016, 28, 70S–76S. [Google Scholar] [CrossRef]
- Usmani, S.; Saboor, A.; Haris, M.; Khan, M.A.; Park, H. Latest Research Trends in Fall Detection and Prevention Using Machine Learning: A Systematic Review. Sensors 2021, 21, 5134. [Google Scholar] [CrossRef] [PubMed]
- Laurence, B.D.; Michel, L. The Fall in Older Adults: Physical and Cognitive Problems. Curr. Aging Sci. 2017, 10, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Bihl, T.; Bresciani, J.P. Identifying Fall Risk Predictors by Monitoring Daily Activities at Home Using a Depth Sensor Coupled to Machine Learning Algorithms. Sensors 2021, 21, 1957. [Google Scholar] [CrossRef]
- Cho, H.; Heijnen, M.; Craig, B.A.; Rietdyk, S. Falls in young adults: The effect of sex, physical activity, and prescription medications. PLoS ONE 2021, 16, e0250360. [Google Scholar]
- Umegaki, H.; Makino, T.; Uemura, K.; Shimada, H.; Cheng, X.W.; Dodge, H.; Kuzuya, M. Falls in community-dwelling prefrail older adults. Health Soc. Care Community 2020, 28, 110–115. [Google Scholar] [CrossRef]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef]
- Park, S.H. Tools for assessing fall risk in the elderly: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Miake-Lye, I.M.; Hempel, S.; Ganz, D.A.; Shekelle, P.G. Inpatient fall prevention programs as a patient safety strategy: A systematic review. Ann. Intern Med. 2013, 158, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, Y.S. The Diagnostic Accuracy of the Berg Balance Scale in Predicting Falls. West J. Nurs. Res. 2017, 39, 1502–1525. [Google Scholar] [CrossRef]
- Omana, H.; Bezaire, K.; Brady, K.; Davies, J.; Louwagie, N.; Power, S.; Santin, S.; Hunter, S.W. Functional Reach Test, Single-Leg Stance Test, and Tinetti Performance-Oriented Mobility Assessment for the Prediction of Falls in Older Adults: A Systematic Review. Phys. Ther. 2021, 101, 10. [Google Scholar] [CrossRef]
- Wrisley, D.M.; Kumar, N.A. Functional gait assessment: Concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys. Ther. 2010, 90, 761–773. [Google Scholar] [CrossRef]
- Shanahan, C.J.; Boonstra, F.; Cofre, L.L.; Strik, M.; Moffat, B.A.; Khan, F.; Kilpatrick, T.J.; van der Walt, A.; Galea, M.P.; Kolbe, S.C. Technologies for Advanced Gait and Balance Assessments in People with Multiple Sclerosis. Front Neurol. 2017, 8, 708. [Google Scholar] [CrossRef]
- Papagiannis, G.I.; Triantafyllou, A.I.; Roumpelakis, I.M.; Zampeli, F.; Garyfallia, E.P.; Koulouvaris, P.; Papadopoulos, E.C.; Papagelopoulos, P.J.; Babis, G.C. Methodology of surface electromyography in gait analysis: Review of the literature. J. Med. Eng. Technol. 2019, 43, 59–65. [Google Scholar] [CrossRef]
- World Confederation for Physical Therapy/World Physiotherapy. Available online: https://www.apta.org/apta-and-you/leadership-and-governance/policies/wcpt (accessed on 1 June 2022).
- Bezold, J.; Krell-Roesch, J.; Eckert, T.; Jekauc, D.; Woll, A. Sensor-based fall risk assessment in older adults with or without cognitive impairment: A systematic review. Eur. Rev. Aging Phys. A 2021, 18, 15. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Zhang, Y.; Zhu, R. A wearable motion capture device able to detect dynamic motion of human limbs. Nat. Commun. 2020, 11, 5615. [Google Scholar] [CrossRef]
- Fang, F.; Aabith, S.; Homer-Vanniasinkam, S.; Tiwari, M.K. 9—High-resolution 3D printing for healthcare underpinned by small-scale fluidics. In 3D Printing in Medicine; Kalaskar, D.M., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 167–206. [Google Scholar]
- Rucco, R.; Sorriso, A.; Liparoti, M.; Ferraioli, G.; Sorrentino, P.; Ambrosanio, M.; Baselice, F. Type and Location of Wearable Sensors for Monitoring Falls during Static and Dynamic Tasks in Healthy Elderly: A Review. Sensors 2018, 18, 1613. [Google Scholar] [CrossRef]
- Haescher, M.; Chodan, W.; Hopfner, F.; Bieber, G.; Aehnelt, M.; Srinivasan, K.; Murphy, M.A. Automated fall risk assessment of elderly using wearable devices. J. Rehabil. Assist Technol. Eng. 2020, 7, 2055668320946209. [Google Scholar] [CrossRef]
- Fino, P.C.; Frames, C.W.; Lockhart, T.E. Classifying step and spin turns using wireless gyroscopes and implications for fall risk assessments. Sensors 2015, 15, 10676–10685. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Lockhart, T.E. Fall risk assessments based on postural and dynamic stability using inertial measurement unit. Saf. Health Work 2012, 3, 192–198. [Google Scholar] [CrossRef]
- Sample, R.B.; Kinney, A.L.; Jackson, K.; Diestelkamp, W.; Bigelow, K.E. Identification of key outcome measures when using the instrumented timed up and go and/or posturography for fall screening. Gait Posture 2017, 57, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Prospective Fall-Risk Prediction Models for Older Adults Based on Wearable Sensors. IEEE Trans Neural. Syst. Rehabil. Eng. 2017, 25, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ko, S.; Lee, S.; Lee, J.A.; Kim, K. Quantitative Assessment of Balance Impairment for Fall-Risk Estimation Using Wearable Triaxial Accelerometer. IEEE Sens. J. 2017, 17, 6743–6751. [Google Scholar] [CrossRef]
- FallSkip—Technology to Evaluate Fall Risk in Older Adults. Available online: http://fallskip.com/en/ (accessed on 5 June 2022).
- Comprehensive Gait and Balance Analysis. Available online: https://apdm.com/mobility/?gclid=EAIaIQobChMIwpbqou_P-QIV4D6tBh3unATjEAAYAiAAEgLkgvD_BwE (accessed on 15 June 2022).
- Mancini, M.; Horak, F.B. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev. Med. Devices 2016, 13, 455–462. [Google Scholar] [CrossRef]
- Morris, R.; Stuart, S.; McBarron, G.; Fino, P.C.; Mancini, M.; Curtze, C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol. Meas. 2019, 40, 095003. [Google Scholar] [CrossRef]
- Greene, B.R.; Redmond, S.J.; Caulfield, B. Fall Risk Assessment Through Automatic Combination of Clinical Fall Risk Factors and Body-Worn Sensor Data. IEEE J. Biomed. Health Inform. 2017, 21, 725–731. [Google Scholar] [CrossRef]
- Drover, D.; Howcroft, J.; Kofman, J.; Lemaire, E.D. Faller Classification in Older Adults Using Wearable Sensors Based on Turn and Straight-Walking Accelerometer-Based Features. Sensors 2017, 17, 1321. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Lemaire, E.D.; Kofman, J. Prospective elderly fall prediction by older-adult fall-risk modeling with feature selection. Biomed Signal Proc. 2018, 43, 320–328. [Google Scholar] [CrossRef]
- Qiu, H.; Rehman, R.; Yu, X.; Xiong, S. Application of Wearable Inertial Sensors and A New Test Battery for Distinguishing Retrospective Fallers from Non-fallers among Community-dwelling Older People. Sci. Rep. 2018, 8, 16349. [Google Scholar] [CrossRef] [PubMed]
- Hellmers, S.; Izadpanah, B.; Dasenbrock, L.; Diekmann, R.; Bauer, J.M.; Hein, A.; Fudickar, S. Towards an Automated Unsupervised Mobility Assessment for Older People Based on Inertial TUG Measurements. Sensors 2018, 18, 3310. [Google Scholar] [CrossRef]
- Ghahramani, M.; Stirling, D.; Naghdy, F.; Naghdy, G.; Potter, J. Body postural sway analysis in older people with different fall histories. Med. Biol. Eng. Comput. 2019, 57, 533–542. [Google Scholar] [CrossRef]
- Buisseret, F.; Catinus, L.; Grenard, R.; Jojczyk, L.; Fievez, D.; Barvaux, V.; Dierick, F. Timed Up and Go and Six-Minute Walking Tests with Wearable Inertial Sensor: One Step Further for the Prediction of the Risk of Fall in Elderly Nursing Home People. Sensors 2020, 20, 3207. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, Y.; Wang, H.; Sun, T.; Murphy, T.E.; Tsui, K. Assessing elderly’s functional balance and mobility via analyzing data from waist-mounted tri-axial wearable accelerometers in timed up and go tests. Bmc. Med. Inform. Decis. 2021, 21, 1. [Google Scholar] [CrossRef]
- Lockhart, T.E.; Soangra, R.; Yoon, H.; Wu, T.; Frames, C.W.; Weaver, R.; Roberto, K.A. Prediction of fall risk among community-dwelling older adults using a wearable system. Sci. Rep. UK 2021, 11, 1. [Google Scholar] [CrossRef]
- Diao, Y.; Lou, N.; Liang, S.; Zhang, Y.; Ning, Y.; Li, G.; Zhao, G. A Novel Environment-Adaptive Timed Up and Go Test System for Fall Risk Assessment With Wearable Inertial Sensors. IEEE Sens. J. 2021, 21, 18287–18297. [Google Scholar] [CrossRef]
- Choi, J.; Parker, S.M.; Knarr, B.A.; Gwon, Y.; Youn, J.H. Wearable Sensor-Based Prediction Model of Timed up and Go Test in Older Adults. Sensors 2021, 21, 6831. [Google Scholar] [CrossRef] [PubMed]
- Atrsaei, A.; Paraschiv-Ionescu, A.; Krief, H.; Henchoz, Y.; Santos-Eggimann, B.; Büla, C.; Aminian, K. Instrumented 5-Time Sit-To-Stand Test: Parameters Predicting Serious Falls beyond the Duration of the Test. Gerontology 2021, 21, 6831. [Google Scholar] [CrossRef] [PubMed]
- Bet, P.; Castro, P.C.; Ponti, M.A. Foreseeing future falls with accelerometer features in active community-dwelling older persons with no recent history of falls. Exp. Gerontol. 2021, 143, 111139. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ou, J.; Shu, L.; Hu, G.; Wu, S.; Xu, X.; Chen, Z. Fall Risk Assessment for the Elderly Based on Weak Foot Features of Wearable Plantar Pressure. IEEE Trans Neural. Syst. Rehabil. Eng. 2022, 30, 1060–1070. [Google Scholar] [CrossRef]
- Wu, S.; Ou, J.; Shu, L.; Hu, G.; Song, Z.; Xu, X.; Chen, Z. MhNet: Multi-scale spatio-temporal hierarchical network for real-time wearable fall risk assessment of the elderly. Comput. Biol. Med. 2022, 144, 105355. [Google Scholar] [CrossRef]
- Ma, L.; Mi, T.M.; Jia, Q.; Han, C.; Chhetri, J.K.; Chan, P. Gait variability is sensitive to detect Parkinson’s disease patients at high fall risk. Int. J. Neurosci. 2022, 132, 888–893. [Google Scholar] [CrossRef]
- Polus, J.S.; Bloomfield, R.A.; Vasarhelyi, E.M.; Lanting, B.A.; Teeter, M.G. Machine Learning Predicts the Fall Risk of Total Hip Arthroplasty Patients Based on Wearable Sensor Instrumented Performance Tests. J. Arthroplast. 2021, 36, 573–578. [Google Scholar] [CrossRef]
- Fan, X.; Wang, H.; Zhao, Y.; Huang, K.H.; Wu, Y.T.; Sun, T.L.; Tsui, K.L. Automatic fall risk assessment with Siamese network for stroke survivors using inertial sensor-based signals. Int. J. Intell. Syst. 2022, 37, 6168–6184. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Zhao, Y.; Huang, K.H.; Wu, Y.T.; Cabrera, J.; Sun, T.L.; Tsui, K.L. A Novel Approach for Fall Risk Prediction Using the Inertial Sensor Data From the Timed-Up-and-Go Test in a Community Setting. IEEE Sens. J. 2020, 20, 9339–9350. [Google Scholar] [CrossRef]
- Tunca, C.; Salur, G.; Ersoy, C. Deep Learning for Fall Risk Assessment With Inertial Sensors: Utilizing Domain Knowledge in Spatio-Temporal Gait Parameters. IEEE J. Biomed Health Inform. 2020, 24, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Roshdibenam, V.; Jogerst, G.J.; Butler, N.R.; Baek, S. Machine Learning Prediction of Fall Risk in Older Adults Using Timed Up and Go Test Kinematics. Sensors 2021, 21, 3481. [Google Scholar] [CrossRef]
- Dierick, F.; Stoffel, P.L.; Schutz, G.; Buisseret, F. High Specificity of Single Inertial Sensor-Supplemented Timed Up and Go Test for Assessing Fall Risk in Elderly Nursing Home Residents. Sensors 2022, 22, 2339. [Google Scholar] [CrossRef]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef]
- Sai, A.J.; Gallagher, J.C.; Smith, L.M.; Logsdon, S. Fall predictors in the community dwelling elderly: A cross sectional and prospective cohort study. J. Musculoskel. Neuron 2010, 10, 142–150. [Google Scholar]
- Sun, W.; Hao, B.; Yu, Y.; Li, J.; Hu, G.; Xie, G. Feature++: Automatic Feature Construction for Clinical Data Analysis. Stud. Health Technol. Inform. 2016, 228, 547–551. [Google Scholar]
- Ferrada, X.; Serpell, A.; Skibniewski, M. Selection of construction methods: A knowledge-based approach. Sci. World J. 2013, 2013, 938503. [Google Scholar] [CrossRef]
- Xi, X.; Tang, M.; Miran, S.M.; Luo, Z. Evaluation of Feature Extraction and Recognition for Activity Monitoring and Fall Detection Based on Wearable sEMG Sensors. Sensors 2017, 17, 1229. [Google Scholar] [CrossRef] [PubMed]
- Caby, B.; Kieffer, S.; de Saint, H.M.; Cremer, G.; Macq, B. Feature extraction and selection for objective gait analysis and fall risk assessment by accelerometry. Biomed Eng. Online 2011, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoltzfus, J.C. Logistic regression: A brief primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Dominguez-Almendros, S.; Benitez-Parejo, N.; Gonzalez-Ramirez, A.R. Logistic regression models. Allergol. Immunopathol. Madr. 2011, 39, 295–305. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, L.; Jimenez, A.R.; Garcia-Villamil, G.; Seco, F. Detecting Fall Risk and Frailty in Elders with Inertial Motion Sensors: A Survey of Significant Gait Parameters. Sensors 2021, 21, 6918. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Review of fall risk assessment in geriatric populations using inertial sensors. J. Neuroeng. Rehabil. 2013, 10, 91. [Google Scholar] [CrossRef]
- Porta, S.; Martinez, A.; Millor, N.; Gomez, M.; Izquierdo, M. Relevance of sex, age and gait kinematics when predicting fall-risk and mortality in older adults. J. Biomech. 2020, 105, 109723. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Nait, A.A.; Englebienne, G.; van Schooten, K.S.; Pijnappels, M.; Krose, B. Deep Learning to Predict Falls in Older Adults Based on Daily-Life Trunk Accelerometry. Sensors 2018, 18, 1654. [Google Scholar] [CrossRef]
- Espy, D.D.; Yang, F.; Bhatt, T.; Pai, Y.C. Independent influence of gait speed and step length on stability and fall risk. Gait Posture 2010, 32, 378–382. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Muir, S.W.; Hall, M.; Doherty, T.J.; Kloseck, M.; Beauchet, O.; Speechley, M. Gait variability is associated with frailty in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Blizzard, L.; McGinley, J.L.; Schmidt, M.D.; Srikanth, V.K. Sensorimotor Factors Affecting Gait Variability in Older People-A Population-Based Study. J. Gerontol. Biol. 2010, 65, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afendi, T.; Kurugollu, F.; Crookes, D.; Bouridane, A. A frontal view gait recognition based on 3D imaging using a time of flight camera. In Proceedings of the 2014 22nd European Signal Processing Conference (EUSIPCO), Lisbon, Portugal, 1–5 September 2014; pp. 2435–2439. [Google Scholar]
- Rantz, M.; Skubic, M.; Abbott, C.; Galambos, C.; Popescu, M.; Keller, J.; Stone, E.; Back, J.; Miller, S.J.; Petroski, G.F. Automated In-Home Fall Risk Assessment and Detection Sensor System for Elders. Gerontologist 2015, 55 (Suppl. 1), S78–S87. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Author (Year) | Participant (Number, Age) | Response Variables | Functional Tests | Number of Sensors | Wearable Sensor Type | Sensor Location | Frequency (Hz) | Feature Engineering | Model | Accuracy | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample et al. (2017) [25] | 150 (74.35 ± 9.00; 91 NF, 59 F) | Retrospective | TUG 1 | 8 | IMU 2 (accelerometer + gyroscope) | Spine (chest), spine (lower back), each foot | - | Y | Stepwise logistic regression | - | 82.10% | 48.10% |
| Howcroft et al. (2017) [26] | 75 (75.2 ± 6.6; 47 NF, 28 F) | Prospective fall risk prediction | 7.62 m under single- and dual-task conditions, 6MWT 3 | 6 | Pressure-sensing insoles and triaxial accelerometers | Head, pelvis, left and right shanks, feet | 50, 120 | Y | Neural network | 57% | 65% | 43% |
| Greene et al. (2017) [32] | 422 (75.4) | 1-year fall history | TUG 1 | 4 | IMU 2 (accelerometer + gyroscope) | Shanks | 102.4 | Y | Regularized discriminant classifier | 72.7% | 54.50% | 90.91% |
| Shahzad et al. (2017) [27] | 23 (72.87 ± 8) | BBS 17 | DR 4 tasks twice (TUG 1, FTSS 5, AST 6) | 1 | Triaxial accelerometer | Spine (lower back) | 41 | Y | Lasso regression | - | - | - |
| Drover et al. (2017) [33] | 76 (74.15 ± 7.0) | 6-month follow-up prospective fall | 6MWT 3 | 3 | Triaxial accelerometer | Posterior pelvis, left and right lateral shanks | 50 | Y | Random forest | 77.30% | 84.70% | 66.10% |
| Howcroft et al. (2018) [34] | 75 (75.2 ± 6.6) | 6-month follow-up prospective fall | 7.62-m walk | 5 | Pressure-sensing insoles, triaxial accelerometer | Insole, left shank, pelvis, head | 100 | Y | Relief-F, SVM 13 | 94.40% | 100.00% | 85.70% |
| HaiQiu et al. (2018) [35] | 196 | Retrospective | SIT 7, LOS 8, 5STS 9, MF 10, CRT 11, FES 12, 3-m TUG 1 | 10 | IMU 2 (accelerometer + gyroscope) | Spine (low back), upper and lower legs | 100 | Y | SVM 13 | 89.40% | 84.90% | 92.70% |
| Hellmers et al. (2018) [36] | 157 (75.22 ± 3.83) | SPPB 14, SCPT 15, 6MWT 3, frailty criteria, counter movement lump | aTUG 27 | 2 | IMU 2 (accelerometer + gyroscope) | Hip | 100 | Y | Hierarchical classification model | 96% | - | - |
| Ghahramani et al. (2019) [37] | 86 (80.4 ± 7.9) | Fall history | Five common standing tests, BBS 17 | 1 | Inertial 3D motion sensor (MTw from Xsens technology) | Pelvis | 50 | Y | GMM 18, EM 19, MML 20 | - | 75.7% and 77.7% 34 | 78.6% and 82.1% 34 |
| Buisseret et al. (2020) [38] | 73 (>65) | 6-month follow-up prospective fall | TUG 1, 6MWT 3 | 2 | IMU 2 (accelerometer + gyroscope) | Spine (lower back) | 100 | N | CNN 21 | 76% | - | - |
| Yu et al. (2021) [39] | 85 (69–105) | SFBBS 31 | TUG 1 | 1 | Triaxial accelerometer | Spine (lower back) | 45 | Y | Lasso regression | - | 79% | 74% |
| Lockhart et al. (2021) [40] | 171 (74.3 ± 7.6) | 6-month follow-up prospective fall | 10-m walking test | 1 | Triaxial accelerometer | Spine (sternum) | 100 | Y | PCA 22, Random Forest predictive model | 81.6 ± 0.7% | 80.3 ± 0.2% | 86.7 ± 0.5% |
| Diao et al. (2021) [41] | 103 | Questionnaires, BBS 17 | EATUG 23 | 4 | IMU 2 (accelerometer + gyroscope) | Shanks (15 cm below knee joint) | 60 | Y | SVM 13 | 90.50% | 92.90% | 85.70% |
| Choi et al. (2021) [42] | 37 (69.6 ± 4.3) | 3-m TUG 1 | Walking a circular sidewalk route for 3 min | 3 | Inertial 3D motion sensor (MTw from Xsens technology) | Pelvis and feet | 60 | Y | Ridge regression | - | - | - |
| Atrsaei et al. (2021) [43] | 458 | 12-month follow-up prospective fall | 5STS 9 | 2 | IMU 2 (accelerometer + gyroscope) | Spine (sternum) | 200 | Y | Logistic regression | - | 69% | 56% |
| Bet et al. (2021) [44] | 74 | 12-month follow-up prospective fall | Variants of TUG 1: TUG-S 24, TUG-M 25, TUG-D 26 | 1 | Triaxial accelerometer | Spine (waist) | 100 | Y | N (Shapiro-Wilk normality test) | 75% | 76% | 71% |
| Song et al. (2022) [45] | 48 | BBS 17 | 20-m long walk for over 2 min | 2 | Pressure sensors | Insole | 20 | Y | DT 28, GBDT 30, AdaBoost | 87.5% | 75% | 100% |
| Wu et al. (2022) [46] | 48 (74.5 ± 6.7) | BBS 17, TUG 1, fall history | Walk for at least 2 min | 2 | Pressure sensors | Insole | - | N | MhNet | 73.27% | 70.4% | 76.72% |
| Lin et al. (2020) [47] | 51 PD 29 patients (65.7 ± 8.4) | 6-month follow-up prospective fall | 7-m TUG 1 | 12 | IMU 2 (accelerometer + gyroscope) | Feet, spine (trunk), spine (sternum), arms | - | Y | Binary logistic regression | - | 78.40% | 71.40% |
| Polus et al. (2021) [48] | 72 patients following total hip arthroplasty (71.87 ± 6.45) | TUG 1 | TUG 1 | 10 | IMU 2 (accelerometer + gyroscope) + iPod touch (3D 16 gyroscope + MEMS 32 accelerometer) | Above and below each knee | - | Y | PCA 22, SVM 13 | 90% | 59% | 93% |
| Xiaomao et al. (2021) [49] | 105 stroke survivors (56 ± 14) | SFBBS 31 | 3-m TUG 1 | 2 | IMU 2 (accelerometer + gyroscope) | Spine (back trunk) | 30 | Y | Siamese network | 85% ± 6% | - | - |
| Yu-Cheng et al. (2020) [50] | 50 post-stroke patients (57.4 ± 14.13) | SFBBS 31 | 3-m TUG 1 | 2 | IMU 2 (accelerometer + gyroscope) | Spine (L4 vertebrae) | 30 | Y | Elastic net, logistic regression | 84% | 94% | 64% ± 5% |
| Tunca et al. (2020) [51] | 76 neurological disorder patients (76.8 ± 10.3) | 12-month fall history | Walk back and forth along an 8-m straight line | 4 | IMU 2 (accelerometer + gyroscope) | Dorsum of both feet | 100 | Y | LSTM 33 (gait parameters as input) | 92.10% | - | - |
| Roshdibenam et al. (2021) [52] | 100 patients with different mental or physical impairments (65–96) | TUG 1, 30-s stand, 4-stage balance tests, measurement of orthostatic blood pressure, clinicians’ observations, and SIB score | TUG 1 | 6 | Run Scribe IMU 2 pods (accelerometer + gyroscope) | Right and left feet and neck | 250 | N | CNN 21 | 71% | 55% | 89% |
| Dierick, F et al. (2022) [53] | 73 patients with different mental or physical impairments | 6-month follow-up prospective fall | TUG 1 | 2 | IMU 2 (accelerometer + gyroscope) | Spine (L4 vertebrae) | 100 | Y | Multiple logistic regressions | - | 95.9% | 29.2% |
| Functional Test | Frequency | Description |
|---|---|---|
| TUG 1 | 12 | Time in seconds taken by the individual to get up from a chair without support, walk straight for 3 m, turn, walk back the 3 m, and sit in the chair without support. |
| Variants of the TUG test: TUG-M 2, TUG-D 3, aTUG, EATUG 4 | 3 | TUG-M: the individual must carry a glass full of water. TUG-D: the individual must perform both a motor and a cognitive task; the motor task is to transfer coins between two pockets of a lab coat and the cognitive task is to calculate successive subtractions of 7, starting from 100, out loud. aTUG: The aTUG system is used for automated TUG tests and includes force sensors (FS) in each chair leg, a laser range scanner (LRS) and a light barrier (LB). EATUG: the individual must bypass and overpass an obstacle, such as ascending and descending stairs. |
| Straight walk | 9 | 6MWT 5: the distance walked in 6 min to the nearest meter is measured; the individual must walk back and forth along a straight line approximately 8 m long; 7.62-m walk test; and 10-m walk test. |
| Five common standing tests | 1 | The individual must: (1) stand with eyes open for 2 min; (2) stand quietly with eyes closed for 30 s; (3) stand on one foot for 10 s without support; (4) stand with feet together for 1 min; and (5) stand with one foot in front of the other with the heel of the forward foot touching the toes of the other foot for 1 min. |
| 5STS 6 | 3 | The total duration to perform postural transitions (sit/stand), traditionally measured by a stopwatch, is used to discriminate between patients with and without balance disorders. |
| AST 7 | 1 | The individual must place the whole of each foot, alternatively and rapidly, on and off of a platform (19 cm high and 40 cm wide). |
| Walking a circular sidewalk route for 3 min | 1 | The individual must walk a circular sidewalk route for 3 min. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wang, H.; Yu, L.; Yeung, E.H.K.; Luo, J.; Tsui, K.-L.; Zhao, Y. A Systematic Review of Wearable Sensor-Based Technologies for Fall Risk Assessment in Older Adults. Sensors 2022, 22, 6752. https://doi.org/10.3390/s22186752
Chen M, Wang H, Yu L, Yeung EHK, Luo J, Tsui K-L, Zhao Y. A Systematic Review of Wearable Sensor-Based Technologies for Fall Risk Assessment in Older Adults. Sensors. 2022; 22(18):6752. https://doi.org/10.3390/s22186752
Chicago/Turabian StyleChen, Manting, Hailiang Wang, Lisha Yu, Eric Hiu Kwong Yeung, Jiajia Luo, Kwok-Leung Tsui, and Yang Zhao. 2022. "A Systematic Review of Wearable Sensor-Based Technologies for Fall Risk Assessment in Older Adults" Sensors 22, no. 18: 6752. https://doi.org/10.3390/s22186752
APA StyleChen, M., Wang, H., Yu, L., Yeung, E. H. K., Luo, J., Tsui, K.-L., & Zhao, Y. (2022). A Systematic Review of Wearable Sensor-Based Technologies for Fall Risk Assessment in Older Adults. Sensors, 22(18), 6752. https://doi.org/10.3390/s22186752









