Abstract
Heat-related illnesses, which range from heat exhaustion to heatstroke, affect thousands of individuals worldwide every year and are characterized by extreme hyperthermia with the core body temperature (CBT) usually > 40 °C, decline in physical and athletic performance, CNS dysfunction, and, eventually, multiorgan failure. The measurement of CBT has been shown to predict heat-related illness and its severity, but the current measurement methods are not practical for use in high acuity and high motion settings due to their invasive and obstructive nature or excessive costs. Noninvasive predictions of CBT using wearable technology and predictive algorithms offer the potential for continuous CBT monitoring and early intervention to prevent HRI in athletic, military, and intense work environments. Thus far, there has been a lack of peer-reviewed literature assessing the efficacy of wearable devices and predictive analytics to predict CBT to mitigate heat-related illness. This systematic review identified 20 studies representing a total of 25 distinct algorithms to predict the core body temperature using wearable technology. While a high accuracy in prediction was noted, with 17 out of 18 algorithms meeting the clinical validity standards. few algorithms incorporated individual and environmental data into their core body temperature prediction algorithms, despite the known impact of individual health and situational and environmental factors on CBT. Robust machine learning methods offer the ability to develop more accurate, reliable, and personalized CBT prediction algorithms using wearable devices by including additional data on user characteristics, workout intensity, and the surrounding environment. The integration and interoperability of CBT prediction algorithms with existing heat-related illness prevention and treatment tools, including heat indices such as the WBGT, athlete management systems, and electronic medical records, will further prevent HRI and increase the availability and speed of data access during critical heat events, improving the clinical decision-making process for athletic trainers and physicians, sports scientists, employers, and military officers.
1. Introduction
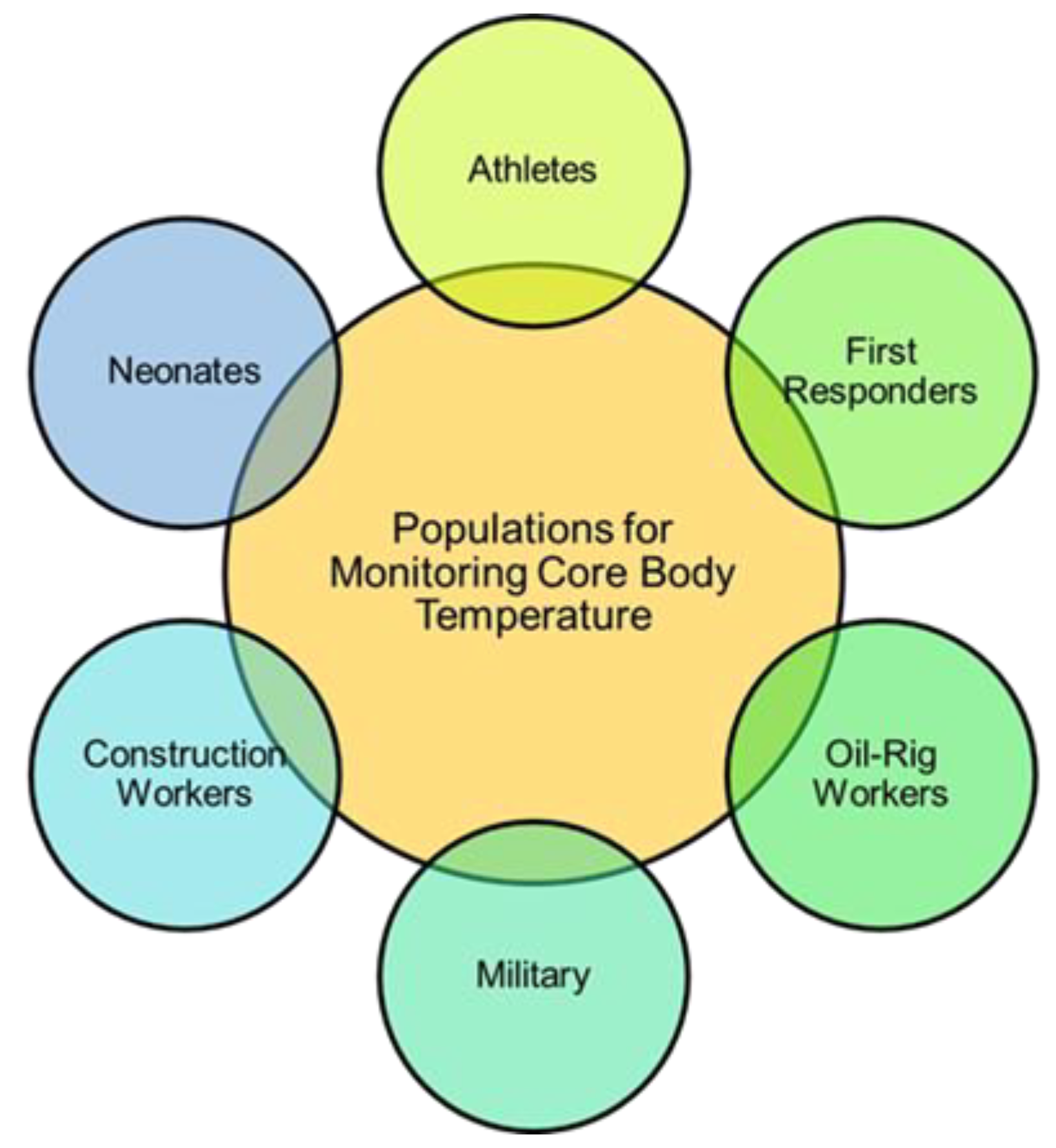
Exertional heat-related illness (HRI) is increasing in incidence as global temperatures rise, with at least 9000 student athletes and 2000 military members affected in the US each year [1,2,3,4]. The spectrum of illness of HRI ranges from heat-induced muscle cramps to life-threatening heat stroke, and a rising core body temperature (CBT) during exercise directly leads to HRI and a subsequent decline in performance [5,6]. Early detection and intervention are critical to improving HRI outcomes, as mortality can drop from 80% in those with delayed cooling to 0% when elevated CBT is detected and reduced to less than 40 °C within 30 min of symptom onset [7,8,9,10,11,12]. Football players, soccer players, long-distance runners, military servicemembers, outdoor and manual workers, and emergency responders are all frequently affected by HRI-related morbidity and mortality (Figure 1) [2,13,14,15,16,17,18,19]. Despite the frequency and potentially fatal consequences of undetected HRI, coaches, trainers, officers, and employers currently rely on visible cues and subjective assessments of their athletes and employees for early detection, such as malaise, confusion, thirst, ataxia, or excessive sweating. This can tragically delay recognition and treatment [7,9,20].
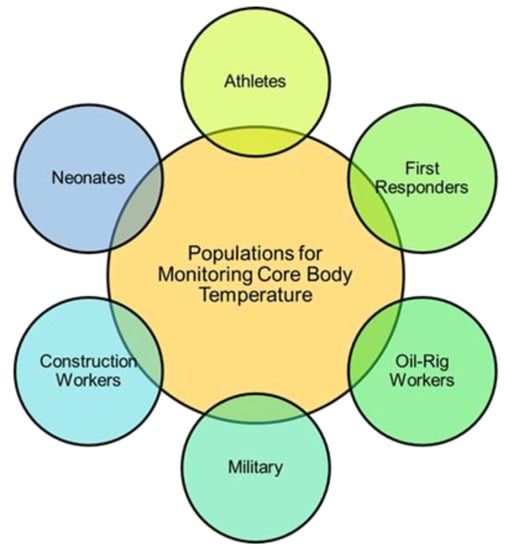
Figure 1.
Populations at a high risk for HRI and, thus, most important for the monitoring of CBT. This review focuses on the monitoring of CBT in athletes and other patients in dynamic environments such as first responders, oil rig and construction workers, and military, as solutions already exist for inpatient and neonate monitoring.
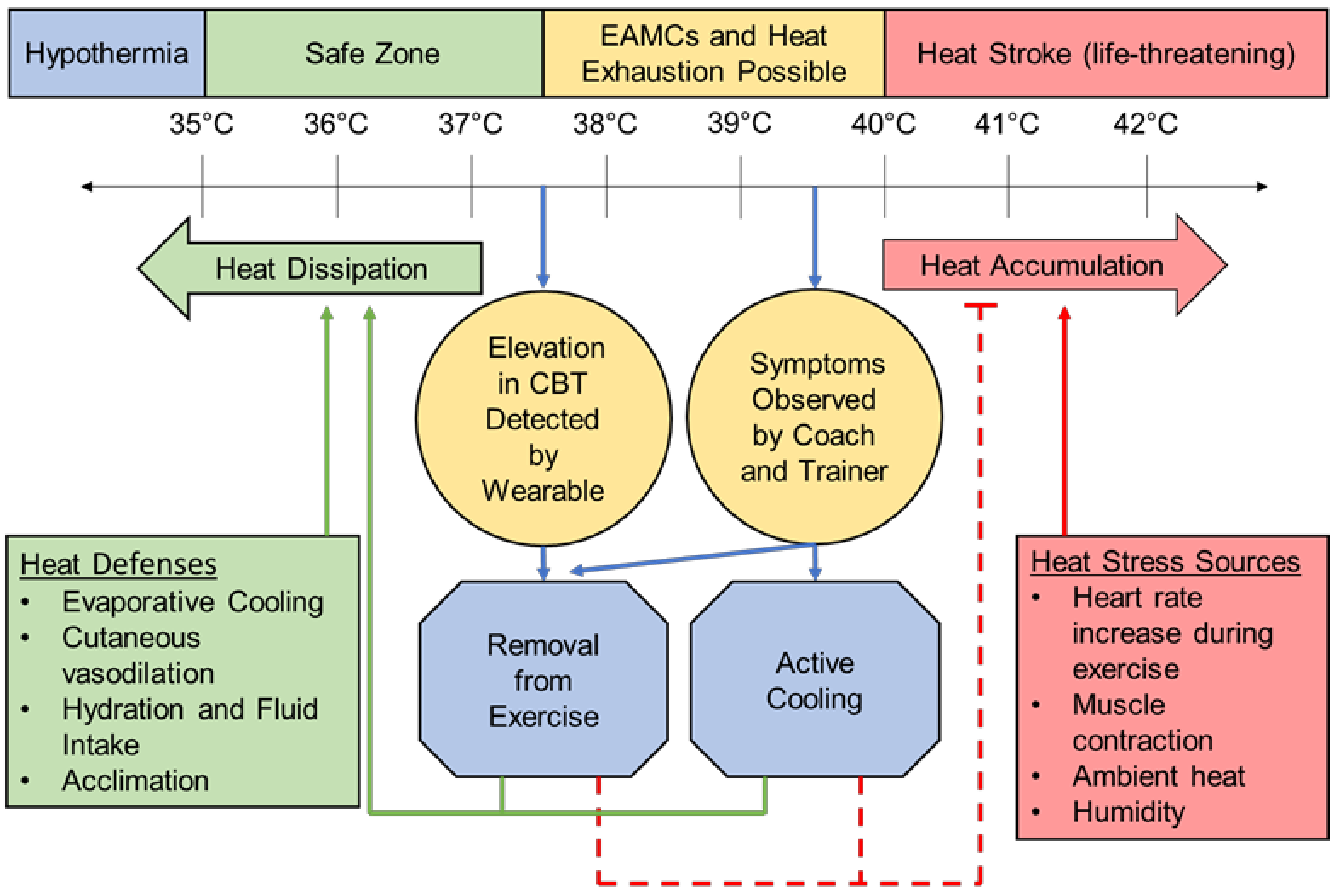
The monitoring of CBT can be instrumental in managing internal and external workloads to prevent the elevation of CBT to the critical temperatures at which HRI occurs and performance declines. A healthy resting CBT is considered to be 37 °C ± 0.5 °C or 98.7 ± 0.9 °F [21], and a slightly elevated CBT of around 38.5 °C/101.3 °F is a normal physiological response to exercise and generally not cause for concern [5,6,22]. Once CBT reaches 40 °C/104 °F, clinical diagnosis and treatment of HRI begins whether or not symptoms are present (Figure 2) [20]. CBT can rise as quickly as 1 °C every 5 min at near-peak exercise intensities due to the heat generated from skeletal muscle contraction, increased metabolic rate, and increased heart rate (HR) [5,22]. Hot and humid climates worsen the rise in CBT and risk for HRI [11], especially if the patient is not acclimatized to exercising in a harsh environment [6].
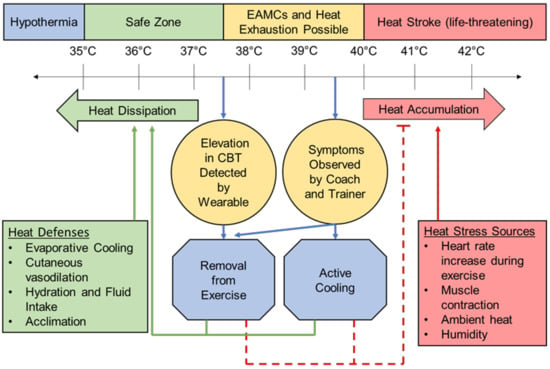
Figure 2.
A schematic representation of the CBT at which heat-related illness occurs [5,6,20,21,22,35]. Includes how wearable CBT measurement systems can provide early intervention to reduce the need for the treatment and, ultimately, morbidity and mortality of HRI. EAMCs = exercise-associated muscle cramps.
Wearable technology is currently playing a role in actively monitoring the physiological status and preventing injury in athletes at all levels, and so, the integration of low-power, high-fidelity predictive analytics with wearable sensors can enable the monitoring of CBT to ultimately provide the early detection and prevention of HRI [23,24,25,26,27]. Current CBT measurement methods during exercise are limited to esophageal, rectal, or telemetric gastrointestinal pill thermometers, which are various combinations of invasive, obstructive to actively exercising athletes, slow to respond to changing CBT, and excessively costly for routine use [28,29]. Other CBT measurement methods used in clinical practice, such as oral, tympanic, axillary skin, and temporal skin, have shown to be unreliable in exercising subjects [30]. Due to these challenges, there has been a significant effort to develop algorithms that provide accurate estimations of CBT by integrating key physiologic parameters such as heart rate, skin temperature, and skin heat flux (Table 1) [29,30,31,32,33,34].

Table 1.
Comparison of methods used to measure CBT in exercising subjects. Summarized from reference [30]. Commercial devices listed are only those marketed or validated as measuring exercising CBT.
CBT has thus far proven challenging to predict via algorithms and noninvasive wearable sensors due to the influence of a myriad of personal, situational, and environmental variables on individual rates of heat accumulation and dissipation. Factors such as gender, age, medication usage, BMI, level of heat acclimatization, exercise workload, sport and position, clothing level, ambient temperature, and ambient humidity all have shown a correlation to an individual’s CBT [22,36]. The risk of HRI is increased in hot and humid environments due to inefficiencies in evaporative cooling mechanisms, but CBT can also reach critical levels in more temperate environments secondary to prolonged high-intensity activity or wearing heavy clothing and equipment that independently prevent effective evaporative cooling [6,11,17,22,28,37,38,39,40,41]. Due to this variability in both individual heat tolerance and the environments in which people experience heat stress, CBT prediction algorithms must be validated on a wide variety of subjects and conditions.
Machine learning (ML) platforms applied to CBT prediction algorithms may provide the opportunity to increase the accuracy of CBT predictions, thereby potentially decreasing the risk of HRI. ML methods have quickly become widespread in medicine and sports science, with applications for reduction of the injury burden already in use in baseball, basketball, soccer, American football, and Australian football [51]. ML can locate and utilize patterns in the data that are difficult or impossible for a human to identify, especially in large, multimodal data sets, and higher predictive power can be expected with increased data input [52]. Causal modeling or dimensionality reduction can be used to distinguish related variables [53,54], which could simplify the process of algorithm development by easily identifying which sensors and at what locations are most correlated with CBT.
The body of literature investigating CBT prediction systems is now growing, and algorithms are becoming more complex. Few of these scientifically evaluated algorithms using wearable devices are being used in practice to reduce the burden of HRI in at-risk populations. Yet, the number of wearable devices that report CBT measurements are increasing on the market. This systematic review provides a comprehensive understanding of how the prediction of CBT using wearable technology has been studied and the accuracy, reliability, and conditions in which CBT predictions have been validated in the literature (e.g., subject characteristics, exercise conditions, study methodology, and generalizability to larger populations). The results disseminating from this review will highlight how CBT is currently being calculated to better understand the gap in translational compatibility of this research into practical systems to prevent HRI and optimize performances in high-risk populations.
2. Materials and Methods
A systematic literature search was conducted in the Web of Science Core Collection database to identify works from 1 January 2000 until 31 December 2021 in which predicted CBT was compared to measured CBT in exercising subjects. This database was selected for its inclusion in both medical and engineering journals. The search used the terms below, where TS stands for topic and searches the title, abstract, author keywords, and Web of Science Keywords Plus for the specified term or terms.
TS = (core body temperature OR core temperature OR deep body temperature) AND TS = (noninvasive OR non-invasive OR wearable OR indirect) AND TS = (exercise* OR hot* OR heat* OR physical*) AND TS = (model* OR predict* or estimate*)
Studies were excluded if they met any of the following criteria:
- non-medical or non-human research;
- systematic and narrative reviews or meta-analyses that did not develop a previously unreported CBT prediction algorithm;
- did not include a wearable device (defined below);
- did not include exercising subjects;
- validated the CBT prediction model with the same data on which it was developed;
- did not use a sufficient comparator method (ingestible telemetric temperature pills, rectal thermometer, or esophageal thermometer);
- did not predict or measure CBT.
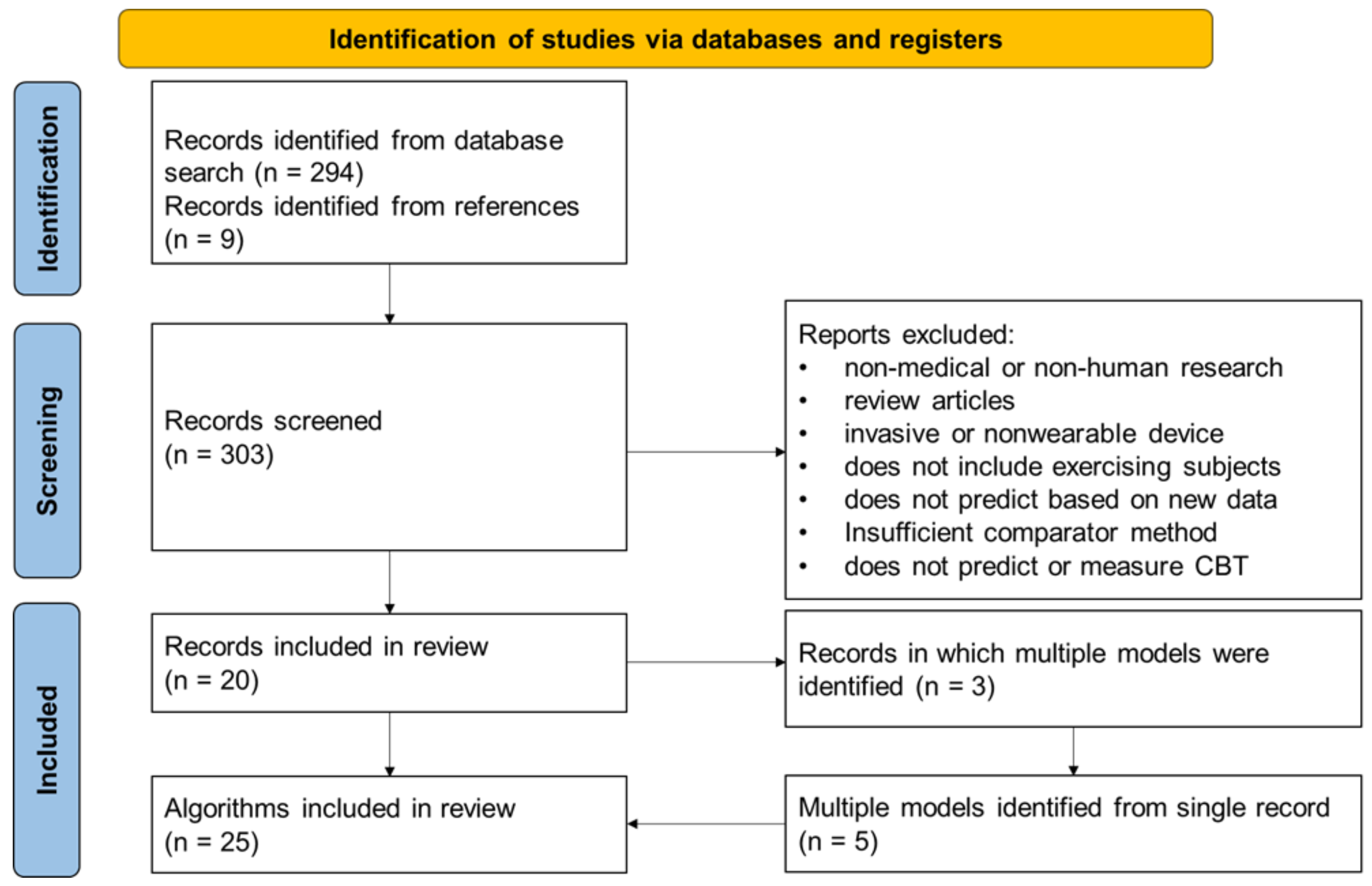
The study selection process and enumeration of CBT prediction algorithms are depicted in Figure 3.
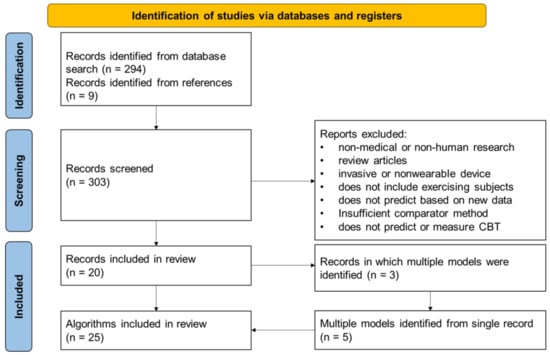
Figure 3.
Flowchart depicting the search results and methodology of the study selection. Additionally depicted is the enumeration of multiple prediction models from some studies.
For this review, wearables were defined as epidermal patches, wrist monitors, and chest straps [54]. Sufficient comparators were defined as ingestible telemetric temperature pills, rectal thermometers, or esophageal thermometers in accordance with the previous literature demonstrating the effectiveness of these methods in measuring the core body temperature [30].
The following data were extracted from papers meeting the inclusion and exclusion criteria:
- Study metrics and methodology, including participant demographics and testing conditions;
- Characteristics of the CBT predictive algorithm, including modeling method and number and locations of sensors;
- Outcome measures of algorithm prediction accuracy, defined as the root mean square error (RMSE), mean difference (MD), or standard error of the estimate (SEE).
Studies were also classified as whether they collected prospective data or retrospective data. The testing conditions were recorded based on the variability of the exercise environment and the exercises performed. Studies in which the testing environment was considered variable included a difference of at least 2.0 °C and/or 10% relative humidity within a testing period or between separate testing periods. Studies in which the exercise intensity or pattern was considered variable between exercises must include at least 2 separate exercises in which the exercise performed, exercise intensity, or duration or sequence of the exercise periods differed.
Each individual algorithm reported and validated by a study was recorded as a separate data point in this review. In cases where values such as age and the RMSE were reported based on differing testing conditions but utilizing the same algorithm, a grand mean of that value was calculated and reported with weighting based on the number of subjects in each condition. Only data from wearables and participants used in the validated algorithm were included. Data from participants or trials used to train or develop algorithms were excluded.
The primary outcome measure collected from the selected studies was the root mean square error or deviation (RMSE or RMSD), a commonly used metric for measuring the goodness-of-fit of a prediction algorithm. The RMSE can be interpreted as the standard deviation of the unexplained variance between the predicted and actual values. It is an absolute measure of fit calculated using the square root of the variance of the residuals and is thus expressed in the same units (°C) as the compared values. A lower RMSE value indicates a better fit [55]. When reported, ± 1 standard deviation (SD) of the RMSE was also recorded.
Where the RMSE was not reported by a study, the mean difference (MD) or standard error of the estimate (SEE) were collected as the outcome measures, along with their respective measures of variability.
3. Results
3.1. Article Search Results
The systematic literature search identified 303 potential studies for analysis, 20 of which reported novel algorithms for CBT predictions using wearable sensors in exercising subjects that met all the inclusion criteria and none of the exclusion criteria. From those 20 study records, a total of 25 CBT prediction algorithms were identified, because 3 of the studies reported multiple algorithms. The root mean square error (RMSE) was reported as an outcome measure in 18 of the 25 algorithms, compared to the mean difference (MD) reported for 5 out of the 25 algorithms and standard error of the estimate (SEE) for 3 out of the 25 algorithms. The data used for validation of the algorithms were collected prospectively with the validation of the algorithm for 20 algorithms, while 6 of the algorithms were validated with retrospective data and one algorithm using both (Table 2).

Table 2.
Methodological and demographic details of the included studies. The clothing level was recorded as follows: light for short sleeve athletic wear, heavy for long sleeves and pants, and occupational for military or other specialized clothing. PC = Prospectively Collected and RC = Retrospectively Collected data. NR = Not Reported, V = Variable, O = Occupational Gear, H = Heavy (long sleeves and pants), and L = light (short sleeves and shorts).
3.2. Aggregate Results
A total of 592 unique subjects were used to validate the analyzed algorithms, with 211 subjects being used to validate more than one algorithm. The average sample size used to validate an algorithm was 32.1 ± 43.2 subjects, and the average age and mass of the subjects were 26.4 ± 4.7 years and 76.6 ± 4.4 kg, respectively (Table 2). The largest sample size used to validate an algorithm was 166 subjects [56], and the smallest sample size was 4 subjects [29]. All studies that reported the sex of their participants included males, with 7 out of 25 algorithms also reporting female subjects in validation. Variable exercise conditions (temperature and relative humidity differences greater than 2.0 °C and/or 10%) were reported in 20 out of 25 algorithms. Variable exercise sequences or intensities were reported in 16 out of 25 algorithms. The telemetric gastrointestinal thermometer pill was the most used comparator device, reported in 17 out of 25 algorithms, followed by a rectal thermometer was reported in 12. Several algorithms were validated using multiple different comparator methods.
Due to the much lower number of algorithms reporting the MD or SEE as compared to the RMSE, and the inability to directly compare these measures to the RMSE, subsequent analyses were performed solely on algorithms reporting the RMSE. In addition, the outlier data from Tsadok et al. DW was excluded from further analysis due to the extremely high RMSE compared to all other data points.
3.3. Results from Studies Reporting RMSE
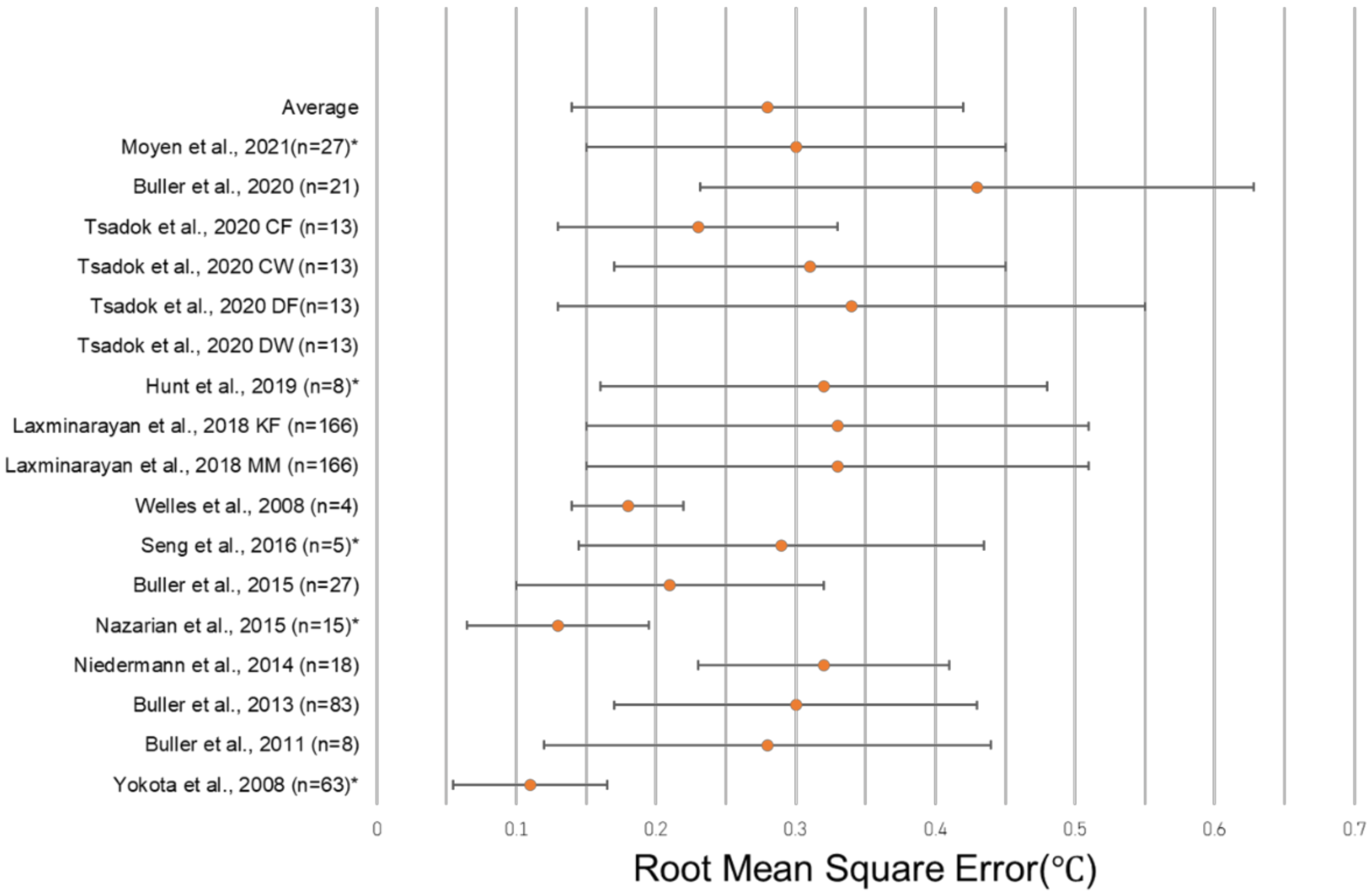
The unweighted average RMSE in the 18 algorithms in which it was reported was 0.38 °C, and the average standard deviation of the RMSE among those algorithms was 0.23 °C. The unweighted average RMSE decreased to 0.28 °C if a single outlier (1.97 °C in Tsadok et al. DW) was removed, and the average RMSE SD similarly decreased to 0.14 °C if the same outlier (1.26 °C in Tsadok et al. DW) was removed. A total of four algorithms reporting the RMSE utilized HR sensors alone to predict CBT, with the average RMSE and SD reported as 0.32 °C ± 0.15 °C across 139 subjects (Table 3). Algorithms using additional sensor modalities, such as skin heat flux, combined with HR reported an average RMSE and SD of 0.25 °C ± 0.13 °C in nine algorithms and 472 subjects. There were three algorithms reviewed that did not include HR, with an average RMSE and SD of 0.29 °C ± 0.15 °C across 39 subjects (Table 3). The most common wearable device location was the chest, with eight algorithms including only a wearable at the chest and two algorithms including a wearable at the chest in addition to devices at other locations. Algorithms with only a wearable at the chest had an average RMSE and SD of 0.29 °C ± 0.14 °C across 322 subjects, while those with a wearable at the chest plus at least one other device reported an average 0.32 °C ± 0.13 °C with 184 subjects. No wearable at the chest was included in five of the algorithms, and the average RMSE of these algorithms was 0.26 °C ± 0.15 °C across 81 subjects. A single wearable device was reported in 11 algorithms, with an average value of 0.27 °C ± 0.15 °C across 269 subjects. Multiple device locations were used in four algorithms with an average RMSE reported as 0.29 °C ± 0.12 °C with 354 subjects. The greatest degree of accuracy (lowest RMSE value) was observed in an algorithm that used a single wearable at the wrist, reporting a 0.13 °C RMSE in 15 subjects [46]. The greatest degree of accuracy (lowest RMSE value) was observed in an algorithm that used a single wearable at the wrist, reporting a 0.13 °C RMSE in 15 subjects [46] (Figure 4). One study did not report the location of the sensors used in its algorithm (Table 4).

Table 3.
Comparison of the overarching design principles. One study did not report the sensor location. Model numbers prior to the parentheses indicate the number of models reporting the RMSE and found in Table 4 only, while the model numbers in parentheses include models listed in Table 4 and Table 5 reporting the RMSE, MD, and SEE. Values in italics indicate the outlier Tsadok et al. DW [47] was excluded from that data set.
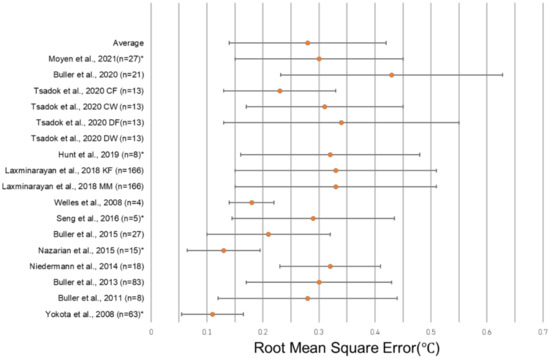
Figure 4.
Forest plot depicting the RMSE ± standard deviation of studies reporting this metric. Tsadok et al. DW [47] is excluded both from the plot and from the displayed average due to its status as an outlier. The asterisks (*) denote models that did not report a RMSE SD, and so, the error bars represent ± ½ of the reported RMSE. [29,31,32,34,37,46,56,58,59,62,64,66].

Table 4.
Data on the model design and outcomes for models that reported the root mean square estimate (RMSE). NR: Not Reported and V = Variable.
3.4. Results from Studies Reporting MD or SEE
The mean difference (MD) was reported for 5 out of the 25 algorithms while the standard error of the estimate (SEE) was reported for 3 out of the 25 algorithms. The lowest MD, i.e., greatest degree of accuracy, was seen in the proprietary ML model using one wearable at the chest at 0.16. The lowest SEE, i.e., greatest degree of accuracy, was seen in an algorithm using bootstrap modeling with a neck wearable at 0.2 (Table 5).

Table 5.
Data on the model design and outcomes for models that reported the mean difference or standard error of the estimate.
4. Discussion
This systematic review investigated the wearable devices and algorithms used in CBT prediction today to better understand the accuracy and reliability of the existing methodologies. In addition, this review identified areas where future research and developments should be directed to improve the translatability of this technology to practical HRI prevention strategies. Our results show that multiple wearable devices ranging from wristwatches to ear sensors are being used in CBT prediction algorithms that utilize steady-state Kalman filters, regression, and ML methods such as Leave-One-Out Cross-Validation (LOOCV). A high accuracy was observed among published algorithms, with 17 out of 18 algorithms meeting a previously reported clinical acceptance threshold of a RMSE less than or equal to 0.5 °C [32,33,37,60]. The average RMSE of the 18 algorithms reporting this value in this report was 0.28 ± 0.14 °C after removal of the outlier, Tsadok et al. DW, which reported an RMSE of 1.97 °C or nearly five times the next-highest reported RMSE, likely due to its reliance on a single, distal measurement site, single sensor, and proprietary algorithm. This outlier was the only algorithm reporting an RMSE above 0.5 °C. These critical findings demonstrate that accurate CBT predictions using wearable devices is achievable under controlled, prescribed conditions, but algorithms must be validated in a wide range of conditions and subjects. Increasing the number of wearable devices worn did not appear to correlate with the increased accuracy in this review (RMSE 0.29 ± 0.15 °C in 233 subjects for single-device algorithms versus 0.29 ± 0.12 °C in 354 subjects for multiple-device algorithms; Table 4), showing that sufficient data can be collected for CBT prediction from a single wearable device.
High accuracy across variable conditions and subjects was observed in the two algorithms in this review that reported the use of ML methods [31,57]. The first algorithm included ambient humidity and temperature sensors with skin temperature and heart rate in a single wearable device, the KENZEN, along with user-input individual characteristics, including age, mass in kilograms, and biological sex [31]. This algorithm showed a high degree of accuracy across variable conditions and exertion levels ranging from 13.4 to 43.2 °C and 32 to 110% predicted max HR (RMSE = 0.30 °C) when tested on 27 subjects [57]. The study used LOOCV to evaluate and compare the accuracy of multiple ML algorithms. The other algorithm using ML methods in this review used a proprietary ML method with the CORE device to predict CBT from the HR, skin temperature, and heat flux in consistent environmental conditions. This study reported the lowest MD and thus highest accuracy of the algorithms reporting that measure but could not be compared against the algorithms reporting the RMSE. In addition, this study did not validate the algorithm in varying environmental conditions or include environmental variables in the algorithm [57]. It also must be noted that, as a proprietary device and algorithm, the exact methods being used are unknown; thus, transparency in methods, development, accuracy, and reliability is critical to using such devices for clinical decision-making. While these studies showed immense promise for the use of ML methods to predict CBT due to the high accuracy observed, accurate predictions in controlled clinical and laboratory settings and in similar subjects may not translate to the variable conditions of a dynamic external environment [30,36]. Thus, the clinical validation of these algorithms in variable environments and larger, more diverse subject pools is still required.
The above studies using ML methods reflected similar limitations to others in this review, namely the lack of validity in dynamic real-world environments and lack of diverse subject pools. Even though 21 out of 25 total algorithms in this review conducted validations in at least two different environmental conditions (ambient humidity and temperature), many of these validations were in laboratory settings rather than in the field. Additionally, all but eight algorithms limited their validation testing to one clothing level [31,34,37,63], and the clothing level was included as a variable in only one algorithm [37]. Environmental conditions were included as variables in only three CBT prediction algorithms reporting the RMSE [31,37,46], including the above-mentioned algorithm using the KENZEN device, and these three algorithms reported a much more accurate average RMSE than algorithms not including environmental variables (0.18 °C across 105 subjects compared to 0.42 °C across 558 subjects). Additionally, the rates of heat accumulation and dissipation and, in turn, individual CBT have shown to be affected by individual variables, including gender, age, level of physical fitness, skin surface to body mass ratio, hydration status, sleep deprivation, and phase of the menstrual cycle [36]. Studies have also shown that the chances of HRI are increased by up to 40% in those with previous episodes of HRI; skin conditions increasing heat storage; cardiac or thyroid conditions preventing proper thermoregulation; those experiencing alcohol or opioid withdrawal; and those taking medications that affect the body’s ability to thermoregulate, including anticholinergics, antipsychotics, antihistamines, and stimulants [9,10,28,35]. However, the subject pools in the reported studies did not show much variability, with the average subject age and mass ranging from 21.6 to 31.1 years and 72.1 to 81.0 kg, only a third of the reviewed algorithms including female subjects in addition to male and most studies standardizing the results by excluding participants with medical conditions or taking medication. Only one algorithm included subject characteristics or demographics as variables [31]. While the reported results were accurate within their constrained conditions and populations, future algorithms must be validated across varied environments and populations at high risk for HRI, including athletes, soldiers, first responders, and those working physically intensive occupations. Ideally, algorithms will also be validated across both normal and pathologic CBTs, although subject safety dictates stopping exercise once reaching 40 °C due to the high risk of harm at this temperature. There remains a need to validate algorithms for high school athletes under 19 years of age, as this demographic makes up the largest proportion (nearly 50%) of HRI presenting to emergency rooms [68].
ML methods offer vast opportunities to address some of the current issues with CBT prediction with the wearable devices described in this review, namely the individualization of predictions, wearable device count, and interoperability with the existing HRI prevention tools. This systematic review demonstrated the ability of the current algorithms to predict CBT using wearable devices accurately under controlled conditions, as well as in varied conditions when including environmental variables such as in the algorithms in Moyen et al., Welles et al. and Nazarian et al. [29,31,46]. While the accuracy from the study is not in question, future studies should strive to validate CBT prediction algorithms using wearable devices in dynamic, real-world environments, such as in Moyen et al. and in a wider variety of subjects at risk for HRI. Rather than limiting the scope of validation conditions, future algorithms using ML methods may be able to personalize their results by including additional data and variables on individual subjects into future algorithms, thereby better controlling for the inherent variability between users. Additionally, ML methods such as causal modeling and dimensionality reduction, used to determine the correlations between variables, can help reduce the number of devices worn and eliminate redundant data sources, as demonstrated in the study by Eggenberger et al., which showed the ability to acquire highly similar correlations between two CBT prediction algorithms using either 18 unique measurement locations or by reducing their algorithms to include only 2 of those measurements (r2 = 0.70 versus r2 = 0.68, respectively) [60].
Most importantly, the interoperability and integration between wearable CBT prediction algorithms and preexisting tools for injury prevention will decrease the athletic trainer burden and provide a wholistic platform for expedited clinical decision-making during times of distress (Figure 5). Existing systems such as EMRs, heat indices such as the wet bulb globe temperature (WBGT), and the integration of CBT prediction algorithms into a holistic platform will provide team physicians, athletic trainers, head coaches, sports scientists, employers, and military officers an integrative platform to ultimately reduce the incidences of HRI and injury burden more broadly while optimizing performances. The algorithm could also integrate with the existing tools used in HRI prevention for athletes and employees, such as the WBGT, which estimates environmental heat stress by accounting for environmental variables, including the air temperature, humidity, wind speed, and thermal radiation [69], to estimate the effects of external influences on a subject’s heat status. This integrated system could also help prevent the under- or overuse of injuries and maximize performances by identifying problematic patterns and informing coaches, trainers, and officers on decisions to stop or alter exercises and keep athletes within an optimal temperature range to prevent HRI and maximize performances [47]. The future of interoperability between wearable CBT prediction algorithms using ML and the preexisting tools for injury prevention such as EMRs, heat indices, AMSs and worker management systems, and treatment algorithms will increase the availability and speed of access of important data for decision-making, thus preventing injuries and improving care at the clinic, athletic field, and battlefield.
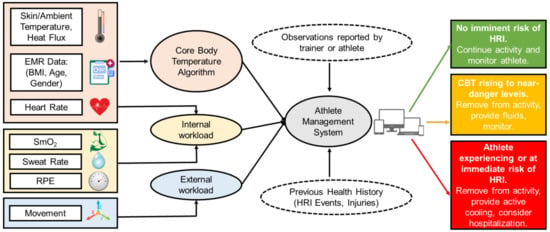
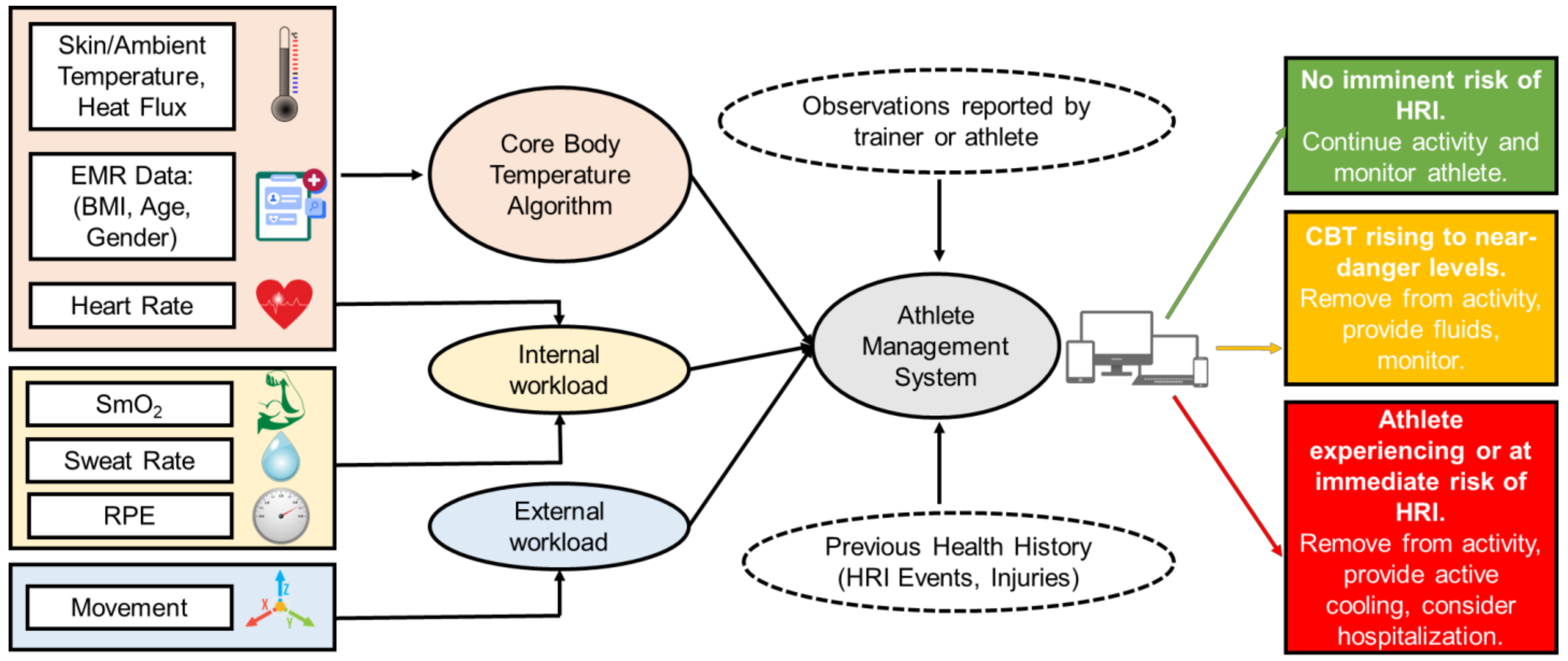
Figure 5.
A workflow schematic depicting the hypothetical interoperability of an algorithm for CBT prediction using wearable devices [25,26,27,54]. Both sensor inputs to the CBT prediction algorithm and its output to other systems are included in the schematic. Heart Rate is used to calculate both the CBT and internal workload. EMR = Electronic Medical Record, RPE = Rating of Perceived Exertion, and SmO2 = Muscle Oxygen Saturation.
5. Conclusions
This systematic review focused on the current state of wearable sensor technology and predictive analytics towards monitoring the core body temperature in athletes, with applications in other fields of work in high acuity environments or situations (Figure 6). The findings of this review demonstrate the opportunity that exists for the development of accurate and versatile wearable sensors that integrate CBT monitoring algorithms to accurately predict the onset of and prevent heat-related illness. The overall volume of currently published literature on the application of wearable technology to monitor the core body temperature is limited, with only 20 studies and 25 CBT prediction algorithms for exercising subjects identified in this systematic review. Of the algorithms reporting a RMSE, 17 out of 18 algorithms reported here met the previously identified clinical validity standards, with an RMSE lower than 0.5 °C. The validation of these algorithms in variable populations and in real-world situations is still needed. A large opportunity exists for ML methods to incorporate individual and environmental characteristics, such as ambient temperature and subject demographics, into CBT prediction algorithms. Improved CBT prediction methods using ML will more reliably predict and prevent HRI across diverse environments and populations, but the future of CBT prediction lies in its integration with the larger HRI and athletic injury prevention space, which includes heat indices such as the WBGT, athlete management systems, electronic medical records, treatment algorithms, and RTP protocols. ML algorithms, with their increased data processing abilities, are ideal candidates for interoperability within these larger systems, as they can both supply input to the CBT prediction algorithm and use its output, along with other data, to assist in clinical decision-making, increasing the amount of data available to clinicians and streamlining transitions of care.
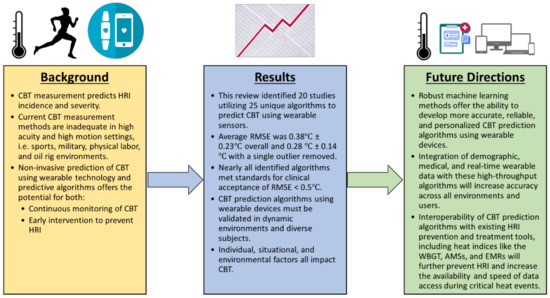
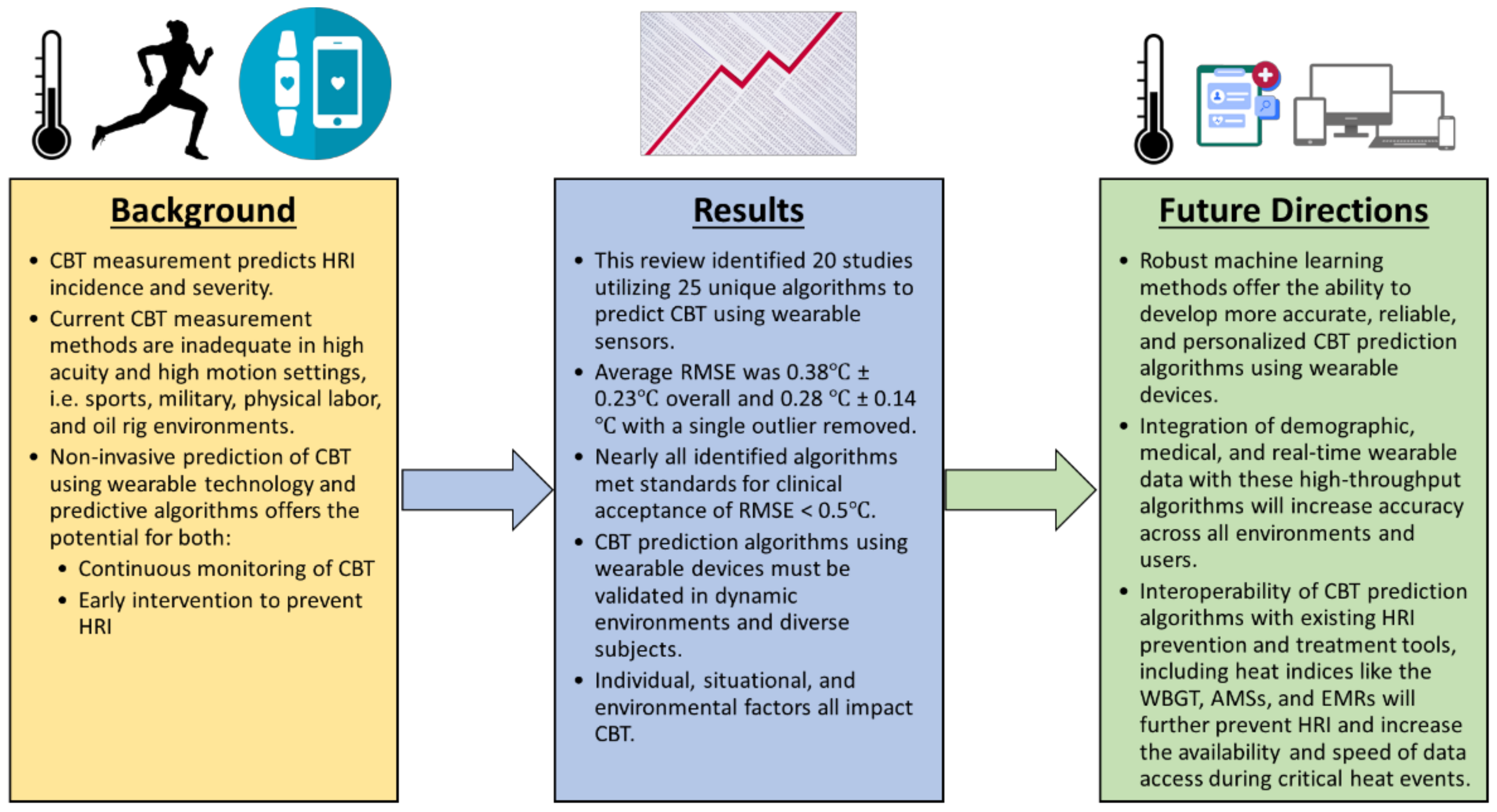
Figure 6.
Conclusions from this review and proposed future directions for the technology. HRI = heat-related illness, CBT = core body temperature, RMSE = root mean square error, WBGT = wet bulb globe temperature, AMS = athlete management system, and EMR = electronic medical record.
Author Contributions
C.M.D., E.R.H. and D.R.S. contributed to the design, collection, analysis, interpretation of data, writing, and editing of the manuscript. All the other authors contributed heavily to the editing and review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This systematic review did not require IRB review as it relied entirely on publicly available data and therefore did not meet the definition of human subjects as in 45 CFR 46.102.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors acknowledge a collaboration between Case Western Reserve University and University Hospitals Cleveland Medical Center.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cramer, M.N.; Gagnon, D.; Laitano, O.; Crandall, C.G. Human Temperature Regulation under Heat Stress in Health, Disease, and Injury. Physiol. Rev. 2022, 102, 1907–1989. [Google Scholar] [CrossRef] [PubMed]
- Bruggers, J. ‘This Was Preventable’: Football Heat Deaths and the Rising Temperature. Inside Climate News, 20 July 2018. [Google Scholar]
- Gilchrist, J.; Haileyesus, T.; Murphy, M.; Comstock, R.; Collins, C.; McIlvain, N.; Yard, E. Heat Illness Among High School Athletes-United States, 2005–2009. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5932a1.htm (accessed on 11 June 2022).
- Armed Forces Health Surveillance Branch. Update: Heat Illness, Active Component, U.S. Armed Forces. 2020. Available online: https://www.health.mil/News/Articles/2021/04/01/Update-Heat-MSMR-2021 (accessed on 11 June 2022).
- Périard, J. Prolonged Exercise in the Heat. Aspetar Sports Med. J. 2013, 2, 10–15. [Google Scholar]
- González-Alonso, J.; Teller, C.; Andersen, S.L.; Jensen, F.B.; Hyldig, T.; Nielsen, B. Influence of Body Temperature on the Development of Fatigue during Prolonged Exercise in the Heat. J. Appl. Physiol. 1999, 86, 1032–1039. [Google Scholar] [CrossRef]
- Heatstroke-Injuries; Poisoning. Available online: https://www.merckmanuals.com/professional/injuries-poisoning/heat-illness/heatstroke (accessed on 11 June 2022).
- Walter, E.; Steel, K. Management of Exertional Heat Stroke: A Practical Update for Primary Care Physicians. Br. J. Gen. Pract. 2018, 68, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; DeMartini, J.K.; Bergeron, M.F.; Csillan, D.; Eichner, E.R.; Lopez, R.M.; Ferrara, M.S.; Miller, K.C.; O’Connor, F.; Sawka, M.N.; et al. National Athletic Trainers’ Association Position Statement: Exertional Heat Illnesses. J. Athl. Train. 2015, 50, 986–1000. [Google Scholar] [CrossRef]
- Navarro, C.S.; Casa, D.J.; Belval, L.N.; Nye, N.S. Exertional Heat Stroke. Curr. Sports Med. Rep. 2017, 16, 304–305. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Casa, D.J.; Millard-Stafford, M.; Moran, D.S.; Pyne, S.W.; Roberts, W.O. Exertional Heat Illness during Training and Competition. Med. Sci. Sports Exerc. 2007, 39, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Demartini, J.K.; Casa, D.J.; Stearns, R.; Belval, L.; Crago, A.; Davis, R.; Jardine, J. Effectiveness of Cold Water Immersion in the Treatment of Exertional Heat Stroke at the Falmouth Road Race. Med. Sci. Sports Exerc. 2015, 47, 240–245. [Google Scholar] [CrossRef]
- Knight, T. Runner Dies after Sweltering Marathon. The Telegraph, 24 April 2007. [Google Scholar]
- Carter, H. Four Runners Die on Great North Run. The Guardian, 18 September 2005. [Google Scholar]
- 30 NCAA Football Players Have Died During Workouts Since 2000, HBO Reveals. Available online: https://www.acsh.org/news/2018/09/28/30-ncaa-football-players-have-died-during-workouts-2000-hbo-reveals-13456 (accessed on 10 June 2022).
- Thibodaux, D.P.; Bourgeois, R.M.; Loeppke, R.R.; Konicki, D.L.; Hymel, P.A.; Dreger, M. Medical Evacuations From Oil Rigs off the Gulf Coast of the United States From 2008 to 2012: Reasons and Cost Implications. J. Occup. Environ. Med. 2014, 56, 681–685. [Google Scholar] [CrossRef]
- Arbury, S.; Jacklitsch, B.; Farquah, O.; Hodgson, M.; Lamson, G.; Martin, H.; Profitt, A. Heat Illness and Death Among Workers—United States, 2012–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 661–665. [Google Scholar]
- Ioannou, L.G.; Foster, J.; Morris, N.B.; Piil, J.F.; Havenith, G.; Mekjavic, I.B.; Kenny, G.P.; Nybo, L.; Flouris, A.D. Occupational Heat Strain in Outdoor Workers: A Comprehensive Review and Meta-Analysis. Temperature 2022, 9, 67–102. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.M. Exertional Heat Stroke within Secondary School Athletics. Curr. Sports Med. Rep. 2019, 18, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Glazer, J.L. Management of Heatstroke and Heat Exhaustion. Am. Fam. Physician 2005, 71, 2133–2140. [Google Scholar] [PubMed]
- Del Bene, V.E. Temperature. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Périard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercise under Heat Stress: Thermoregulation, Hydration, Performance Implications, and Mitigation Strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, D.R.; Rowbottom, J.R.; Drummond, C.; Voos, J.E.; Craker, J. A Review of Wearable Technology: Moving beyond the Hype: From Need through Sensor Implementation. In Proceedings of the 2016 8th Cairo International Biomedical Engineering Conference (CIBEC), Cairo, Egypt, 15–17 December 2016; pp. 52–55. [Google Scholar]
- Seshadri, D.R.; Magliato, S.; Voos, J.E.; Drummond, C. Clinical Translation of Biomedical Sensors for Sports Medicine. J. Med. Eng. Technol. 2019, 43, 66–81. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable Sensors for Monitoring the Physiological and Biochemical Profile of the Athlete. NPJ Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable Sensors for Monitoring the Internal and External Workload of the Athlete. NPJ Digit. Med. 2019, 2, 71. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Drummond, C.; Craker, J.; Rowbottom, J.R.; Voos, J.E. Wearable Devices for Sports: New Integrated Technologies Allow Coaches, Physicians, and Trainers to Better Understand the Physical Demands of Athletes in Real Time. IEEE Pulse 2017, 8, 38–43. [Google Scholar] [CrossRef]
- Epstein, Y.; Yanovich, R. Heatstroke. N. Engl. J. Med. 2019, 380, 2449–2459. [Google Scholar] [CrossRef]
- Welles, A.P.; Xu, X.; Santee, W.R.; Looney, D.P.; Buller, M.J.; Potter, A.W.; Hoyt, R.W. Estimation of Core Body Temperature from Skin Temperature, Heat Flux, and Heart Rate Using a Kalman Filter. Comput. Biol. Med. 2018, 99, 1–6. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Belval, L.N.; Stearns, R.; Casa, D.J.; Hosokawa, Y. Monitoring Internal Body Temperature. Sports Sci. Exch. 2019, 32, 1–5. [Google Scholar]
- Moyen, N.E.; Bapat, R.C.; Tan, B.; Hunt, L.A.; Jay, O.; Mündel, T. Accuracy of Algorithm to Non-Invasively Predict Core Body Temperature Using the Kenzen Wearable Device. Int. J. Environ. Res. Public Health 2021, 18, 13126. [Google Scholar] [CrossRef] [PubMed]
- Niedermann, R.; Wyss, E.; Annaheim, S.; Psikuta, A.; Davey, S.; Rossi, R.M. Prediction of Human Core Body Temperature Using Non-Invasive Measurement Methods. Int. J. Biometeorol. 2014, 58, 7–15. [Google Scholar] [CrossRef]
- Gunga, H.-C.; Sandsund, M.; Reinertsen, R.E.; Sattler, F.; Koch, J. A Non-Invasive Device to Continuously Determine Heat Strain in Humans. J. Therm. Biol. 2008, 33, 297–307. [Google Scholar] [CrossRef]
- Buller, M.J.; Tharion, W.J.; Cheuvront, S.N.; Montain, S.J.; Kenefick, R.W.; Castellani, J.; Latzka, W.A.; Roberts, W.S.; Richter, M.; Jenkins, O.C.; et al. Estimation of Human Core Temperature from Sequential Heart Rate Observations. Physiol. Meas. 2013, 34, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Pryor, J.L.; Périard, J.D.; Pryor, R.R. Predisposing Factors for Exertional Heat Illness. In Exertional Heat Illness: A Clinical and Evidence-Based Guide; Adams, W.M., Jardine, J.F., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 29–57. ISBN 978-3-030-27805-2. [Google Scholar]
- Aoyagi, Y.; McLellan, T.M.; Shephard, R.J. Interactions of Physical Training and Heat Acclimation: The Thermophysiology of Exercising in a Hot Climate. Sports Med. 1997, 23, 173–210. [Google Scholar] [CrossRef] [PubMed]
- Yokota, M.; Berglund, L.; Cheuvront, S.; Santee, W.; Latzka, W.; Montain, S.; Kolka, M.; Moran, D. Thermoregulatory Model to Predict Physiological Status from Ambient Environment and Heart Rate. Comput. Biol. Med. 2008, 38, 1187–1193. [Google Scholar] [CrossRef]
- Casa, D.J.; Armstrong, L.E.; Hillman, S.K.; Montain, S.J.; Reiff, R.V.; Rich, B.S.E.; Roberts, W.O.; Stone, J.A. National Athletic Trainers’ Association Position Statement: Fluid Replacement for Athletes. J. Athl. Train. 2000, 35, 212–224. [Google Scholar]
- Lipman, G.S.; Eifling, K.P.; Ellis, M.A.; Gaudio, F.G.; Otten, E.M.; Grissom, C.K. Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Heat-Related Illness: 2014 Update. Wilderness Environ. Med. 2014, 25, S55–S65. [Google Scholar] [CrossRef]
- Shapiro, Y.; Seidman, D.S. Field and Clinical Observations of Exertional Heat Stroke Patients. Med. Sci. Sports Exerc. 1990, 22, 6–14. [Google Scholar] [CrossRef]
- Leon, L.R.; Bouchama, A. Heat Stroke. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 611–647. ISBN 978-0-470-65071-4. [Google Scholar]
- CorTemp. Available online: https://www.hqinc.net/cortemp/ (accessed on 13 June 2022).
- Rectal Probes and Thermometer Information. Available online: https://ksi.uconn.edu/wp-content/uploads/sites/1222/2019/08/RectalProbes_ResourceUpdate_8.19new-GG-1.pdf (accessed on 30 June 2022).
- Mon-a-therm Esophageal Stethoscope with Temperature Sensor 400TM. Available online: https://www.medtronic.com/covidien/en-us/products/temperature-management/mon-a-therm-esophageal-stethoscope-400-tm.html (accessed on 30 June 2022).
- CIRCA S-CATHTM–CIRCA’s S-CATHTM Hot & Cold Esophageal Temperature Monitoring System. Available online: https://www.circascientific.com/products/circa-s-cath-us/ (accessed on 30 June 2022).
- Nazarian, N.; Liu, S.; Kohler, M.; Lee, J.K.W.; Miller, C.; Chow, W.T.L.; Alhadad, S.B.; Martilli, A.; Quintana, M.; Sunden, L.; et al. Project Coolbit: Can Your Watch Predict Heat Stress and Thermal Comfort Sensation? Environ. Res. Lett. 2021, 16, 034031. [Google Scholar] [CrossRef]
- Tsadok, I.; Scheinowitz, M.; Shpitzer, S.A.; Ketko, I.; Epstein, Y.; Yanovich, R. Assessing Rectal Temperature with a Novel Non-Invasive Sensor. J. Therm. Biol. 2021, 95, 102788. [Google Scholar] [CrossRef] [PubMed]
- CORE Body Temperature Sensor. Available online: https://corebodytemp.com/ (accessed on 13 June 2022).
- Eq02+ LifeMonitor. Available online: https://www.equivital.com/products/eq02-lifemonitor (accessed on 14 June 2022).
- ZephyrTM Performance Systems | Performance Monitoring Technology. Available online: https://www.zephyranywhere.com/ (accessed on 14 June 2022).
- Claudino, J.G.; de Oliveira Capanema, D.; de Souza, T.V.; Serrão, J.C.; Machado Pereira, A.C.; Nassis, G.P. Current Approaches to the Use of Artificial Intelligence for Injury Risk Assessment and Performance Prediction in Team Sports: A Systematic Review. Sports Med.-Open 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.I.; Mitchell, T.M. Machine Learning: Trends, Perspectives, and Prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, D.R.; Thom, M.L.; Harlow, E.R.; Gabbett, T.J.; Geletka, B.J.; Hsu, J.J.; Drummond, C.K.; Phelan, D.M.; Voos, J.E. Wearable Technology and Analytics as a Complementary Toolkit to Optimize Workload and to Reduce Injury Burden. Front. Sports Act. Living 2021, 2, 630576. [Google Scholar] [CrossRef] [PubMed]
- Grace-Martin, K. Assessing the Fit of Regression Models. Available online: https://cscu.cornell.edu/wp-content/uploads/68_regfit.pdf (accessed on 13 June 2022).
- Laxminarayan, S.; Rakesh, V.; Oyama, T.; Kazman, J.B.; Yanovich, R.; Ketko, I.; Epstein, Y.; Morrison, S.; Reifman, J. Individualized Estimation of Human Core Body Temperature Using Noninvasive Measurements. J. Appl. Physiol. 2018, 124, 1387–1402. [Google Scholar] [CrossRef]
- Verdel, N.; Podlogar, T.; Ciuha, U.; Holmberg, H.-C.; Debevec, T.; Supej, M. Reliability and Validity of the CORE Sensor to Assess Core Body Temperature during Cycling Exercise. Sensors 2021, 21, 5932. [Google Scholar] [CrossRef]
- Buller, M.J.; Davey, T.; Fallowfield, J.L.; Montain, S.J.; Hoyt, R.W.; Delves, S.K. Estimated and Measured Core Temperature Responses to High-Intensity Warm Weather Military Training: Implications for Exertional Heat Illness Risk Assessment. Physiol. Meas. 2020, 41, 065011. [Google Scholar] [CrossRef]
- Hunt, A.P.; Buller, M.J.; Maley, M.J.; Costello, J.T.; Stewart, I.B. Validity of a Noninvasive Estimation of Deep Body Temperature When Wearing Personal Protective Equipment during Exercise and Recovery. Mil. Med. Res. 2019, 6, 20. [Google Scholar] [CrossRef]
- Eggenberger, P.; MacRae, B.A.; Kemp, S.; Bürgisser, M.; Rossi, R.M.; Annaheim, S. Prediction of Core Body Temperature Based on Skin Temperature, Heat Flux, and Heart Rate Under Different Exercise and Clothing Conditions in the Heat in Young Adult Males. Front. Physiol. 2018, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Mazgaoker, S.; Ketko, I.; Yanovich, R.; Heled, Y.; Epstein, Y. Measuring Core Body Temperature with a Non-Invasive Sensor. J. Therm. Biol. 2017, 66, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Seng, K.-Y.; Chen, Y.; Chai, K.M.A.; Wang, T.; Fun, D.C.Y.; Teo, Y.S.; Sze Tan, P.M.; Ang, W.H.; Lee, J.K.W. Tracking Body Core Temperature in Military Thermal Environments: An Extended Kalman Filter Approach. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; IEEE: New York, NY, USA, 2016; pp. 296–299. [Google Scholar]
- Seo, Y.; DiLeo, T.; Powell, J.B.; Kim, J.-H.; Roberge, R.J.; Coca, A. Comparison of Estimated Core Body Temperature Measured with the BioHarness and Rectal Temperature under Several Heat Stress Conditions. J. Occup. Environ. Hyg. 2016, 13, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Buller, M.J.; Tharion, W.J.; Duhamel, C.M.; Yokota, M. Real-Time Core Body Temperature Estimation from Heart Rate for First Responders Wearing Different Levels of Personal Protective Equipment. Ergonomics 2015, 58, 1830–1841. [Google Scholar] [CrossRef]
- Richmond, V.L.; Wilkinson, D.M.; Blacker, S.D.; Horner, F.E.; Carter, J.; Havenith, G.; Rayson, M.P. Insulated Skin Temperature as a Measure of Core Body Temperature for Individuals Wearing CBRN Protective Clothing. Physiol. Meas. 2013, 34, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Buller, M.J.; Castellani, J.; Roberts, W.S.; Hoyt, R.W.; Jenkins, O.C. Human Thermoregulatory System State Estimation Using Non-Invasive Physiological Sensors. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 3290–3293. [Google Scholar] [CrossRef]
- Teunissen, L.P.J.; de Haan, A.; de Koning, J.J.; Daanen, H.A.M. Telemetry Pill versus Rectal and Esophageal Temperature during Extreme Rates of Exercise-Induced Core Temperature Change. Physiol. Meas. 2012, 33, 915–924. [Google Scholar] [CrossRef]
- Nelson, N.G.; Collins, C.L.; Comstock, R.D.; McKenzie, L.B. Exertional Heat-Related Injuries Treated in Emergency Departments in the U.S., 1997–2006. Am. J. Prev. Med. 2011, 40, 54–60. [Google Scholar] [CrossRef]
- US Army. Heat Stress Control and Heat Stress Management; 2022. Available online: https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN35159-TB_MED_507-000-WEB-1.pdf (accessed on 6 July 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).