Adjusting the Connection Length of Additively Manufactured Electrodes Changes the Electrochemical and Electroanalytical Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Additive Manufacturing
2.3. Electrochemical Experiments
3. Results and Discussion
3.1. Effect on the Electrochemical Performance
| Conductive Filament | Shape | Size | Thickness | Connection Length | Application | Reference |
|---|---|---|---|---|---|---|
| PLA/CB (PP) | Disc | D: 5 mm | 1 mm | 34 mm | Electroanalytical detection of NBOMes | [11] |
| PLA/CB (PP) | Disc | D: 54 mm | N/A | N/A | Electroanalytical detection of dopamine | [12] |
| PLA/NG | Disc | D: 3 mm | 1 mm | 34 mm | Electroanalytical detection of Mn | [13] |
| PLA/nanocarbon | Disc | D: 11 mm | 0.3 mm | N/A | Electrochemical capacitors | [16] |
| PLA/CB (PP) | Disc | D: 5.4 mm | N/A | N/A | Electroanalytical detection of Cd, Pb and Cu | [18] |
| PLA/CB (PP) | Disc | D: 3 mm | 0 mm (Defined) | 18 mm | Electroanalytical detection of ascorbic acid and acetaminophen | [20] |
| PLA/G (BM) | Lollipop | D: 5 mm | 1 mm | 20 mm | Electroanalytical detection of L-methionine | [22] |
| PLA/CB (PP) | Lollipop | D: ~5 mm | N/A | ~20 mm | Electroanalytical detection of Hg, glucose, caffeine | [23] |
| PLA/G (BM) | Disc | D: 15 mm | 2 mm | N/A | Electroanalytical detection of picric acid | [34] |
| PLA/G (BM) | Lollipop | D: 5 mm | 2 mm | 20 mm | Oxygen evolution reaction | [35] |
| PLA/G (BM) | Disc | D: 15 mm | 2 mm | N/A | Electroanalytical detection of 1-naphthol | [36] |
| PLA/G (BM) | Disc | D: ~5 mm | N/A | ~40 mm | Pseudo-reference electrode | [37] |
| PLA/G (BM) | Disc | D: 3 mm | 1 mm | N/A | Electrochemical energy storage | [38] |
| PLA/NG | Lollipop | D: 3 mm | 1 mm | N/A | Electroanalytical detection of Pb and Cd | [39] |
| PLA/CB/2D-MoSe2 | Lollipop | D: 3.5 mm | 1.5 mm | 10 mm | Electrochemical water splitting | [40] |
| PLA/G (BM) | Lollipop | D: 8 mm | 1.6 mm | 45 mm | Electroanalytical detection of mycotoxin zearalenone | [41] |
| PLA/G (BM) | Lollipop | D: 8 mm | 2 mm | 37 mm | Electroanalytical detection of hydrogen peroxide | [42] |
| PLA/G (BM) | Lollipop | D: 6 mm | 1 mm | N/A | Electrode benchmarking | [43] |
| PLA/G (BM) | Lollipop | D: 5 mm | 1 mm | “few cm” | Hydrogen evolution reaction | [44] |
| PLA/CB (PP) | Lollipop | D: 5 mm | 1 mm | 50 mm | Hydrogen evolution reaction | [45] |
| PLA/G (BM) | Disc | D: 4.8 mm | N/A | N/A | Electroanalytical detection of cocaine | [46] |
| PLA/CB (PP) | Disc | D: 3.8 mm | N/A | N/A | Electroanalytical detection of 2,4,6-trinitrotoluene | [47] |
| PLA/CB (PP) | Lollipop | D: ~4 mm | N/A | ~11 mm | Electroanalytical detection of glucose | [48] |
| PLA/CB (PP) | Disc | D: 5 mm | N/A | N/A | Electroanalytical detection of forensic drugs | [49] |
| PLA/G (BM) | Lollipop | D: 6 mm | N/A | N/A | Electroanalytical detection of COVID-19 | [50] |
| PLA/CB (PP) | Disc | D: 2.85 mm | 10 mm | N/A | Electroanalytical detection of Hantavirus Araucaria nucleoprotein | [51] |
| PLA/CB (PP) | Lollipop | D: 5 mm | 2 mm | 25 mm | Effect of water ingress on AMEs | [52] |
| PLA/CB (PP) | Disc | D: 4 mm | N/A | ~20 mm | Electroanalytical Sensors | [53] |
| PLA/CB (PP) | Disc | D: 3.55 mm | N/A | N/A | Electroanalytical detection of adrenaline | [54] |
| PLA/CB (PP) | Disc | D: 5.4 mm | 1.8 mm | 40 mm | Electroanalytical detection of naproxen | [55] |
| PLA/G (BM) | Lollipop | D: 6 mm | N/A | 20 mm | Electroanalytical detection of L-cysteine | [56] |
3.2. Effect on the Electroanalytical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ryan, K.R.; Down, M.P.; Banks, C.E. Future of additive manufacturing: Overview of 4D and 3D printed smart and advanced materials and their applications. Chem. Eng. J. 2020, 403, 126162. [Google Scholar] [CrossRef]
- Kumar, L.J.; Pandey, P.M.; Wimpenny, D.I. 3D Printing and Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2019; Volume 311. [Google Scholar]
- Tully, J.J.; Meloni, G.N. A Scientist’s Guide to Buying a 3D Printer: How to Choose the Right Printer for Your Laboratory. Anal. Chem. 2020, 92, 14853–14860. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.J.; Crapnell, R.D.; Rothwell, E.J.; Hurst, N.J.; Banks, C.E. Additive manufacturing for electrochemical labs: An overview and tutorial note on the production of cells, electrodes and accessories. Talanta Open 2021, 4, 100051. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.; Ng, S.; Iffelsberger, C.; Pumera, M. Inherent Impurities in Graphene/Polylactic Acid Filament Strongly Influence on the Capacitive Performance of 3D-Printed Electrode. Chem.—A Eur. J. 2020, 26, 15746–15753. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Pumera, M. Free-standing electrochemically coated MoS x based 3D-printed nanocarbon electrode for solid-state supercapacitor application. Nanoscale 2021, 13, 5744–5756. [Google Scholar] [CrossRef]
- Foster, C.W.; Metters, J.P.; Kampouris, D.K.; Banks, C.E. Ultraflexible Screen-Printed Graphitic Electroanalytical Sensing Platforms. Electroanalysis 2014, 26, 262–274. [Google Scholar] [CrossRef]
- Rocha, D.P.; Rocha, R.G.; Castro, S.V.F.; Trindade, M.A.G.; Munoz, R.A.A.; Richter, E.M.; Angnes, L. Posttreatment of 3D-printed surfaces for electrochemical applications: A critical review on proposed protocols. Electrochem. Sci. Adv. 2021, 2, e2100136. [Google Scholar] [CrossRef]
- Kalinke, C.; Neumsteir, N.V.; de Oliveira Aparecido, G.; de Barros Ferraz, T.V.; dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3D printed PLA-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Anal. 2019, 145, 1207–1218. [Google Scholar] [CrossRef]
- Elbardisy, H.M.; Richter, E.M.; Crapnell, R.D.; Down, M.P.; Gough, P.G.; Belal, T.S.; Talaat, W.; Daabees, H.G.; Banks, C.E. Versatile additively manufactured (3D printed) wall-jet flow cell for high performance liquid chromatography-amperometric analysis: Application to the detection and quantification of new psychoactive substances (NBOMes). Anal. Methods 2020, 12, 2152–2165. [Google Scholar] [CrossRef]
- Richter, E.M.; Rocha, D.P.; Cardoso, R.M.; Keefe, E.M.; Foster, C.W.; Munoz, R.A.A.; Banks, C.E. Complete Additively Manufactured (3D-Printed) Electrochemical Sensing Platform. Anal. Chem. 2019, 91, 12844–12851. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.P.; Foster, C.W.; Munoz, R.A.A.; Buller, G.A.; Keefe, E.M.; Banks, C.E. Trace manganese detection via differential pulse cathodic stripping voltammetry using disposable electrodes: Additively manufactured nanographite electrochemical sensing platforms. Analyst 2020, 145, 3424–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusmão, R.; Browne, M.P.; Sofer, Z.; Pumera, M. The capacitance and electron transfer of 3D-printed graphene electrodes are dramatically influenced by the type of solvent used for pre-treatment. Electrochem. Commun. 2019, 102, 83–88. [Google Scholar] [CrossRef]
- Redondo, E.; Muñoz, J.; Pumera, M. Green activation using reducing agents of carbon-based 3D printed electrodes: Turning good electrodes to great. Carbon 2021, 175, 413–419. [Google Scholar] [CrossRef]
- Redondo, E.; Ng, S.; Muñoz, J.; Pumera, M. Tailoring capacitance of 3D-printed graphene electrodes by carbonisation temperature. Nanoscale 2020, 12, 19673–19680. [Google Scholar] [CrossRef] [PubMed]
- Novotný, F.; Urbanová, V.; Plutnar, J.; Pumera, M. Preserving fine structure details and dramatically enhancing electron transfer rates in graphene 3D-printed electrodes via thermal annealing: Toward nitroaromatic explosives sensing. ACS Appl. Mater. Interfaces 2019, 11, 35371–35375. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.P.; Ataide, V.N.; de Siervo, A.; Gonçalves, J.M.; Muñoz, R.A.; Paixão, T.R.; Angnes, L. Reagentless and sub-minute laser-scribing treatment to produce enhanced disposable electrochemical sensors via additive manufacture. Chem. Eng. J. 2021, 425, 130594. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Hurst, N.J.; Bernalte, E.; Crapnell, R.D.; Whittingham, M.J.; Brownson, D.A.C.; Banks, C.E. Exploration of defined 2-dimensional working electrode shapes through additive manufacturing. Analyst 2022, 147, 5121–5129. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Bernalte, E.; Ferrari, A.G.-M.; Whittingham, M.J.; Williams, R.J.; Hurst, N.J.; Banks, C.E. All-in-One Single-Print Additively Manufactured Electroanalytical Sensing Platforms. ACS Meas. Sci. Au 2021, 2, 167–176. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Mendonça, D.M.; Silva, W.P.; Silva, M.N.; Nossol, E.; da Silva, R.A.; Richter, E.M.; Muñoz, R.A. 3D printing for electroanalysis: From multiuse electrochemical cells to sensors. Anal. Chim. Acta 2018, 1033, 49–57. [Google Scholar] [CrossRef]
- Kalinke, C.; Neumsteir, N.V.; de Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A. Sensing of L-methionine in biological samples through fully 3D-printed electrodes. Anal. Chim. Acta 2021, 1142, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Katseli, V.; Economou, A.; Kokkinos, C. Single-step fabrication of an integrated 3D-printed device for electrochemical sensing applications. Electrochem. Commun. 2019, 103, 100–103. [Google Scholar] [CrossRef]
- Katseli, V.; Thomaidis, N.; Economou, A.; Kokkinos, C. Miniature 3D-printed integrated electrochemical cell for trace voltammetric Hg(II) determination. Sens. Actuators B Chem. 2020, 308, 127715. [Google Scholar] [CrossRef]
- Sibug-Torres, S.M.; Go, L.P.; Castillo, V.C.G.; Pauco, J.L.R.; Enriquez, E.P. Fully integrated 3D-printed electrochemical cell with a modified inkjet-printed Ag electrode for voltammetric nitrate analysis. Anal. Chim. Acta 2021, 1160, 338430. [Google Scholar] [CrossRef] [PubMed]
- Stefano, J.S.; e Silva, L.R.G.; Rocha, R.G.; Brazaca, L.C.; Richter, E.M.; Muñoz, R.A.A.; Janegitz, B.C. New conductive filament ready-to-use for 3D-printing electrochemical (bio) sensors: Towards the detection of SARS-CoV-2. Anal. Chim. Acta 2021, 1191, 339372. [Google Scholar] [CrossRef]
- Abdalla, A.; Hamzah, H.H.; Keattch, O.; Covill, D.; Patel, B. Augmentation of conductive pathways in carbon black/PLA 3D-printed electrodes achieved through varying printing parameters. Electrochimica Acta 2020, 354, 136618. [Google Scholar] [CrossRef]
- Tirado-Garcia, I.; Garcia-Gonzalez, D.; Garzon-Hernandez, S.; Rusinek, A.; Robles, G.; Martinez-Tarifa, J.; Arias, A. Conductive 3D printed PLA composites: On the interplay of mechanical, electrical and thermal behaviours. Compos. Struct. 2021, 265, 113744. [Google Scholar] [CrossRef]
- Iffelsberger, C.; Jellett, C.W.; Pumera, M. 3D Printing Temperature Tailors Electrical and Electrochemical Properties through Changing Inner Distribution of Graphite/Polymer. Small 2021, 17, e2101233. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Neumsteir, N.V.; Henriques, B.F.; Aparecido, G.D.O.; Loureiro, H.C.; Janegitz, B.C.; Bonacin, J.A. Influence of filament aging and conductive additive in 3D printed sensors. Anal. Chim. Acta 2021, 1191, 339228. [Google Scholar] [CrossRef]
- Whittingham, M.J.; Hurst, N.J.; Crapnell, R.D.; Ferrari, A.G.-M.; Blanco, E.; Davies, T.J.; Banks, C.E. Electrochemical Improvements Can Be Realized via Shortening the Length of Screen-Printed Electrochemical Platforms. Anal. Chem. 2021, 93, 16481–16488. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin Hamzah, H.H.; Keattch, O.; Covill, D.; Patel, B.A. The effects of printing orientation on the electrochemical behaviour of 3D printed acrylonitrile butadiene styrene (ABS)/carbon black electrodes. Sci. Rep. 2018, 8, 9135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palenzuela, C.L.M.; Novotný, F.; Krupička, P.; Sofer, Z.; Pumera, M. 3D-Printed Graphene/Polylactic Acid Electrodes Promise High Sensitivity in Electroanalysis. Anal. Chem. 2018, 90, 5753–5757. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.L.; Rowley-Neale, S.J.; Ferrari, A.G.M.; Bonacin, J.A.; Banks, C.E. Ni−Fe (Oxy) hydroxide Modified Graphene Additive Manufactured (3D-Printed) Electrochemical Platforms as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemElectroChem 2019, 6, 5633–5641. [Google Scholar] [CrossRef]
- Manzanares-Palenzuela, C.L.; Hermanova, S.; Sofer, Z.; Pumera, M. Proteinase-sculptured 3D-printed graphene/polylactic acid electrodes as potential biosensing platforms: Towards enzymatic modeling of 3D-printed structures. Nanoscale 2019, 11, 12124–12131. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Novotný, F.; Webster, R.D.; Pumera, M. 3D-printed Ag/AgCl pseudo-reference electrodes. Electrochem. Commun. 2019, 103, 104–108. [Google Scholar] [CrossRef]
- Foster, C.; Down, M.P.; Zhang, Y.; Ji, X.; Rowley-Neale, S.J.; Smith, G.C.; Kelly, P.J.; Banks, C.E. 3D Printed Graphene Based Energy Storage Devices. Sci. Rep. 2017, 7, 42233. [Google Scholar] [CrossRef]
- Foster, C.W.; Elbardisy, H.M.; Down, M.P.; Keefe, E.M.; Smith, G.C.; Banks, C.E. Additively manufactured graphitic electrochemical sensing platforms. Chem. Eng. J. 2020, 381, 122343. [Google Scholar] [CrossRef]
- Hughes, J.P.; dos Santos, P.L.; Down, M.P.; Foster, C.W.; Bonacin, J.A.; Keefe, E.M.; Rowley-Neale, S.J.; Banks, C.E. Single step additive manufacturing (3D printing) of electrocatalytic anodes and cathodes for efficient water splitting. Sustain. Energy Fuels 2020, 4, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Nasir, M.Z.M.; Novotný, F.; Alduhaish, O.; Pumera, M. 3D-printed electrodes for the detection of mycotoxins in food. Electrochem. Commun. 2020, 115, 106735. [Google Scholar] [CrossRef]
- Marzo, A.M.L.; Mayorga-Martinez, C.C.; Pumera, M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2019, 151, 111980. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Lynch, S.; Foster, C.W.; Banks, C.E. Electrochemical Decoration of Additively Manufactured Graphene Macroelectrodes with MoO2Nanowires: An Approach to Demonstrate the Surface Morphology. J. Phys. Chem. C 2020, 124, 15377–15385. [Google Scholar] [CrossRef]
- Iffelsberger, C.; Ng, S.; Pumera, M. Catalyst coating of 3D printed structures via electrochemical deposition: Case of the transition metal chalcogenide MoSx for hydrogen evolution reaction. Appl. Mater. Today 2020, 20, 100654. [Google Scholar] [CrossRef]
- Hüner, B.; Demir, N.; Kaya, M.F. Electrodeposition of NiCu bimetal on 3D printed electrodes for hydrogen evolution reactions in alkaline media. Int. J. Hydrogen Energy 2022, 47, 12136–12146. [Google Scholar] [CrossRef]
- Rocha, R.G.; Ribeiro, J.S.; Santana, M.H.P.; Richter, E.M.; Muñoz, R.A.A. 3D-printing for forensic chemistry: Voltammetric determination of cocaine on additively manufactured graphene–polylactic acid electrodes. Anal. Methods 2021, 13, 1788–1794. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Rocha, D.P.; Rocha, R.G.; Stefano, J.S.; Silva, R.A.; Richter, E.M.; Muñoz, R.A. 3D-printing pen versus desktop 3D-printers: Fabrication of carbon black/polylactic acid electrodes for single-drop detection of 2,4,6-trinitrotoluene. Anal. Chim. Acta 2020, 1132, 10–19. [Google Scholar] [CrossRef]
- Katseli, V.; Economou, A.; Kokkinos, C. Smartphone-Addressable 3D-Printed Electrochemical Ring for Nonenzymatic Self-Monitoring of Glucose in Human Sweat. Anal. Chem. 2021, 93, 3331–3336. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.A.; de Oliveira, F.M.; de Melo, E.I.; de Carvalho, A.E.; Lucca, B.G.; Ferreira, V.S.; da Silva, R.A.B. Multi sensor compatible 3D-printed electrochemical cell for voltammetric drug screening. Anal. Chim. Acta 2021, 1169, 338568. [Google Scholar] [CrossRef]
- Muñoz, J.; Pumera, M. 3D-Printed COVID-19 immunosensors with electronic readout. Chem. Eng. J. 2021, 425, 131433. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Gogola, J.L.; Budni, L.H.; Janegitz, B.C.; Marcolino-Junior, L.H.; Bergamini, M.F. 3D-printed electrode as a new platform for electrochemical immunosensors for virus detection. Anal. Chim. Acta 2021, 1147, 30–37. [Google Scholar] [CrossRef]
- Williams, R.J.; Brine, T.; Crapnell, R.D.; Ferrari, A.G.-M.; Banks, C.E. The effect of water ingress on additively manufactured electrodes. Mater. Adv. 2022, 3, 7632–7639. [Google Scholar] [CrossRef]
- Silva-Neto, H.A.; Santhiago, M.; Duarte, L.C.; Coltro, W.K. Fully 3D printing of carbon black-thermoplastic hybrid materials and fast activation for development of highly stable electrochemical sensors. Sens. Actuators B Chem. 2021, 349, 130721. [Google Scholar] [CrossRef]
- Silva-Neto, H.A.; Dias, A.A.; Coltro, W.K.T. 3D-printed electrochemical platform with multi-purpose carbon black sensing electrodes. Mikrochim. Acta 2022, 189, 235. [Google Scholar] [CrossRef] [PubMed]
- João, A.F.; de Faria, L.V.; Ramos, D.L.; Rocha, R.G.; Richter, E.M.; Muñoz, R.A. 3D-printed carbon black/polylactic acid electrochemical sensor combined with batch injection analysis: A cost-effective and portable tool for naproxen sensing. Microchem. J. 2022, 180, 107565. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A. Prussian blue nanoparticles anchored on activated 3D printed sensor for the detection of L-cysteine. Sens. Actuators B Chem. 2022, 362, 131797. [Google Scholar] [CrossRef]

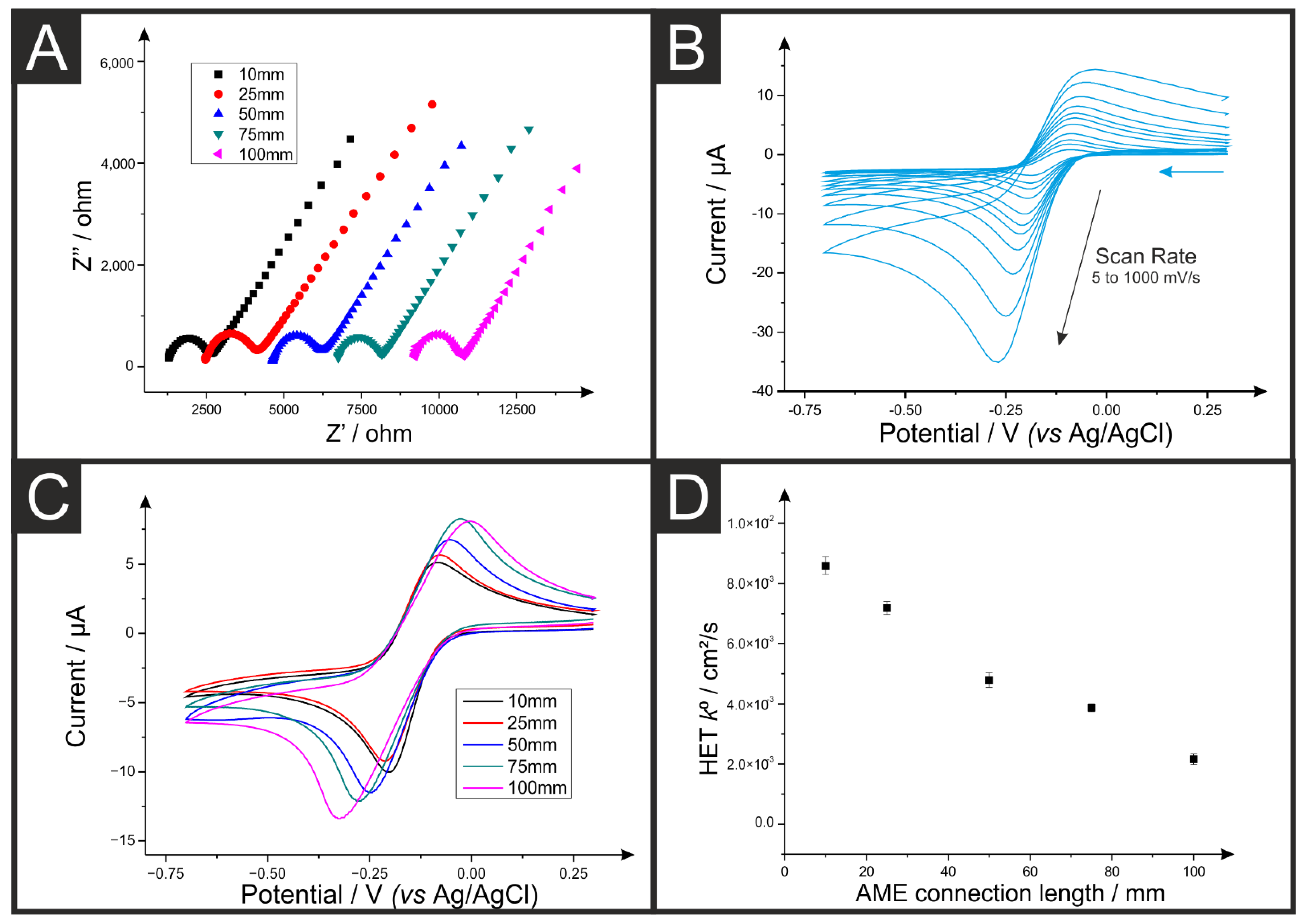
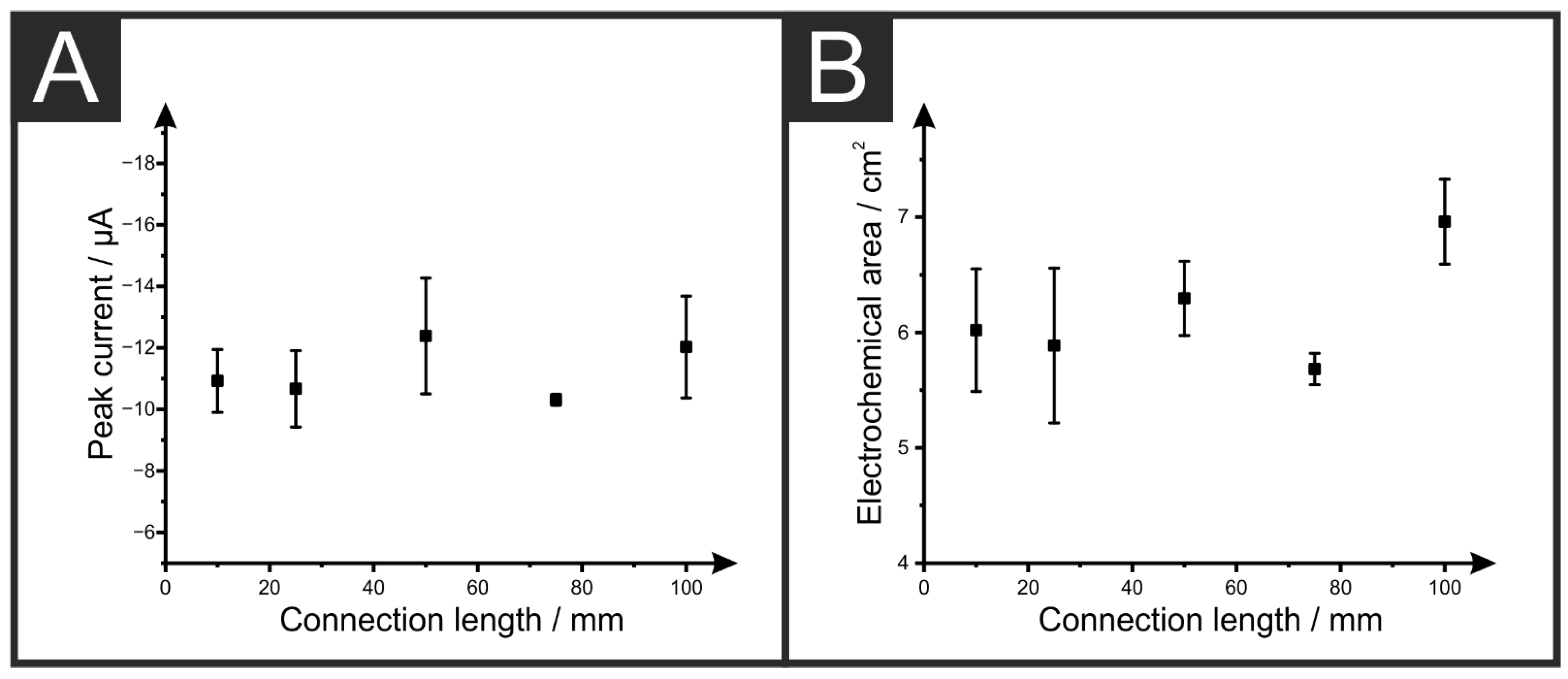
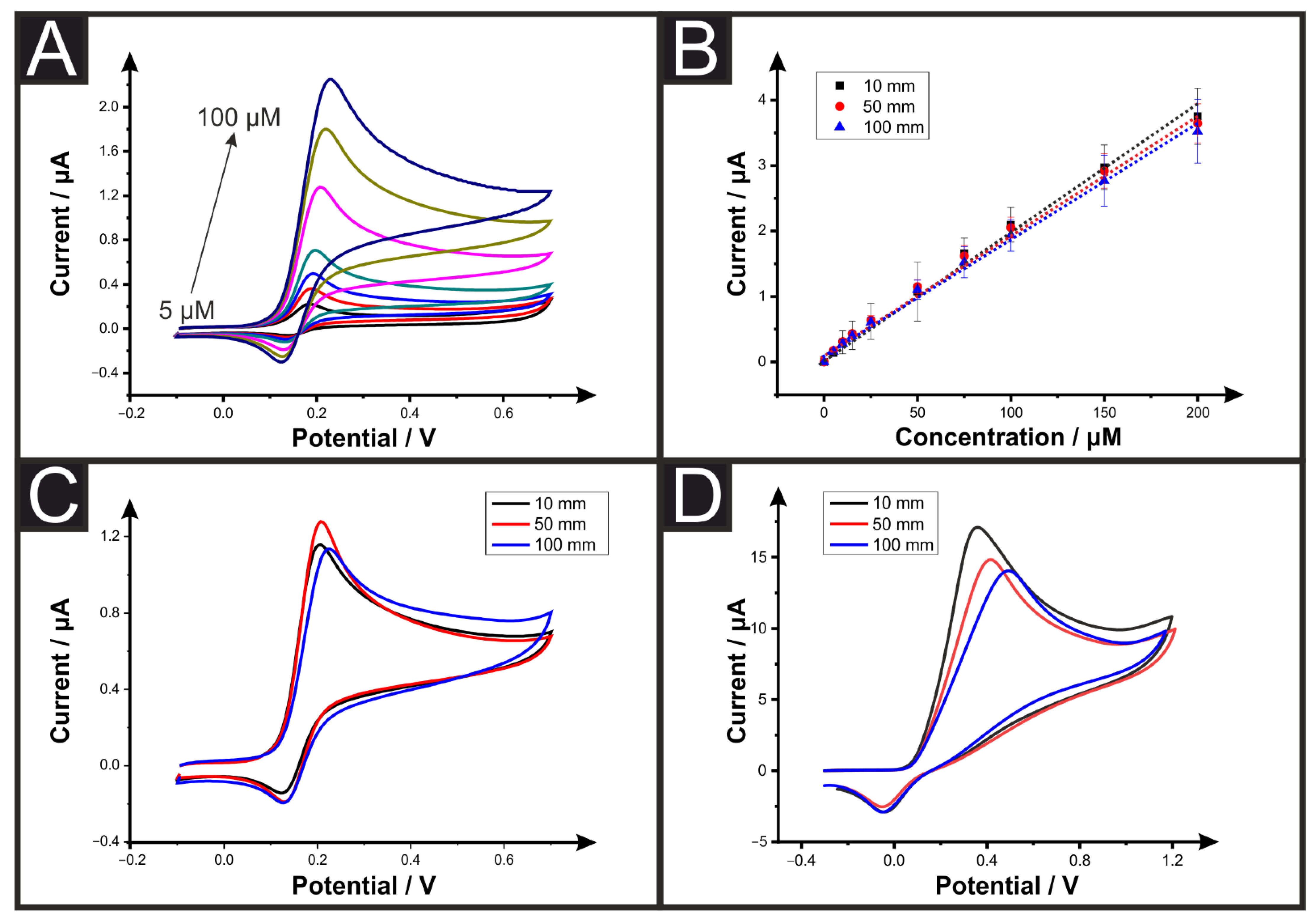
| Electrode Connection Length (mm) | Multimeter Resistance (n = 12, kΩ) | EIS Resistance (n = 5, kΩ) |
|---|---|---|
| 10 | 1.32 ± 0.20 | 1.24 ± 0.03 |
| 25 | 2.65 ± 0.11 | 2.55 ± 0.06 |
| 50 | 4.85 ± 0.12 | 4.67 ± 0.08 |
| 75 | 7.18 ± 0.33 | 6.81 ± 0.07 |
| 100 | 9.07 ± 0.13 | 9.00 ± 0.18 |
| AME Connection Length (mm) | R2 | Sensitivity (µA µM−1) | LOD (µM) |
|---|---|---|---|
| 10 | 0.9957 | 0.0188 | 1.19 |
| 50 | 0.9945 | 0.0182 | 1.73 |
| 100 | 0.9959 | 0.0175 | 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crapnell, R.D.; Garcia-Miranda Ferrari, A.; Whittingham, M.J.; Sigley, E.; Hurst, N.J.; Keefe, E.M.; Banks, C.E. Adjusting the Connection Length of Additively Manufactured Electrodes Changes the Electrochemical and Electroanalytical Performance. Sensors 2022, 22, 9521. https://doi.org/10.3390/s22239521
Crapnell RD, Garcia-Miranda Ferrari A, Whittingham MJ, Sigley E, Hurst NJ, Keefe EM, Banks CE. Adjusting the Connection Length of Additively Manufactured Electrodes Changes the Electrochemical and Electroanalytical Performance. Sensors. 2022; 22(23):9521. https://doi.org/10.3390/s22239521
Chicago/Turabian StyleCrapnell, Robert D., Alejandro Garcia-Miranda Ferrari, Matthew J. Whittingham, Evelyn Sigley, Nicholas J. Hurst, Edmund M. Keefe, and Craig E. Banks. 2022. "Adjusting the Connection Length of Additively Manufactured Electrodes Changes the Electrochemical and Electroanalytical Performance" Sensors 22, no. 23: 9521. https://doi.org/10.3390/s22239521










